Abstract
Background
We evaluated the safety and efficiency of flow diverters (FDs) in treating small intracranial aneurysms (IAs).
Materials and Methods
We reviewed the literature published in PubMed and EMBASE. R for Project software was used to calculate the complete aneurysm occlusion rates, procedure-related neurologic mortality, procedure-related neurologic morbidity and procedure-related permanent morbidity.
Results
Ten observational studies were included in this analysis. The complete aneurysm occlusion rate was 84.23% (80.34%–87.76%), the procedure-related neurologic mortality was 0.87% (0.29%–1.74%), the procedure-related neurologic morbidity rate was 5.22% (3.62%–7.1%), the intracerebral haemorrhage rate was 1.42% (0.64%–2.49%), the ischemic rate was 2.35% (1.31%–3.68%), the subarachnoid haemorrhage rate was 0.03% (0%–0.32%) and the procedure-related permanent morbidity was 2.41% (0.81%–4.83%).
Conclusions
Treatment of small IAs with FDs may be correlated with high complete occlusion rates and low complication rates. Future long-term follow-up randomized trials will determine the optimal treatment for small IAs.
Keywords: flow diverters, small intracranial aneurysms, pipeline, SILK, systematic review, meta-analysis
Introduction
Intracranial aneurysms (IAs) have a prevalence of almost 5% in the general population.1,2 A devastating consequence may be subarachnoid haemorrhage, which accounts for the unstable status of IAs.3–7 Flow diverters (FDs) have become vital tools for treating intracranial aneurysms (IAs)8,9 and many kinds have emerged. In 2008, the SILK flow diverter (SFD; Balt Extrusion, Montmorency, France) was the first flow diverter to receive European Commission approval.10,11 The China Food and Drug Administration accepted the use of Tubridge (China) in 2010.12 The popularity of FDs also grew in the United States after 2011 when the U.S. Food and Drug Administration approved the pipeline embolization device (PED).13–16 Over the next several years, the Surpass flow diverter,17 the flow re-direction endoluminal device18–20 and the P64 flow modulation device21,22 have been investigated in various trials. FDs employ two mechanisms: redirecting blood flow from the aneurysm, averting the development of a thrombosis; and promoting neo-intimal growth along the mesh, reconstructing the parent artery.23,24 FDs have been used to treat complex aneurysms, including large, giant, wide-neck and fusiform aneurysms. Numerous reports have documented the safety and effectiveness of FD treatment of many complex IAs.25–27
More than half of small IAs (56%) were treated by FDs in a recent systematic review.28 Previously, Brinjikji29 and Arrese30 conducted meta-analyses of FD treatment for a wide spectrum of IAs, but these did not focus on small aneurysms. Our aim was to conduct a systematic review and meta-analysis into the safety and efficiency of small IAs treated by FDs. PED and SFD were the most commonly used FDs in our study.28 We calculated the aneurysm occlusion rates, neurologic mortality, procedure-related neurologic morbidity and other measures of the safety and efficiency of FD treatment of small IAs.
Methods
Search strategy
We performed a systematic review and meta-analysis based on a predefined protocol in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.31 We searched PubMed and EMBASE databases for related reports published from January 2005 to December 2015. We used the following medical subject headings and keywords to retrieve articles of interest in English: small intracranial aneurysm, small intracerebral aneurysm, small brain aneurysm, small aneurysm, flow diverter, flow diverting, flow diversion, pipeline and silk. We also reviewed the bibliographies of retrieved studies for additional relevant publications.
For the broadest possible representation of the available data, we included the larger and more recent study if two studies described the same patient population. We excluded single-centre studies when they were included in a multi-centre study.
Inclusion and exclusion criteria
Three reviewers (Xiyang Yao, Junwei Ma and Gang Chen) independently evaluated the eligibility of the studies. The reviewers resolved initial disagreements and reached a consensus regarding the inclusion or exclusion of studies.
The inclusion criteria were: (i) At least five patients with small (<10 mm, according to the International Study of Unruptured Intracranial Aneurysms size classification32) IAs receiving treatment with FDs; (ii) calculation of aneurysm occlusion rate; (iii) documentation of neurological complications during follow-up, including the neurologic death rate and neurologic morbidity; (iv) only PED or SFD (additional coiling/stent placement) patients were tested.
The exclusion criteria were: (i) review articles; (ii) technique notes; (iii) guidelines; (iv) technique notes disaster series (series in which all patients were selected because of certain major complications); (v) abstracts from meetings; (vi) in vitro or cadaveric studies; (vii) studies with animal models.
Data extraction
Three investigators (Xiyang Yao, Junwei Ma and Gang Chen) independently collected data from the articles using data abstraction forms containing the following: baseline data, the study name, year of publication, number and country of centres, sources of funding, number of patients in each study, number of small IAs treated by FDs, number of FD types used, number of additional coils used, number of ruptured aneurysms, complete occlusion rate and death and complication rates during the procedure. Aneurysm characteristics were also recorded including the location and morphology.
The aneurysm location was classified as either anterior or posterior circulation. The morphology was classified as saccular, fusiform, dissecting or blister. Wide-neck FDs were defined as a neck diameter >4 mm or a dome to neck ratio <1.5.
Endpoints
Aneurysm occlusion rate was defined as the complete occlusion rate during the final follow-up.
Procedure-related neurologic mortality was defined as death resulting from neurological events during the procedure.
Procedure-related neurologic morbidity was defined as neurological events that occurred during the procedure.
Procedure-related permanent morbidity was defined as a modified Rankin scale (mRS) ≥2. The mRS ranges from 0 (no symptoms) to 6 (death).33
Ischemic rate (diagnosed clinically or radiologically).
Intracerebral haemorrhage (ICH) rate.
Subarachnoid haemorrhage (SAH) rate.
Disagreements between the three reviewers were resolved by consensus.
Quality assessment
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were used to evaluate the quality of the chosen studies.34 Three reviewers (Xiyang Yao, Junwei Ma and Gang Chen) independently performed quality assessments. In cases when the researchers disagreed in their assessment of study quality, the first author made the final decision.
Data synthesis and analysis
We used the fixed-effect model to estimate the event rate (e.g., mortality and morbidity) and corresponding 95% confidence intervals (CI) of each study.35 Heterogeneity among trials was investigated using the Cochran Q test and measured by the I2 statistic. I2 values exceeding 25%, 50% and 75% represented low, moderate and high heterogeneity, respectively.36 Sensitivity analysis assessed the effect of each individual study on the overall results.37 Publication bias was quantified using the Egger’s test.38 The R Project (www.r-project.org/) was performed for all analyses.
Results
Search results and study characteristics
Our search retrieved 185 studies; 80 from PubMed and 105 from EMBASE. We excluded 56 duplicated studies. After scanning the titles and abstracts, we excluded 101 studies as not relevant. The full text of 28 studies was assessed and 20 of these were excluded for the following reasons: three studies included less than five small IAs, eight studies had no available data, five studies only contained abstracts and four studies did not classify the IA size. In the end, eight studies39–46 were included and two15,47 additional studies were identified from a search of the bibliographies. Figure 1 shows the flow diagram of the study selection process.
Figure 1.
Flow diagram of the study selection process.
IAs, intracranial aneurysms.
Five studies15,39,41,42,47 were retrospective case series and five40,43–46 were prospective studies. Eight studies15,39–41,43–45,47 reported aneurysm occlusion rates; nine39–47 reported procedure-related neurologic morbidity, mortality and complication rates; and seven39–41,43–45,47 reported both. The analysis included 783 patients from nine studies and 824 treated small IAs from ten studies. Characteristics of the included studies are presented in Table 1 and the aneurysm characteristics are presented in Table 2.
Table 1.
Characteristics of included studies.
| Author | Year | Study design | Patients (n) | Mean age (years) | Females (n) | Small IAs treated (n) | FD type |
|---|---|---|---|---|---|---|---|
| Puri et al.39 | 2015 | Retrospective | 7 | 65 | 6 | 7 | PED |
| Strauss et al.47 | 2015 | Retrospective | / | / | / | 28/67 | SFD |
| Briganti et al.40 | 2015 | Prospective | 14 | 59 | 10 | 15 | PED |
| Chalouhi et al.41 | 2015 | Retrospective | 100 | Range: 17–80 | 89 | 100 | PED |
| Kallmes et al.42 | 2014 | Retrospective | 386 | / | / | 386 | PED |
| Chalouhi et al.43 | 2014 | Prospective | 40 | 52.1 | / | 40 | PED |
| Lin et al.44 | 2013 | Prospective | 41 | 54.9 | 38 | 53 | PED |
| Yavuz et al.45 | 2013 | Prospective | 22 | / | / | 22 | PED |
| Saatci et al.15 | 2012 | Retrospective | 155 | / | / | 155 | PED |
| Byrne et al.46 | 2010 | Prospective | 18 | / | / | 18 | SFD |
IAs, intracranial aneurysms; FD, flow diverter; SFD, SILK flow diverter; /, not specified.
Table 2.
Characteristics of small IAs.
| Characteristic | Puri et al.39 | Strauss et al.47 | Briganti et al.40 | Chalouhi et al.41 | Kallmes et al.42 |
|---|---|---|---|---|---|
| Size (mean ± SD, mm) | 3.23 ± 1.23 | / | 6.9 ± 1.16 | 5.2 ± 1.5 | / |
| Wide neck (n) | 0 | 22 | 6 | / | / |
| Location | |||||
| Anterior | 7 | / | 15 | 95 | 372 |
| Posterior | 0 | / | 0 | 5 | 14 |
| Morphology | |||||
| Saccular | / | / | 14 | 89 | / |
| Fusiform | / | / | 1 | 11 | / |
| Dissecting | / | / | 0 | 0 | / |
| Blister | / | / | 0 | 5 | / |
| Additional or previous coil treatments (n) | 3 | / | 4 | 2 | / |
| Ruptures (n) | 3 | / | 0 | 7 | / |
| Characteristic |
Chalouhi et al.43 | Lin et al.44 | Yavuz et al.45 | Saatci et al.15 | Byrne et al.46 |
| Size (mean ± SD, mm) | 6.2 ± 2.4 | 5.34 ± 0.3 | / | / | / |
| Wide neck (n) | / | / | / | 22 | / |
| Location | |||||
| Anterior | 40 | 53 | 22 | / | 12 |
| Posterior | 0 | 0 | 0 | 6 | 25 |
| Morphology | |||||
| Saccular | 40 | 35 | / | / | 11 |
| Fusiform | 0 | 17 | / | / | 7 |
| Dissecting | 0 | 1 | / | / | 0 |
| Blister | 0 | 0 | / | / | 0 |
| Additional or previous coil treatments (n) | 0 | 2 | 0 | / | / |
| Ruptures (n) | 0 | 0 | 1 | / | / |
SD, standard deviation; /, not specified.
Quality analysis
The STROBE score ranged from 12 to 20, with a mean ± SD of 15.8 ± 1.8. The included studies were published between 2010 and 2015 and 40% were published in 2015.
Study results
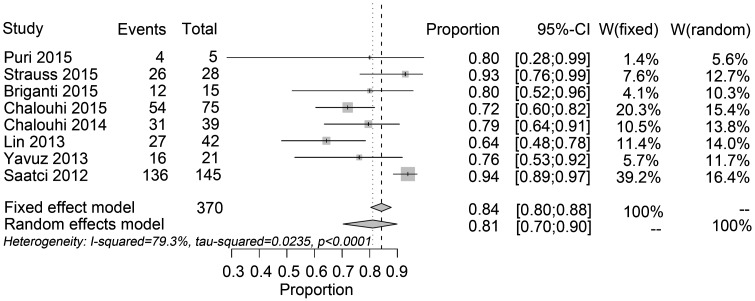
The complete aneurysm occlusion rate was 84.23% (95% CI: 80.34, 87.76%; I2 = 79.3%) at the most recent follow-up (Figure 2). The mean imaging and clinical follow-ups were 6 months. The procedure-related neurologic mortality was 0.87% (95% CI: 0.29%, 1.74%; I2 = 32.4%, P = 0.1693). The procedure-related neurologic morbidity rate was 5.22% (95% CI: 3.62%, 7.1%; I2 = 35.5%, P = 0.1432). The ICH rate was 1.42% (95% CI: 0.64%, 2.49%; I2 = 0%, P = 0.7227). Ischemic rate was 2.35% (95% CI: 1.31%, 3.68%; I2 = 63.3%). The SAH rate was 0.03% (95% CI: 0%, 0.32%; I2 = 4.1%, P = 0.3982). The procedure-related permanent morbidity was 2.41% (95% CI: 0.81%, 4.83%; I2 = 56.5%).
Figure 2.
Forest plots of the complete aneurysm occlusion rate.
Sensitivity analysis and publication bias
We omitted each study in turn from the sensitivity analysis to determine the effect of each single study on the overall risk estimate. When the results from Saatci15 were eliminated, the pooled aneurysm occlusion rate was 76.22% (95% CI: 70.45%, 81.55%, which indicated lower than the overall outcome. When the findings from Briganti40 were omitted, the ischemic rate was 2.07% (95% CI: 1.1%, 3.35%; I2 = 36.4%, P = 0.1508), the heterogeneity was obviously declined. When all studies were included, the Egger’s test gave a P-value of >0.01, suggesting there was little evidence of publication bias.
Discussion
The International Study of Unruptured Intracranial Aneurysms found that 73.9% of 1449 IAs were less than 10 mm in size and characterized these as small.48 Moreover, 89.6% of the observed 6679 aneurysms were reported in a prospective Japanese cohort of unruptured IAs.49 FDs were used to treat complex and fusiform IAs, rather than small IAs. However, some studies have reported the treatment of small and less complex IAs with FDs. Our systematic review and meta-analysis of the available data indicates that the use of FD to treat small IAs is safe and effective. Our analysis indicated 84.23% complete aneurysm occlusion, 0.87% neurologic mortality and 5.22% procedure-related neurologic morbidity.
Our main reservation is that the aneurysm occlusion result was derived from heterogeneous studies. This could not be regulated by subgroup analysis, because the aneurysm occlusion rate was not classified by subgroup such as location, wide neck or rupture. Although SFD and PED were both used, SFD was only used in one of the included studies. When the results from Saatci’s study were omitted from the sensitivity analysis, the heterogeneity clearly decreased. In Brinjikji’s meta-analysis, the aneurysm occlusion rate for the small aneurysm subgroup was 80% at 6 months.29 Brinjikji and colleagues found no effects of aneurysm size on the rates of aneurysm occlusion in a population containing 1451 patients with 1654 treated aneurysms. However, increased size of the aneurysms associated with the negative clinical results was the current issue.50 Arrese et al. reported a 76.2% aneurysm occlusion rate30 at the 9 month follow-up. No other differences were found except for the types of devices used and aneurysm occlusions had a high heterogeneity in the fixed-effects model in the meta-analysis of Arrese et al.
Among the large studies, mortality ranged from 0% to 2%,39,42 whereas procedural-related neurologic morbidity ranged from 3% to 29%.41,47 Our meta-analysis revealed more typical results on mortality and morbidity associated with the treatment of small IAs with FDs. Ischemic rates were associated with high neurologic morbidity. Multiple devices were used for technical reasons and the platelet function and clopidogrel resistance of the patients were determined. Platelet function and clopidogrel resistance may cause distal thromboembolic events and parent artery or stent occlusion.40,51 As indicated in one cohort,52 the last-recorded P2Y12 reaction units (PRU) measurement was <60 or >240 and this was an independent predictor of thromboembolic and haemorrhagic complications occurring up to 6 months after IA treatment with PED. To maintain the PRU between 60 and 240, most patients needed two adjustments of the dose or type of P2Y12 receptor antagonist.52 Therefore, decreasing the number of FDs, adjusting the clopidogrel dose and type of P2Y12 receptor antagonist and determining platelet function could reduce the number of ischemic events. However, decreasing the long-term danger of thromboembolic events caused by FD use is difficult.53 The mechanism of ICH is not well understood. A haemorrhagic change of ischemic events, embolization from FDs, or dual antiplatelet treatment have been proposed.54,55 Another severe haemorrhagic complication was SAH, but this was not very frequent in our population; SAH occurred in two patients and one case was caused by a wire perforation during the course of the procedure.44 However, some studies showed that intraprocedural ruptures were more common during the treatment of small aneurysms.56,57
Coiling is the traditional choice of endovascular treatment for small IAs and has favourable clinical and angiographic outcomes.58 Lanzino et al.59 compared FD-treated paraclinoid aneurysms with standard endovascular approaches-treated historic controls with aneurysms of similar size and location in a small population. A significantly higher complete occlusion rate was found in PD patients (76%) compared with coiled-treated patients (21%) with similar morbidity. Chalouhi et al.46 reported similar clinical results and morbidity after treatment with stent-assisted coiling. The occlusion rate was higher following stent-assisted coiling compared with FD treatment. Cost comparisons have indicated that FDs are 27% cheaper per millimetre than stent-assisted coiling, although the final cost depends on different factors, including the number of FDs, type of coil and aneurysm volume.60 Considering the higher occlusion rate and reduced need for dual antiplatelet treatment, stent-assisted coiling may be the preferred treatment method.
Several limitations might have affected our analysis of the selected studies. First, the observational studies analysed were restricted by selection and recall bias. Second, data was not always available from full text articles and we did not contact authors for the additional information. Hence, eligible studies may have been eliminated. Third, some studies did not describe the number of patients lost to follow-up. Based on the initial sample size, we were concerned that this loss of participants might cause variations in the results. We did not correct for changes in the study population by establishing a control group, such as patients using conventional endovascular coils. Last, we only included only English-language publications in our systematic meta-analysis, therefore language bias must be considered. Consequently, our results should be interpreted with caution when making patient care decisions.
Conclusions
Small IAs can be effectively treated by FDs with high complete occlusion rates. However, there is a risk of procedural-related neurologic morbidity and ischemic events, such as ICH and SAH. Future studies should include a suitable control group, such as coil-treated patients for comparison. Before deciding the optimal approach for treating small IAs, clinicians should take other aneurysm characteristics into account.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This work was supported by Suzhou Key Medical Center (Szzx201501), grants from the National Natural Science Foundation of China (No. 81571115, 81422013, and 81471196), Scientific Department of Jiangsu Province (No. BL2014045), Suzhou Government (No. LCZX201301, SZS201413, and SYS201332), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- 1.Caranci F, Briganti F, Cirillo L, et al. Epidemiology and genetics of intracranial aneurysms. Eur J Radiol 2013; 82: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 2.Backes D, Vergouwen MD, Tiel Groenestege AT, et al. PHASES score for prediction of intracranial aneurysm growth. Stroke 2015; 46: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 3.Hasegawa Y, Suzuki H, Uekawa K, et al. Characteristics of cerebrovascular injury in the hyperacute phase after induced severe subarachnoid hemorrhage. Transl Stroke Res 2015; 6: 458–466. [DOI] [PubMed] [Google Scholar]
- 4.Cetas JS, McFarlane R, Kronfeld K, et al. Brainstem opioidergic system is involved in early response to experimental SAH. Transl Stroke Res 2015; 6: 140–147. [DOI] [PubMed] [Google Scholar]
- 5.Cheng C, Jiang L, Yang Y, et al. Effect of APOE gene polymorphism on early cerebral perfusion after aneurysmal subarachnoid hemorrhage. Transl Stroke Res 2015; 6: 446–450. [DOI] [PubMed] [Google Scholar]
- 6.Etminan N. Aneurysmal subarachnoid hemorrhage–status quo and perspective. Transl Stroke Res 2015; 6: 167–170. [DOI] [PubMed] [Google Scholar]
- 7.Guresir E, Schuss P, Borger V, et al. Experimental subarachnoid hemorrhage: double cisterna magna injection rat model–assessment of delayed pathological effects of cerebral vasospasm. Transl Stroke Res 2015; 6: 242–251. [DOI] [PubMed] [Google Scholar]
- 8.Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke 2007; 38: 2346–2352. [DOI] [PubMed] [Google Scholar]
- 9.D’Urso PI, Lanzino G, Cloft HJ, et al. Flow diversion for intracranial aneurysms: a review. Stroke 2011; 42: 2363–2368. [DOI] [PubMed] [Google Scholar]
- 10.Shankar JJ, Tampieri D, Iancu D, et al. SILK flow diverter for complex intracranial aneurysms: a Canadian registry. J Neurointerv Surg 2016; 8: 273–278. [DOI] [PubMed] [Google Scholar]
- 11.Wagner A, Cortsen M, Hauerberg J, et al. Treatment of intracranial aneurysms. Reconstruction of the parent artery with flow-diverting (silk) stent. Neuroradiology 2012; 54: 709–718. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Yang PF, Fang YB, et al. A novel flow-diverting device (Tubridge) for the treatment of 28 large or giant intracranial aneurysms: a single-center experience. AJNR Am J Neuroradiol 2014; 35: 2326–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu AH, Cheung AK, Wenderoth JD, et al. Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the pipeline embolization device. AJNR Am J Neuroradiol 2015; 36: 1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Kelly CJ, Spears J, Chow M, et al. Canadian experience with the pipeline embolization device for repair of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 2013; 34: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saatci I, Yavuz K, Ozer C, et al. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 2012; 33: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu SC, Kwok CK, Cheng PW, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device–a prospective study in 143 patients with 178 aneurysms. Radiology 2012; 265: 893–901. [DOI] [PubMed] [Google Scholar]
- 17.Wakhloo AK, Lylyk P, de Vries J, et al. Surpass flow diverter in the treatment of intracranial aneurysms: a prospective multicenter study. AJNR Am J Neuroradiol 2015; 36: 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briganti F, Leone G, Ugga L, et al. Safety and efficacy of flow re-direction endoluminal device (FRED) in the treatment of cerebral aneurysms: a single center experience. Acta Neurochir (Wien) 2016; 158: 1745–1755. [DOI] [PubMed] [Google Scholar]
- 19.Mohlenbruch MA, Herweh C, Jestaedt L, et al. The FRED flow-diverter stent for intracranial aneurysms: clinical study to assess safety and efficacy. AJNR Am J Neuroradiol 2015; 36: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kocer N, Islak C, Kizilkilic O, et al. Flow re-direction endoluminal device in treatment of cerebral aneurysms: initial experience with short-term follow-up results. J Neurosurg 2014; 120: 1158–1171. [DOI] [PubMed] [Google Scholar]
- 21.Briganti F, Leone G, Ugga L, et al. Mid-term and long-term follow-up of intracranial aneurysms treated by the p64 flow modulation device: a multicenter experience. J Neurointerv Surg 2016. [DOI] [PMC free article] [PubMed]
- 22.Fischer S, Aguilar-Perez M, Henkes E, et al. Initial experience with p64: a novel mechanically detachable flow diverter for the treatment of intracranial saccular sidewall aneurysms. AJNR Am J Neuroradiol 2015; 36: 2082–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canton G, Levy DI, Lasheras JC, et al. Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J Neurosurg 2005; 103: 891–902. [DOI] [PubMed] [Google Scholar]
- 24.Lopes D, Sani S. Histological postmortem study of an internal carotid artery aneurysm treated with the Neuroform stent. Neurosurgery 2005; 56: E416–E416. [DOI] [PubMed] [Google Scholar]
- 25.Nelson PK, Lylyk P, Szikora I, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piano M, Valvassori L, Quilici L, et al. Midterm and long-term follow-up of cerebral aneurysms treated with flow diverter devices: a single-center experience. J Neurosurge 2013; 118: 408–416. [DOI] [PubMed] [Google Scholar]
- 27.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]
- 28.Briganti F, Leone G, Marseglia M, et al. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol J 2015; 28: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 30.Arrese I, Sarabia R, Pintado R, et al. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery 2013; 73: 193–200. [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097–e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiebers DO, Whisnant JP, Huston J, 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362: 103–110. [DOI] [PubMed] [Google Scholar]
- 33.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–607. [DOI] [PubMed] [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 35.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 36.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi ZY, Shao C, Zhang X, et al. Exogenous and endogenous hormones in relation to glioma in women: a meta-analysis of 11 case-control studies. PloS One 2013; 8: e68695–e68695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puri AS, Massari F, Asai T, et al. Safety, efficacy, and short-term follow-up of the use of Pipeline Embolization Device in small (<2.5 mm) cerebral vessels for aneurysm treatment: single institution experience. Neuroradiology 2016; 58: 267–275. [DOI] [PubMed] [Google Scholar]
- 40.Briganti F, Delehaye L, Leone G, et al. Flow diverter device for the treatment of small middle cerebral artery aneurysms. J Neurointerv Surg 2016; 8: 287–294. [DOI] [PubMed] [Google Scholar]
- 41.Chalouhi N, Zanaty M, Whiting A, et al. Safety and efficacy of the pipeline embolization device in 100 small intracranial aneurysms. J Neurosurg 2015; 122: 1498–1502. [DOI] [PubMed] [Google Scholar]
- 42.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chalouhi N, Starke RM, Yang S, et al. Extending the indications of flow diversion to small, unruptured, saccular aneurysms of the anterior circulation. Stroke 2014; 45: 54–58. [DOI] [PubMed] [Google Scholar]
- 44.Lin LM, Colby GP, Kim JE, et al. Immediate and follow-up results for 44 consecutive cases of small (<10 mm) internal carotid artery aneurysms treated with the pipeline embolization device. Surg Neurol Int 2013; 4: 114–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yavuz K, Geyik S, Saatci I, et al. Endovascular treatment of middle cerebral artery aneurysms with flow modification with the use of the pipeline embolization device. AJNR Am J Neuroradiol 2014; 35: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byrne JV, Beltechi R, Yarnold JA, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: a multicentre prospective study. PloS One 2010; 5: e12492–e12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strauss I, Maimon S. Silk flow diverter in the treatment of complex intracranial aneurysms: a single-center experience with 60 patients. Acta Neurochir (Wien) 2016; 158: 247–254. [DOI] [PubMed] [Google Scholar]
- 48.UCAS Japan Investigators, Morita A, Kirino T, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012; 366: 2474–2482. [DOI] [PubMed] [Google Scholar]
- 49.Vlak MH, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011; 10: 626–636. [DOI] [PubMed] [Google Scholar]
- 50.Im SH, Han MH, Kwon OK, et al. Endovascular coil embolization of 435 small asymptomatic unruptured intracranial aneurysms: procedural morbidity and patient outcome. AJNR Am J Neuroradiol 2009; 30: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pierot L. Flow diverter stents in the treatment of intracranial aneurysms: where are we? J Neuroradiol 2011; 38: 40–46. [DOI] [PubMed] [Google Scholar]
- 52.Delgado Almandoz JE, Crandall BM, Scholz JM, et al. Last-recorded P2Y12 reaction units value is strongly associated with thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in patients with cerebral aneurysms treated with the pipeline embolization device. AJNR Am J Neuroradiol 2014; 35: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tahtinen OI, Manninen HI, Vanninen RL, et al. The silk flow-diverting stent in the endovascular treatment of complex intracranial aneurysms: technical aspects and midterm results in 24 consecutive patients. Neurosurgery 2012; 70: 617–623. [DOI] [PubMed] [Google Scholar]
- 54.Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: current status. Stroke 2013; 44: 2046–2054. [DOI] [PubMed] [Google Scholar]
- 55.Hu YC, Deshmukh VR, Albuquerque FC, et al. Histopathological assessment of fatal ipsilateral intraparenchymal hemorrhages after the treatment of supraclinoid aneurysms with the pipeline embolization device. J Neurosurg 2014; 120: 365–374. [DOI] [PubMed] [Google Scholar]
- 56.Pierot L, Spelle L, Vitry F, et al. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke 2008; 39: 2497–2504. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell PJ, Muthusamy S, Dowling R, et al. Does small aneurysm size predict intraoperative rupture during coiling in ruptured and unruptured aneurysms? J Stroke Cerebrovasc Dis 2013; 22: 1298–1303. [DOI] [PubMed] [Google Scholar]
- 58.Zanaty M, Chalouhi N, Tjoumakaris SI, et al. Endovascular management of cerebral aneurysm: review of the literature. Transl Stroke Res 2014; 5: 199–206. [DOI] [PubMed] [Google Scholar]
- 59.Lanzino G, Crobeddu E, Cloft HJ, et al. Efficacy and safety of flow diversion for paraclinoid aneurysms: a matched-pair analysis compared with standard endovascular approaches. AJNR Am J Neuroradiol 2012; 33: 2158–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colby GP, Lin LM, Paul AR, et al. Cost comparison of endovascular treatment of anterior circulation aneurysms with the pipeline embolization device and stent-assisted coiling. Neurosurgery 2012; 71: 944–950. [DOI] [PubMed] [Google Scholar]