Abstract
Objective
To investigate the cardiometabolic effects of a severe hypothyroid state induced by withdrawal of thyroid hormone replacement before radioactive iodine therapy.
Methods
Patients with thyroid cancer who were scheduled to receive radioactive iodine ablation were enrolled. Cardiometabolic parameters were measured using blood samples taken immediately before levothyroxine withdrawal, 4 weeks following withdrawal (on radiotherapy day), and 4 weeks following reinstitution of levothyroxine.
Results
Out of 48 patients (age 49.4 ± 10.5 years; 77.1% [37/48] female), the severe hypothyroid state induced by levothyroxine withdrawal significantly aggravated the majority of lipid parameters, particularly in patients with a greater number of metabolic syndrome components. Fasting plasma glucose levels and homeostatic model assessment values for insulin resistance and β-cell function significantly decreased following levothyroxine withdrawal. Serum high-sensitivity C-reactive protein, fibrinogen and cystatin C levels significantly decreased, and homocysteine levels increased during the severe hypothyroid state. All of these changes were reversed by levothyroxine reinstitution.
Conclusions
Severe hypothyroid state induced pronounced changes in cardiometabolic parameters. Further studies should identify the long-term effects of changes in these parameters on cardiovascular morbidity and mortality in relation to thyroid disease.
Keywords: Hypothyroidism, atherosclerosis, metabolic syndrome, thyroid hormone withdrawal, thyroid cancer
Introduction
Hypothyroidism adversely affects cardiovascular morbidity and mortality,1,2 as thyroid hormone regulates lipid metabolism and cardiovascular haemodynamics. Hypothyroidism leads to lipid abnormalities that are characterized by hypercholesterolaemia with increased levels of low-density lipoprotein (LDL) and apolipoprotein B, due to reduced numbers of LDL receptors in the liver and subsequent decrease in LDL clearance.3 Furthermore, hypothyroidism increases systemic vascular resistance and impairs cardiac contractility and diastolic function.4 These changes in lipid profiles and haemodynamics are potentially associated with atherosclerosis and an increased risk of cardiovascular disease and mortality among patients with subclinical or overt hypothyroidism.5–7
Thyroid hormone status has also been demonstrated to affect various adipocytokines8–10 and atherogenic inflammatory markers,11–15 which may lead to additional harmful effects that promote the progression of cardiovascular disease. In addition, patients with hypothyroidism exhibit elevated serum levels of high-sensitivity C-reactive protein (hs-CRP),11,15 homocysteine,11–13 and fibrinogen,13,14 which are well-known biomarkers and independent risk factors for atherosclerosis. Serum levels of cystatin C are a marker for renal function and cardiovascular disease, and have been reported to exhibit changes according to thyroid function.16–18 Whether these markers are independently correlated with thyroid function and whether they can be reversed following treatment of thyroid dysfunction, however, remains controversial. In addition, the abovementioned studies have included heterogeneous populations with different severities, durations, and aetiologies of thyroid dysfunction, which might confound any analyses of the effects of thyroid hormone on these markers.
During radioactive iodine therapy following thyroidectomy for differentiated thyroid cancer, patients experience a severe hypothyroid state after thyroid hormone treatment withdrawal, and they subsequently recover once thyroid hormone treatment is reinstated.9,10 Such serial changes in thyroid hormone status may cause drastic changes in cardiometabolic parameters, and produce a representative and well-controlled group of patients with homogeneous thyroid hormone status.
The present study aimed to evaluate the effects of the severe hypothyroid state following thyroid hormone withdrawal on cardiometabolic and vascular inflammatory parameters among patients who underwent radioactive iodine therapy after thyroidectomy, and to identify any patient subgroups who experienced more pronounced changes in these parameters.
Patients and methods
Study population and design
This prospective cohort study enrolled consecutive patients with differentiated thyroid cancer who were scheduled to receive radioactive iodine ablation following total thyroidectomy at Konkuk University Medical Center, Seoul, Korea between May 2012 and May 2013. Patients with chronic disease (including kidney, liver, and heart failure), acute infection or inflammatory conditions, or who were receiving drugs for diabetes mellitus, hypertension, dyslipidaemia, or other conditions that might affect metabolic parameters were excluded.
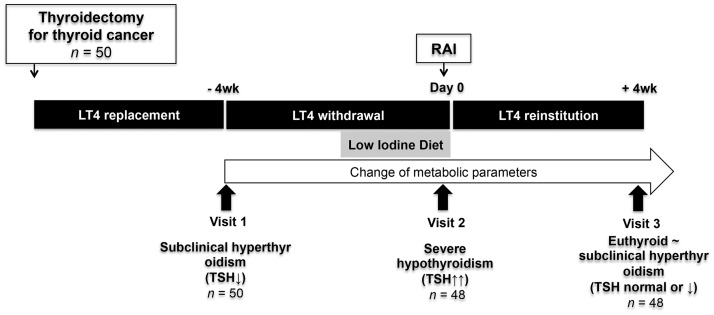
Blood samples were obtained at three time points to identify serial changes in various metabolic parameters (Figure 1): Visit 1, immediately before withdrawal of levothyroxine in preparation for radioactive iodine ablation (a mild thyrotoxic state due to receiving levothyroxine for thyroid stimulating hormone [TSH] suppression); Visit 2, day of radioactive iodine ablation following 4 weeks of levothyroxine withdrawal (a severe hypothyroid state with TSH levels ≥30 mU/l); and Visit 3, at 4 weeks following reinstitution of levothyroxine (recovery to a mild thyrotoxic state).
Figure 1.
Schematic of study design in patients with differentiated thyroid cancer who received radioactive iodine ablation following total thyroidectomy. LT4, levothyroxine; RAI, radioactive iodine; TSH, thyroid stimulating hormone.
The study protocol was reviewed and approved by the institutional review board of Konkuk University Medical Center (KUH1010507), and this study was registered in the ClinicalTrial.gov registry (NCT01744769). All participants provided written informed consent.
Outcome measures
Blood pressure and heart rate were recorded three times between 7:00 and 9:00 h after the patients had been in a relaxed state for ≥10 min, and a 5-min rest period was provided between each measurement. The mean of three readings was used for analysis.
Between 7:00 and 9:00 h, and following a 14 h overnight fast, 20 ml of venous blood was drawn from the antecubital vein of each patient into tubes without anticoagulant and tubes containing ethylenediaminetetra-acetic acid-2Na (1 mg/ml blood). Plasma was immediately separated by centrifugation at 2000 g at 4 ℃ for 10 min, and serum was prepared by allowing the blood sample to clot for 30 min at room temperature, followed by centrifugation at 2000 g at 4 ℃ for 10 min.
Biochemical measurements of cardiometabolic parameters were conducted immediately following sample preparation. Fasting plasma glucose (FPG), serum triglycerides, free fatty acid, homocysteine, cystatin C, fibrinogen, hs-CRP, total cholesterol, high-density lipoprotein cholesterol (HDL-C), LDL cholesterol (LDL-C), and apolipoprotein A and B levels were measured using a Toshiba 200FR automatic analyser (Toshiba Medical System Co., Ltd, Tokyo, Japan) according to the manufacturer’s instructions. Plasma insulin levels were determined using a MODULAR analytics E170 module (Roche Diagnostics, Manheim, Germany) and glycosylated haemoglobin (HbA1c) levels were measured using a VARIANT II TURBO 2.0 kit (Bio-Rad Laboratories, Hercules, CA, USA), both according to the manufacturer’s instructions. Free thyroxine and TSH were measured using an automatic SR300 radioimmunoassay analyser (STRATEC Biomedical AG, Birkenfeld, Germany) and associated reagents, according to the manufacturer’s instructions.
Primary outcome measures were changes in cardiometabolic parameters, comprising blood lipids (total cholesterol, LDL-C, HDL-C, triglycerides, free fatty acids, and apolipoproteins A and B), glycaemic parameters (FPG, fasting insulin, HbA1c, and homeostatic model assessment values for insulin resistance and β-cell function [HOMA-IR and HOMA-B], and vascular inflammation and atherosclerosis markers (uric acid, hs-CRP, fibrinogen, homocysteine, and cystatin C), according to thyroid hormone status.
Secondary outcome measures were the correlation between TSH levels and metabolic parameters, and the baseline characteristics that predicted changes in metabolic parameters. The signs and symptoms of hypothyroidism were assessed using the Zulewski score as previously described,19 and metabolic syndrome was defined according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III report.20
Statistical analyses
All normally distributed continuous variables are presented as mean ± SD, and categorical variables as presented as n (%) prevalence. Student’s paired t-test or analysis of variance was used to compare continuous variables, and χ2-test was used to compare categorical variables. The relationship between changes in TSH levels and other parameters was evaluated using Pearson’s correlation coefficient. All analyses were performed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA), and differences with a P-value < 0.05 were considered statistically significant.
Results
Baseline characteristics
Among 50 patients who were enrolled, two patients withdrew during the study period and 48 patients completed the study. Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of patients with differentiated thyroid cancer who were scheduled to receive radioactive iodine ablation following total thyroidectomy.
| Characteristic | Patient cohort (n = 48) |
|---|---|
| Age, years | 49.4 ± 10.5 |
| Female | 37 (77.1) |
| Menopausal | 21 (56.8) |
| Smoker | 0 (0) |
| Levothyroxine dosage, µg/day | 126.8 ± 12.0 |
| Duration between thyroidectomy and radioactive iodine therapy, days | 134.3 ± 42.1 |
| Radioactive iodine therapy dose, mCi | 123.8 ± 21.1 |
Data presented as mean ± SD or n (%) patient prevalence.
Changes in metabolic parameters according to changes in thyroid hormone status
Changes in haemodynamic and metabolic parameters according to changes in thyroid hormone status were investigated by comparing parameters immediately before levothyroxine withdrawal (Visit 1), at 4 weeks following levothyroxine withdrawal (on day of radiotherapy; Visit 2), and at 4 weeks following levothyroxine reinstitution (Visit 3; Table 2). TSH levels had significantly increased and free thyroxine levels significantly decreased at Visit 2 (66.9 ± 30.3 mU/l and 0.19 ± 0.17 ng/dl, respectively; both P < 0.05 compared with Visit 1), and these changes were subsequently reversed at Visit 3 (2.4 ± 5.0 mU/l and 2.03 ± 0.35 ng/dl, respectively; both P < 0.05 compared with Visit 2). The mean Zulewski score increased, and mean systolic blood pressure and heart rate (HR) decreased, during the severe hypothyroid state; all changes were reversed following reinstitution of levothyroxine. Most lipid parameters (total cholesterol, LDL-C, triglycerides, free fatty acids and apolipoprotein B levels) significantly increased following levothyroxine withdrawal, including HDL-C levels (Table 2); all of these changes were reversed by levothyroxine replacement. In addition, FPG, insulin, HOMA-B, and HOMA-IR significantly decreased following levothyroxine withdrawal, and these changes were also reversed after levothyroxine reinstitution. Among the markers of vascular inflammation and atherosclerosis, levothyroxine withdrawal was associated with decreased levels of hs-CRP, fibrinogen, and cystatin C, although serum homocysteine levels were significantly increased. All of these changes were reversed by levothyroxine reinstitution, with the exception of the decreased cystatin C levels (Table 2).
Table 2.
Change of metabolic parameters in patients with differentiated thyroid cancer who received radioactive iodine ablation following total thyroidectomy (n = 48). Metabolic parameters were measured immediately before withdrawal of levothyroxine in preparation for radioactive iodine ablation (Visit 1), at 4 weeks following levothyroxine withdrawal (on day of radiotherapy; Visit 2) and at 4 weeks following reinstitution of levothyroxine (Visit 3).
| Blood sampling day |
|||
|---|---|---|---|
| Parameter | Visit 1 | Visit 2 | Visit 3 |
| TSH, mU/l | 0.14 ± 0.32 | 66.9 ± 30.3* | 2.4 ± 5.0*† |
| Free thyroxine, ng/dl | 2.17 ± 0.37 | 0.19 ± 0.17* | 2.03 ± 0.35† |
| Zulewski score18 | 0.48 ± 0.65 | 1.00 ± 1.15* | 0.68 ± 0.96† |
| Body mass index, kg/m2 | 24.3 ± 3.6 | 24.0 ± 3.5 | 24.4 ± 3.8 |
| Waist circumference, cm | 81.6 ± 10.2 | 81.5 ± 10.0 | 81.9 ± 10.0 |
| Systolic blood pressure, mmHg | 126.0 ± 14.0 | 115.4 ± 13.4* | 120.7 ± 14.0† |
| Diastolic blood pressure, mmHg | 74.6 ± 14.0 | 74.3 ± 10.1 | 72.3 ± 10.4 |
| Heart rate, beats/min | 84.0 ± 13.2 | 69.4 ± 10.8* | 81.3 ± 13.2† |
| AST, IU/l | 24.8 ± 13.0 | 41.8 ± 21.9* | 23.1 ± 7.6† |
| ALT, IU/l | 27.1 ± 18.7 | 41.4 ± 28.3* | 23.1 ± 7.6† |
| Creatinine, mg/dl | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.7 ± 0.2 |
| Total cholesterol, mg/dl | 178.7 ± 33.6 | 238.7 ± 50.8* | 186.7 ± 37.0† |
| Triglycerides, mg/dl | 141.1 ± 66.7 | 159.1 ± 90.6* | 117.3 ± 62.0*† |
| HDL-cholesterol, mg/dl | 54.0 ± 13.5 | 64.8 ± 18.9* | 55.7 ± 16.6† |
| LDL-cholesterol, mg/dl | 96.5 ± 27.6 | 142.1 ± 44.7* | 107.6 ± 31.5† |
| Apolipoprotein A, mg/dl | 156.6 ± 40.1 | 165.4 ± 27.5 | 159.1 ± 36.7 |
| Apolipoprotein B, mg/dl | 71.2 ± 27.3 | 105.7 ± 35.2* | 74.5 ± 29.4† |
| Free fatty acid, µEq/l | 222.8 ± 172.0 | 512.4 ± 255.0* | 297.2 ± 202.3† |
| Fasting plasma glucose, mg/dl | 107.4 ± 19.0 | 91.3 ± 9.2* | 104.7 ± 20.3† |
| Fasting insulin, μIU/ml | 26.8 ± 26.0 | 6.3 ± 4.6* | 20.0 ± 22.3 |
| HbA1c, % | 5.6 ± 0.5 | 5.7 ± 0.5 | 5.6 ± 0.4 |
| HOMA-B | 204.3 ± 157.6 | 86.9 ± 67.4* | 182.1 ± 165.6 |
| HOMA-IR | 7.8 ± 8.6 | 1.5 ± 1.1* | 5.7 ± 7.2 |
| Uric acid, mg/dl | 4.8 ± 1.3 | 5.2 ± 1.5* | 4.5 ± 1.1† |
| Homocysteine, µmol/l | 9.5 ± 3.0 | 12.7 ± 4.7* | 10.1 ± 3.1† |
| hs-CRP, mg/l | 0.17 ± 0.08 | 0.08 ± 0.07* | 0.10 ± 0.09† |
| Fibrinogen, mg/dl | 315.6 ± 53.3 | 288.7 ± 37.8* | 308.2 ± 45.6† |
| Cystatin C, mg/l | 1.36 ± 0.31 | 1.22 ± 0.33* | 1.28 ± 0.27* |
Data presented as mean ± SD.
P < 0.05 versus Visit 1; †P < 0.05 versus Visit 2 (Student’s paired t-test or analysis of variance).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, glycosylated haemoglobin; HOMA-IR, homoeostasis model assessment for insulin resistance; HOMA-B, homoeostasis model assessment for β-cell function; hs-CRP, high-sensitivity C-reactive protein.
Changes metabolic syndrome components during a severe hypothyroid state
Most metabolic syndrome components were aggravated following levothyroxine withdrawal, with the exception of glycaemic profiles during the severe hypothyroid phase. Thus, the proportions of patients who fulfilled the metabolic syndrome criteria for each related component, based on the NCEP Adult Treatment Panel III definition of metabolic syndrome,20 were analysed according to changes in thyroid hormone status (Figure 2). Among the metabolic syndrome components, proportions of male and female patients with high triglyceride levels (≥150 mg/dl) increased significantly during the severe hypothyroid state (Visit 2), however, proportions of other metabolic syndrome components, such as high blood pressure, high FPG levels (among female patients), and high HDL-C levels decreased during the hypothyroid state (P < 0.05 versus Visit 1; Figure 2). The proportion of all patients who fulfilled the criteria for metabolic syndrome decreased from 29.2 % (14/48) at baseline (Visit 1) to 25.0 % (12/48) during the hypothyroid state (P < 0.05). There were no sex-specific differences in these changes, and the proportions returned to baseline values following reinstitution of levothyroxine.
Figure 2.
Serial changes in the proportion of patients with metabolic syndrome components associated with withdrawal and reinstitution of thyroid hormone in patients with differentiated thyroid cancer who received radioactive iodine ablation following total thyroidectomy (n = 48). Proportion of patients with (a) waste circumference (WC) ≥ 90 cm among males and ≥ 80 cm among females; (b) blood pressure (BP) ≥ 130/85 mmHg; (c) fasting plasma glucose (FPG) ≥ 100 mg/dl; (d) triglyceride (TG) ≥ 150 mg/dl; (e) high-density lipoprotein-cholesterol (HDL-C) levels < 40 mg/dl among males and < 50 mg/dl among females; and (f) metabolic syndrome defined according to the National Cholesterol Education Program Adult Treatment Panel III report.20 Data presented as % patient prevalence. Visit 1, immediately before withdrawal of levothyroxine in preparation for radioactive iodine ablation; Visit 2, at 4 weeks following levothyroxine withdrawal (on day of radiotherapy); and Visit 3, at 4 weeks following reinstitution of levothyroxine. *P < 0.05 versus Visit 1; #P < 0.05 versus Visit 2 (χ2-test).
Correlation between TSH levels and metabolic parameters following thyroid hormone withdrawal
The correlation between TSH levels and the metabolic parameters that changed during the severe hypothyroid state were further analysed. During the severe hypothyroid state (Visit 2) TSH levels exhibited a significant positive correlation with LDL-C levels (r = 0.3, P = 0.04) and HDL-C levels (r = 0.39, P = 0.01), and a significant negative correlation with hs-CRP levels (r = −0.32, P = 0.03) (Figure 3). TSH levels were not significantly correlated with other metabolic parameters (triglycerides, free fatty acids, uric acid, homocysteine, and fibrinogen levels).
Figure 3.
Correlation between thyroid stimulating hormone (TSH) levels and metabolic parameters in patients with differentiated thyroid cancer who received radioactive iodine ablation following total thyroidectomy (n = 48). Levels of (a) triglyceride (TG); (b) free fatty acid (FFA); (c) low-density lipoprotein cholesterol (LDL-C); (d) high-density lipoprotein cholesterol (HDL-C); (e) uric acid; (f) high-sensitivity C-reactive protein (HS-CRP); (g) homocysteine; and (H) fibrinogen in patients in a severe hypothyroid state at 4 weeks following levothyroxine withdrawal (Pearson’s correlation coefficient).
Baseline characteristics of patients with different metabolic responses following thyroid hormone withdrawal
To identify characteristics that may predict significant changes in cardiometabolic parameters during a hypothyroid state, patients were divided into tertiles according to magnitude of change in lipid parameters. Patients in the upper tertile for change in triglyceride levels exhibited higher baseline levels of aspartate aminotransferase (AST)/ alanine aminotransferase (ALT), triglycerides, free fatty acids, fibrinogen and uric acid, and a higher baseline proportion of metabolic syndrome, compared with the lower tertile group (P < 0.05; Table 3). Patients in the upper tertile for change in LDL-C levels exhibited a similar pattern (higher baseline body mass index, AST/ALT, total cholesterol, LDL-C, triglycerides, free fatty acids, apolipoprotein B, hs-CRP, homocysteine, fibrinogen, cystatin C, and uric acid levels and a higher baseline proportion of metabolic syndrome) compared with the lower tertile group (P < 0.05; Table 3). The severe hypothyroid state also induced an increase in HDL-C levels, and the upper tertile group for changes in HDL-C levels exhibited a significantly greater proportion of female patients, a lower baseline proportion of patients with metabolic syndrome, a lower body mass index, and a smaller waist circumference. Furthermore, these patients exhibited lower baseline levels of FPG, hs-CRP, and uric acid (P < 0.05; Table 3).
Table 3.
Comparison of baseline characteristics between upper and lower tertiles grouped according to change of lipid profiles during levothyroxine withdrawal in patients with differentiated thyroid cancer who received radioactive iodine ablation following total thyroidectomy (n = 48).
|
|
Blood lipid parameter |
|
||||
|---|---|---|---|---|---|---|
| Characteristic | Change in triglycerides |
Change in LDL-C |
Change in HDL-C |
|||
| Lowest tertile (n = 15) | Highest tertile (n = 16) | Lowest tertile (n = 16) | Highest tertile (n = 17) | Lowest tertile (n = 15) | Highest tertile (n = 16) | |
| Age, years | 55.2 ± 7.0 | 53.8 ± 10.0 | 54.2 ± 7.2 | 53.5 ± 10.2 | 49.7 ± 13.4 | 50.2 ± 9.7 |
| Female | 12 (80.0) | 12 (75.0) | 12 (75.0) | 13 (76.5) | 10 (66.7) | 15 (93.8)* |
| Metabolic syndrome | 3 (20.0) | 5 (31.3)* | 3 (18.8) | 6 (35.3)* | 6 (40.0) | 3 (18.8)* |
| Body mass index, kg/m2 | 23.4 ± 2.6 | 25.0 ± 2.4 | 23.6 ± 3.2 | 25.3 ± 2.8* | 25.1 ± 4.2 | 23.3 ± 3.5* |
| Waist circumference, cm | 80.2 ± 11.6 | 83.5 ± 8.3 | 80.3 ± 10.6 | 82.1 ± 8.8 | 84.2 ± 10.5 | 75.5 ± 4.4* |
| Systolic blood pressure, mmHg | 130.0 ± 10.1 | 126.5 ± 12.4 | 127.0 ± 10.2 | 126.8 ± 10.4 | 123.0 ± 11.3 | 122.8 ± 11.7 |
| TSH, mU/l | 0.08 ± 0.06 | 0.04 ± 0.02 | 0.07 ± 0.05 | 0.05 ± 0.02 | 0.08 ± 0.05 | 0.08 ± 0.04 |
| Free thyroxine, ng/dl | 2.25 ± 0.37 | 2.22 ± 0.17 | 2.20 ± 0.40 | 2.21 ± 0.28 | 2.03 ± 0.31 | 2.28 ± 0.38 |
| AST, IU/l | 20.8 ± 6.5 | 37.7 ± 7.9* | 22.2 ± 5.5 | 35.8 ± 8.8* | 24.8 ± 9.5 | 21.8 ± 4.8 |
| ALT, IU/l | 20.1 ± 5.5 | 39.8 ± 9.5* | 23.5 ± 7.5 | 38.2 ± 9.7* | 26.9 ± 10.6 | 19.5 ± 5.6 |
| Fasting glucose, mg/dl | 115.1 ± 25.4 | 108.9 ± 12.0 | 110.1 ± 13.4 | 107.9 ± 11.1 | 116.6 ± 19.7 | 96.5 ± 18.7* |
| Total cholesterol, mg/dl | 172.0 ± 28.9 | 182.0 ± 36.6 | 163.0 ± 28.0 | 193.1 ± 36.8* | 196.1 ± 38.3 | 168.7 ± 36.3 |
| Triglycerides, mg/dl | 115.3 ± 46.5 | 163.1 ± 46.7* | 115.3 ± 46.5 | 163.1 ± 46.7* | 141.1 ± 54.6 | 141.3 ± 79.8 |
| HDL-cholesterol, mg/dl | 51.2 ± 14.2 | 50.8 ± 12.7 | 51.3 ± 13.2 | 50.1 ± 11.9 | 54.3 ± 13.1 | 53.2 ± 11.8 |
| LDL-cholesterol, mg/dl | 95.5 ± 22.5 | 105.3 ± 23.7 | 91.5 ± 21.0 | 109.2 ± 23.8* | 112.5 ± 25.0 | 109.3 ± 25.3 |
| Apolipoprotein A, mg/dl | 158.6 ± 38,3 | 156.1 ± 34.5 | 158.6 ± 38,3 | 151.0 ± 32.1 | 153.6 ± 38,3 | 162.0 ± 28.3 |
| Apolipoprotein B, mg/dl | 71.5 ± 25.3 | 75.5 ± 27.3 | 70.2 ± 24.2 | 79.3 ± 22.1* | 76.3 ± 25.1 | 72.5 ± 29.4 |
| Free fatty acid, µEq/l | 205.6 ± 78.3 | 293.1 ± 95.5* | 210.6 ± 74.3 | 295.2 ± 83.5* | 164.9 ± 98.5 | 207.8 ± 110.2 |
| hs-CRP, mg/l | 0.10 ± 0.07 | 0.26 ± 0.09* | 0.11 ± 0.08 | 0.24 ± 0.09* | 0.18 ± 0.09 | 0.08 ± 0.05* |
| Homocysteine, µmol/l | 10.5 ± 3.6 | 8.8 ± 2.1 | 7.9 ± 3.1 | 10.3 ± 2.5* | 10.2 ± 4.3 | 9.4 ± 2.0 |
| Fibrinogen, mg/dl | 332.3 ± 44.5 | 356.8 ± 52.5* | 321.3 ± 45.5 | 366.2 ± 53.2* | 328.9 ± 55.7 | 303.2 ± 46.5 |
| Cystatin C, mg/l | 1.32 ± 0.30 | 1.35 ± 0.31 | 1.18 ± 0.41 | 1.39 ± 0.49* | 1.26 ± 0.32 | 1.10 ± 0.31 |
| Uric acid, mg/dl | 4.0 ± 1.2 | 5.3 ± 1.1* | 4.1 ± 1.1 | 5.4 ± 1.0* | 5.2 ± 1.8 | 4.2 ± 1.1* |
Data presented as mean ± SD or n (%) patient prevalence.
P < 0.05 versus lower tertile (Student’s paired t-test or analysis of variance for continuous variables; χ2-test for categorical variables).
TSH, thyroid stimulating hormone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein.
Discussion
In the present study among patients who underwent total thyroidectomy for differentiated thyroid cancer, severe hypothyroidism due to thyroid hormone withdrawal produced noticeable changes in various cardiometabolic parameters, particularly among patients with underlying metabolic syndrome. Interestingly, these changes were fully reversed following reinstitution of thyroid hormone treatment. Furthermore, the present study revealed the direction of changes in these metabolic parameters, which have been reported with conflicting results in previous studies.15,21
As expected, a severe hypothyroid state significantly aggravated the majority of lipid parameters that were evaluated in the present study, such as levels of LDL-C, triglycerides, free fatty acids and apolipoprotein B. In addition, patients who had a greater number of metabolic syndrome components prior to levothyroxine withdrawal subsequently experienced greater changes in these parameters during the severe hypothyroid state. Furthermore, changes in LDL-C levels were significantly correlated with TSH levels, although not with triglycerides and free fatty acids levels. The results of the present study concur with previously published findings. For example, differences in lipid profiles according to thyroid function have been reported,15 with levels of total cholesterol, triglycerides, LDL-C, and apolipoprotein B increasing in patients with hypothyroidism and decreasing in patients with hyperthyroidism. TSH levels were also reported to be significantly correlated with changes in triglycerides, apolipoprotein B, and LDL-C levels,15 although this relationship was not observed in the present study. The discrepancy between the present and published results may be related to the fact that correlations between TSH levels and lipid parameters were examined during a severe hypothyroid state in the present study, when TSH levels were 20–125 mU/l. HDL-C levels were also elevated during the hypothyroid state in the present study, and may have resulted from decreased hepatic lipase and cholesterol-ester transport protein activity, leading to decreased HDL catabolism, reduced transfer of cholesteryl esters from HDL2 to very low- and intermediate-density lipoproteins, reduced transport of HDL2 to HDL3, and ultimately increased levels of HDL2.21
Several studies have reported that thyroid hormone replacement exerts favourable effects on lipid profiles and haemodynamic factors, which may eventually reduce the risk of cardiovascular disease morbidity and mortality. Levothyroxine replacement in patients with hypothyroidism reduces body weight22 and improves various haemodynamic factors, such as central arterial stiffness and endothelium-dependent vasodilatation.23 In addition, atherogenic lipid levels (e.g., total cholesterol, LDL-C, triglycerides, and apolipoprotein B) are reported to be reduced following levothyroxine replacement in patients with overt hypothyroidism.24 Studies have also reported, however, that levothyroxine replacement does not affect these cardiometabolic parameters among patients with subclinical disease.24,25 In the present study, levothyroxine reinstitution fully reversed the changes in cardiometabolic parameters that were induced by short-term levothyroxine withdrawal, although further studies are needed to determine if levothyroxine has different effects in the acute and chronic hypothyroid states.
In addition to lipid profiles, changes in atherogenic markers (i.e., homocysteine, CRP, fibrinogen, and cystatin C levels) associated with the withdrawal and reinstitution of levothyroxine were also investigated in the present study. Plasma levels of homocysteine levels were observed to be significantly elevated during the severe hypothyroid state, and levothyroxine replacement reversed this elevation. Interestingly, homocysteine is an atherogenic factor that promotes the production of vascular inflammatory chemokines, reactive oxygen species, and oxidized LDL-C.26,27 A meta-analysis has also revealed that plasma homocysteine levels are elevated in patients with hypothyroidism, compared with those in healthy individuals, although levothyroxine replacement can reverse this elevation.28 Another study has suggested that these hypothyroidism-induced elevated homocysteine levels may induce insulin resistance and increase the risk of cardiovascular disease.12 In the present study, however, HOMA-IR was significantly decreased during the hypothyroid state.
Although homocysteine levels and the majority of lipid parameters exhibited unfavourable changes during the severe hypothyroid state in the present study, favourable changes in blood pressure, FPG levels, and atherosclerosis markers (e.g., hs-CRP, fibrinogen, and cystatin C levels) were observed during this state. Furthermore, HOMR-B was shown to be reduced during the severe hypothyroid state. Although several studies have reported increased insulin resistance in cases of hypothyroidism,12,29 the present results are consistent with findings of one study that showed a 7.4% decrease in FPG levels in cases of overt hypothyroidism compared with cases of subclinical disease, and that the decrease was correlated with reduced insulin resistance.10 Interestingly, hypothyroidism induces reductions in energy expenditure and metabolic rate, which may lead to lower levels of FPG and insulin.30
Previous studies have reported conflicting results regarding changes in CRP, fibrinogen and cystatin C levels among patients with hypothyroidism.11,13–17 These factors are well-known inflammatory markers of cardiovascular disease, and many of the previous studies have focused on the increase in these markers and their association with the risk of cardiovascular disease during the hypothyroid state.11,14,16 Thus, the favourable changes in hs-CRP, fibrinogen, and cystatin C levels observed during the hypothyroid state in the present study are difficult to interpret. Nevertheless, the authors speculate that the low-iodine diet recommended for patients undergoing radioactive iodine therapy could affect these parameters, and that acute withdrawal of levothyroxine may induce different responses in these inflammatory markers, compared with the responses observed during chronic hypothyroidism.31
The present study has several clinical implications. First, patients with hyperlipidaemia should be tested to determine their thyroid function, and should be actively treated using thyroid hormone if hypothyroidism is detected, as levothyroxine reinstatement restored lipid profiles to their baseline state within 4 weeks. Furthermore, patients with baseline metabolic syndrome components experienced pronounced changes in their lipid profiles after levothyroxine withdrawal in the present study, which suggests that these patients should be carefully assessed before withdrawing levothyroxine in preparation for radioactive iodine therapy. Given that these changes were fully reversed following levothyroxine reinstitution, however, and that several cardiometabolic markers exhibited favourable changes during the severe hypothyroid state, short-term withdrawal of levothyroxine may not significantly affect the development of cardiovascular disease.
The results of the present study may be limited by the relatively small sample size and the fact that this was a single centre study. Further multicentre studies should be conducted, with larger sample sizes. In addition, further studies are needed to identify the long-term effects of changes in these parameters on cardiovascular morbidity and mortality in relation to thyroid disease.
In conclusion, levothyroxine withdrawal-related hypothyroidism induced pronounced changes in various cardiometabolic parameters among patients who had undergone total thyroidectomy and were preparing to undergo radioactive iodine therapy. These changes may increase the risk of atherosclerosis and cardiovascular diseases, especially among patients with metabolic syndrome components, although these changes were rapidly reversed after reinstituting thyroid hormone treatment.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by a grant from the Korean Society of Lipidology and Atherosclerosis.
References
- 1.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001; 344: 501–509. [DOI] [PubMed] [Google Scholar]
- 2.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab 2003; 88: 2438–2444. [DOI] [PubMed] [Google Scholar]
- 3.Duntas LH. Thyroid disease and lipids. Thyroid 2002; 12: 287–293. [DOI] [PubMed] [Google Scholar]
- 4.Klein I, Danzi S. Thyroid disease and the heart. Circulation 2007; 116: 1725–1735. [DOI] [PubMed] [Google Scholar]
- 5.Collet TH, Bauer DC, Cappola AR, et al. Thyroid antibody status, subclinical hypothyroidism, and the risk of coronary heart disease: an individual participant data analysis. J Clin Endocrinol Metab 2014; 99: 3353–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McQuade C, Skugor M, Brennan DM, et al. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: a PreCIS database study. Thyroid 2011; 21: 837–843. [DOI] [PubMed] [Google Scholar]
- 7.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 2010; 304: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iglesias P, Díez JJ. Influence of thyroid dysfunction on serum concentrations of adipocytokines. Cytokine 2007; 40: 61–70. [DOI] [PubMed] [Google Scholar]
- 9.Iglesias P, Alvarez Fidalgo P, Codoceo R, et al. Serum concentrations of adipocytokines in patients with hyperthyroidism and hypothyroidism before and after control of thyroid function. Clin Endocrinol (Oxf) 2003; 59: 621–629. [DOI] [PubMed] [Google Scholar]
- 10.Botella-Carretero JI, Alvarez-Blasco F, Sancho J, et al. Effects of thyroid hormones on serum levels of adipokines as studied in patients with differentiated thyroid carcinoma during thyroxine withdrawal. Thyroid 2006; 16: 397–402. [DOI] [PubMed] [Google Scholar]
- 11.Christ-Crain M, Meier C, Guglielmetti M, et al. Elevated C-reactive protein and homocysteine values: cardiovascular risk factors in hypothyroidism? A cross-sectional and a double-blind, placebo-controlled trial. Atherosclerosis 2003; 166: 379–386. [DOI] [PubMed] [Google Scholar]
- 12.Yang N, Yao Z, Miao L, et al. Novel clinical evidence of an association between homocysteine and insulin resistance in patients with hypothyroidism or subclinical hypothyroidism. Plos One 2015; 10: e0125922–e0125922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cakal B, Cakal E, Demirbaş B, et al. Homocysteine and fibrinogen changes with L-thyroxine in subclinical hypothyroid patients. J Korean Med Sci 2007; 22: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toplan S, Dariyerli N, Ozdemir S, et al. The effects of experimental hypothyroidism on hemorheology and plasma fibrinogen concentration. Endocrine 2005; 28: 153–156. [DOI] [PubMed] [Google Scholar]
- 15.Lee WY, Suh JY, Rhee EJ, et al. Plasma CRP, apolipoprotein A-1, apolipoprotein B and Lpa levels according to thyroid function status. Arch Med Res 2004; 35: 540–545. [DOI] [PubMed] [Google Scholar]
- 16.Fricker M, Wiesli P, Brändle M, et al. Impact of thyroid dysfunction on serum cystatin C. Kidney Int 2003; 63: 1944–1947. [DOI] [PubMed] [Google Scholar]
- 17.Ozden TA, Tekerek H, Baş F, et al. Effect of hypo-and euthyroid status on serum cystatin C levels. J Clin Res Pediatr Endocrinol 2010; 2: 155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem 2009; 55: 1932–1943. [DOI] [PubMed] [Google Scholar]
- 19.Zulewski H, Müller B, Exer P, et al. Estimation of tissue hypothyroidism by a new clinical score: evaluation of patients with various grades of hypothyroidism and controls. J Clin Endocrinol Metab 1997; 82: 771–776. [DOI] [PubMed] [Google Scholar]
- 20.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421. [PubMed]
- 21.Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids 2011; 2011: 575840–575840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razvi S, Ingoe L, Keeka G, et al. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab 2007; 92: 1715–1723. [DOI] [PubMed] [Google Scholar]
- 23.Adrees M, Gibney J, El-Saeity N, et al. Effects of 18 months of L-T4 replacement in women with subclinical hypothyroidism. Clin Endocrinol (Oxf) 2009; 71: 298–303. [DOI] [PubMed] [Google Scholar]
- 24.Tzotzas T, Krassas GE, Konstantinidis T, et al. Changes in lipoprotein(a) levels in overt and subclinical hypothyroidism before and during treatment. Thyroid 2000; 10: 803–808. [DOI] [PubMed] [Google Scholar]
- 25.Anagnostis P, Efstathiadou ZA, Slavakis A, et al. The effect of L-thyroxine substitution on lipid profile, glucose homeostasis, inflammation and coagulation in patients with subclinical hypothyroidism. Int J Clin Pract 2014; 68: 857–863. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Mao JM, Wang X, et al. Effect of homocysteine on plaque formation and oxidative stress in patients with acute coronary syndromes. Chin Med J (Engl) 2004; 117: 1650–1654. [PubMed] [Google Scholar]
- 27.Wang H, Jiang X, Yang F, et al. Hyperhomocysteinemia accelerates atherosclerosis in cystathionine beta-synthase and apolipoprotein E double knock-out mice with and without dietary perturbation. Blood 2003; 101: 3901–3907. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Chen Y, Cao X, et al. Association between plasma homocysteine status and hypothyroidism: a meta-analysis. Int J Clin Exp Med 2014; 7: 4544–4553. [PMC free article] [PubMed] [Google Scholar]
- 29.Mehran L, Amouzegar A, Tohidi M, et al. Serum free thyroxine concentration is associated with metabolic syndrome in euthyroid subjects. Thyroid 2014; 24: 1566–1574. [DOI] [PubMed] [Google Scholar]
- 30.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev 2014; 94: 355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26: 1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]