Abstract
recA protein coats DNA co-operatively to form filaments approximately 100 A thick, which in the presence of ATP, and more stably so in the presence of the non-hydrolyzable analog ATP gamma S, have a helical appearance with a deep cleft in the protein coat. This protein helix follows the DNA helix, to which it imparts a new helicity of 18.5 bp per turn of 97 A pitch. Here we test the accessibility of the DNA in the complex to modification by dimethylsulfate, and find that the complexed DNA is approximately 2-fold more reactive on the major groove side than it was in B-DNA (methylation of guanine N7), while it is protected approximately 2-fold on the minor groove side (methylation of adenine N3), suggesting that the protein coats the DNA along the minor groove. Furthermore, N3 of cytosine, a residue involved in base pairing, is found exposed in complexes with single strands as it is in naked single-stranded DNA, while it remains inaccessible in complexes with double strands, suggesting that the latter is not melted at this stage of the strand exchange reaction.
Full text
PDF
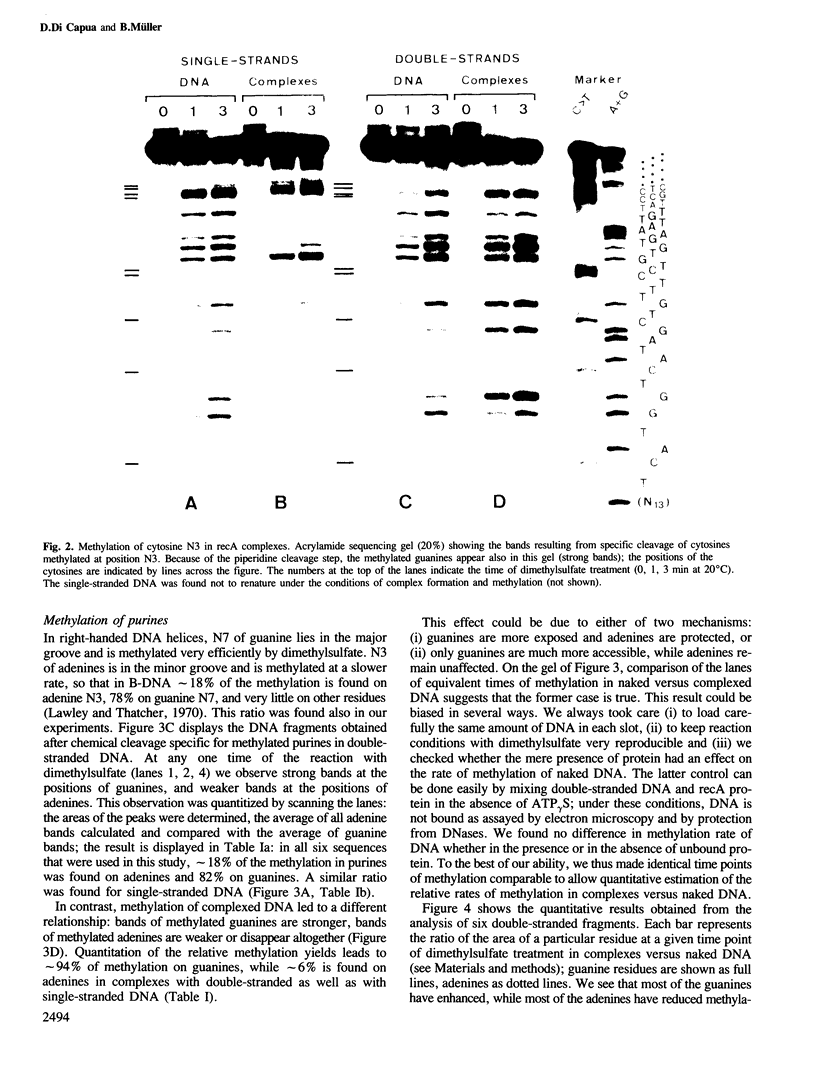
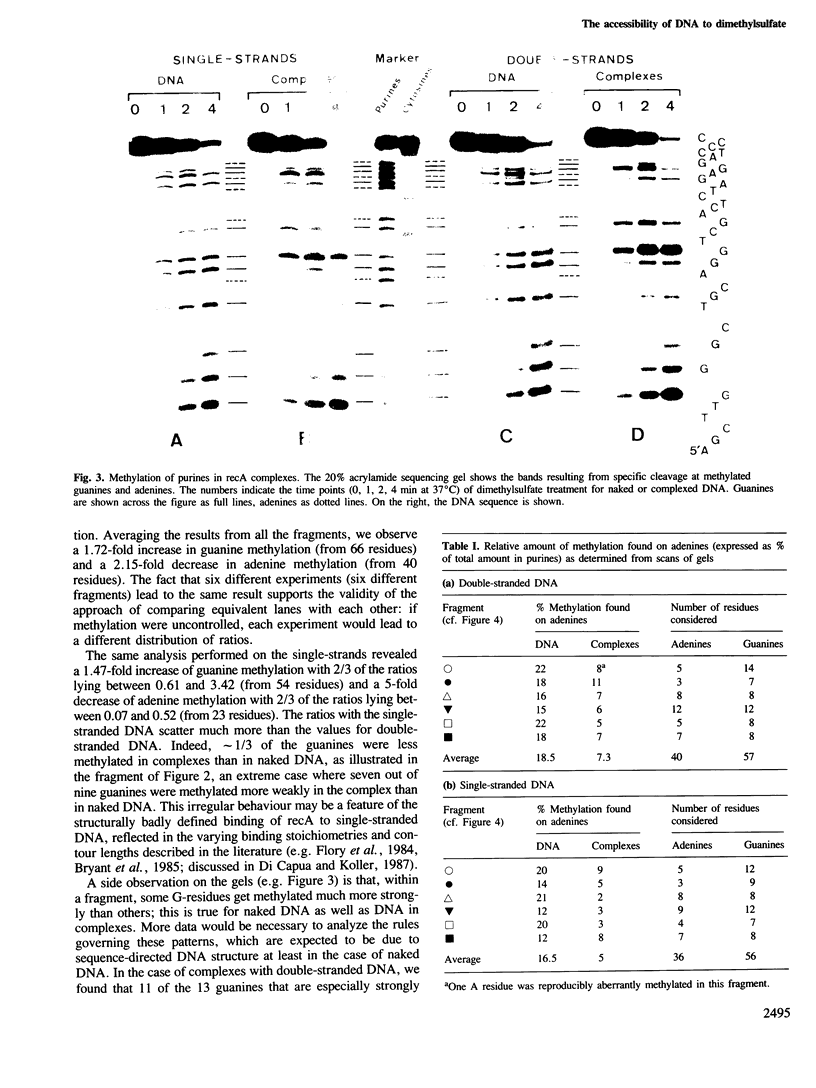
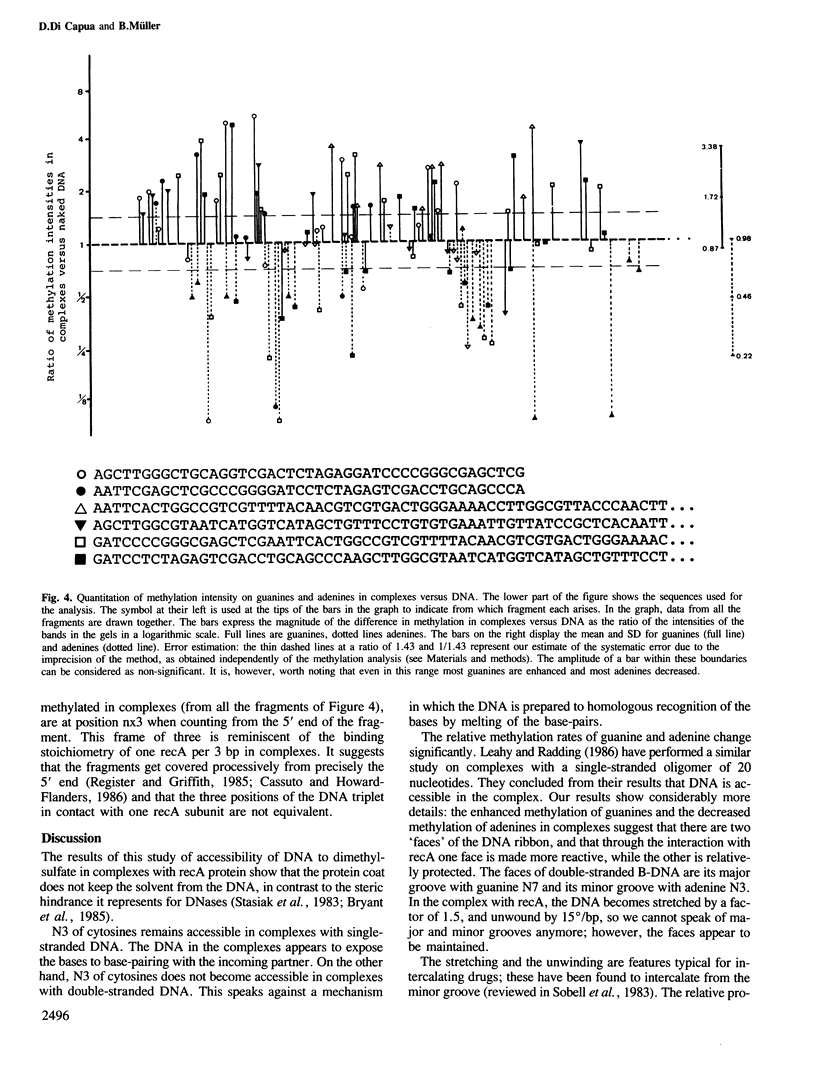


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryant F. R., Taylor A. R., Lehman I. R. Interaction of the recA protein of Escherichia coli with single-stranded DNA. J Biol Chem. 1985 Jan 25;260(2):1196–1202. [PubMed] [Google Scholar]
- Cassuto E., Howard-Flanders P. The binding of RecA protein to duplex DNA molecules is directional and is promoted by a single stranded region. Nucleic Acids Res. 1986 Feb 11;14(3):1149–1157. doi: 10.1093/nar/14.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. M., McEntee K., Lehman I. R. A simple and rapid procedure for the large scale purification of the recA protein of Escherichia coli. J Biol Chem. 1981 May 10;256(9):4676–4678. [PubMed] [Google Scholar]
- Di Capua E., Engel A., Stasiak A., Koller T. Characterization of complexes between recA protein and duplex DNA by electron microscopy. J Mol Biol. 1982 May 5;157(1):87–103. doi: 10.1016/0022-2836(82)90514-9. [DOI] [PubMed] [Google Scholar]
- Flory J., Tsang S. S., Muniyappa K. Isolation and visualization of active presynaptic filaments of recA protein and single-stranded DNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7026–7030. doi: 10.1073/pnas.81.22.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., West S. C., Stasiak A. Role of RecA protein spiral filaments in genetic recombination. Nature. 1984 May 17;309(5965):215–219. doi: 10.1038/309215a0. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Buc H., Spassky A., Wang J. C. Mapping of single-stranded regions in duplex DNA at the sequence level: single-strand-specific cytosine methylation in RNA polymerase-promoter complexes. Proc Natl Acad Sci U S A. 1983 May;80(9):2544–2548. doi: 10.1073/pnas.80.9.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy M. C., Radding C. M. Topography of the interaction of recA protein with single-stranded deoxyoligonucleotides. J Biol Chem. 1986 May 25;261(15):6954–6960. [PubMed] [Google Scholar]
- Register J. C., 3rd, Griffith J. The direction of RecA protein assembly onto single strand DNA is the same as the direction of strand assimilation during strand exchange. J Biol Chem. 1985 Oct 5;260(22):12308–12312. [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Sakore T. D., Jain S. C., Banerjee A., Bhandary K. K., Reddy B. S., Lozansky E. D. beta-kinked DNA--a structure that gives rise to drug intercalation and DNA breathing--and its wider significance in determining the premelting and melting behavior of DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):293–314. doi: 10.1101/sqb.1983.047.01.035. [DOI] [PubMed] [Google Scholar]
- Stasiak A., Di Capua E. The helicity of DNA in complexes with recA protein. Nature. 1982 Sep 9;299(5879):185–186. doi: 10.1038/299185a0. [DOI] [PubMed] [Google Scholar]
- Stasiak A., DiCapua E., Koller T. Unwinding of duplex DNA in complexes with recA protein. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):811–820. doi: 10.1101/sqb.1983.047.01.093. [DOI] [PubMed] [Google Scholar]
- Tsang S. S., Muniyappa K., Azhderian E., Gonda D. K., Radding C. M., Flory J., Chase J. W. Intermediates in homologous pairing promoted by recA protein. Isolation and characterization of active presynaptic complexes. J Mol Biol. 1985 Sep 20;185(2):295–309. doi: 10.1016/0022-2836(85)90405-x. [DOI] [PubMed] [Google Scholar]
- West S. C., Cassuto E., Mursalim J., Howard-Flanders P. Recognition of duplex DNA containing single-stranded regions by recA protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2569–2573. doi: 10.1073/pnas.77.5.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]