Abstract
Objective
The CYP2C19 loss-of-function (LoF) allele is present in half of the East Asian population and is associated with high on-treatment platelet reactivity (HTPR). This study aimed to investigate whether a rapid genotyping-guided approach is feasible and efficacious for selecting P2Y12 receptor blockers in Chinese patients suffering from acute coronary syndrome (ACS).
Methods
This was a single-centre, prospective, randomized, open-label study. A total of 132 patients with ACS were randomized to the rapid genotyping-guided treatment group (GG, N = 65) or the standard treatment group (SG, N = 67). Patients in the GG group were genotyped by the Verigene system. Patients with the CYP2C19 LoF allele were switched to ticagrelor and all remaining patients continued on clopidogrel. The endpoints were HTPR at 24 hours after the first loading dose of clopidogrel and 1 month afterwards.
Results
Forty patients in the GG group switched to ticagrelor, while others continued on clopidogrel. The incidence of HTPR in the GG vs SG groups was 9.2% vs 40.3% at 24 hours and 6.5% vs 32.3% at 1 month, respectively. Rapid point-of-care genotyping showed 100% concordance with conventional genotyping by real-time polymerase chain reaction.
Conclusions
In Chinese patients suffering from ACS, the rapid genotyping-guided approach for selecting P2Y12 receptor blockers is feasible and reduces the incidence of HTPR.
Clinical Trial Registration
URL: http://clinicaltrials.gov. Unique identifier: NCT01994941.
Keywords: P2Y12 receptor, CYP2C19, acute coronary syndrome
Introduction
P2Y12 receptor blockers are cornerstones for management of acute coronary syndrome (ACS). Clopidogrel is used for treating ACS, but there is substantial evidence to suggest its marked inter-individual variability in response.1,2 Ticagrelor is another P2Y12 receptor blocker, which has more potent and consistent antiplatelet activity than clopidogrel. Ticagrelor improves cardiovascular mortality and all-cause mortality at the expense of increased non-procedural-related bleeding compared with clopidogrel in patients with ACS.3,4 Despite approval of ticagrelor as class I indication in American and European guidelines,5–7 its use in East Asian populations is still not popular because of concerns of a high propensity for bleeding complications.8,9
Cytochrome P450 (CYP) 2C19 polymorphism may partially explain the heterogeneity in clopidogrel response. The CYP2C19 loss-of-function (LoF) allele is associated with an increased risk of adverse cardiovascular outcomes,10,11 particularly in East Asian patients with ACS after percutaneous coronary intervention (PCI).12 While clopidogrel is commonly used in East Asian patients with ACS, approximately 60% of them have CYP2C19 LoF alleles13 and they have a high incidence of high on-treatment platelet reactivity (HTPR)14 and major adverse cardiovascular events.15,16 Pharmacogenetics is an attractive option for selecting East Asian patients with a suboptimal response to clopidogrel for potent antiplatelet agents without exposing others to excessive bleeding risks and treatment costs. Use of rapid genotyping technology with a turnaround time of 2 to 4 hours is technically feasible, even in the context of ACS. The RAPIDGENE study17 demonstrated that this approach significantly reduced the incidence of HTPR in patients taking clopidogrel.
This study aimed to investigate the feasibility and efficacy of the rapid genotyping-guided approach to select P2Y12 receptor blockers in Chinese patients suffering from ACS. We hypothesize that this strategy reduces HTPR, which is an important surrogate marker of cardiovascular mortality in our patients.
Materials and methods
Study design and patients
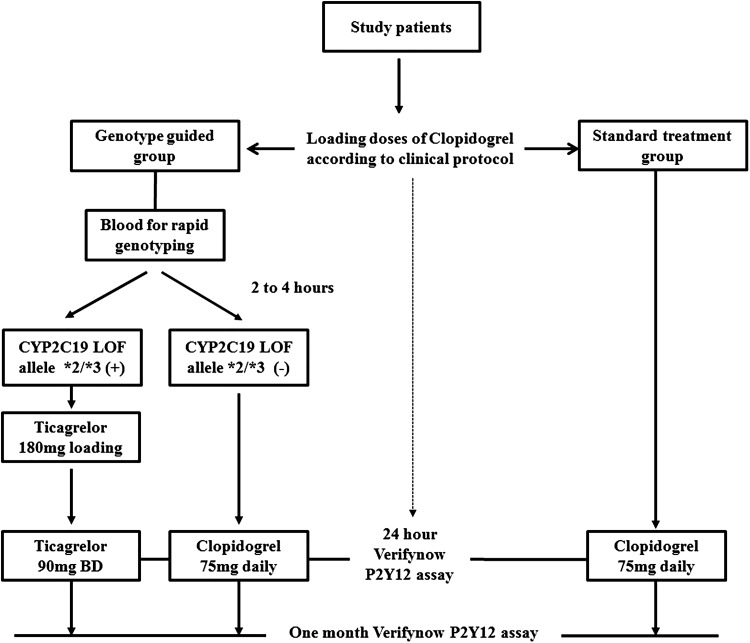
This was a single-centre, prospective, randomized, open-label study. From August 2013 to May 2015, consecutive patients who were admitted for ACS and planned to have treatment with P2Y12 receptor blockers were eligible for the study. The diagnosis of ACS included ST elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI), and unstable angina according to the American Heart Association/American College of Cardiology criteria.6 Exclusion criteria included exposure to P2Y12 receptor blockers in the previous 6 months; an active bleeding disorder, chronic renal failure on dialysis or planning for dialysis within 1 year; serious hepatic disease, contraindicated or allergic to clopidogrel or ticagrelor; a history of intracranial bleeding; and women who were pregnant or who were of childbearing potential who did not use adequate contraception. The study design is shown in Figure 1. Enrolled patients were randomized at a 1:1 ratio to the rapid genotyping-guided treatment group (GG) or standard treatment group (SG). Randomization was performed using computerized random number generation by an independent investigator. All of the patients were provided loading doses of clopidogrel according to the clinical protocol as follows: 600 mg for patients with STEMI undergoing primary PCI, and 300 mg for patients with STEMI not undergoing primary PCI, those with NSTEMI, and those with unstable angina. For patients in the GG group, blood was collected for rapid genotyping (see below). As soon as the genotyping results were available, which was usually in 2 to 4 hours, additional loading doses of ticagrelor 180 mg were administered to individuals with CYP2C19 LoF alleles *2/*3, and they were then continued with ticagrelor 90 mg twice daily. Individuals without CYP2C19 LoF alleles *2/*3 continued to take clopidogrel 75 mg daily. All of the patients in the SG group continued to take clopidogrel 75 mg daily. Additionally, blood was collected from all of the study patients for conventional genotyping (see below) for which results were available at the end of the study. Subsequent clinical management was left to the discretion of attending physicians. Informed consent was obtained from each patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Figure 1.
Study design.
Patients were provided loading doses of clopidogrel and then randomized into the genotyping-guided treatment group or the standard treatment group. Patients in the genotyping-guided treatment group were genotyped with the Verigene system and those with CYP2C19 LoF allele were switched to ticagrelor. All of the patients in the standard treatment group continued on clopidogrel. Platelet reactivity was assessed at 24 hours after the first loading dose of clopidogrel and 1 month afterwards.
CYP2C19 genotyping
Rapid genotyping
For patients who were randomized to the GG group, blood was drawn for rapid genotyping by the Verigene CYP2C19 system (Nanosphere, Northbrook, IL). This system is an FDA approved, microarray-based genotyping assay for rapid detection of CYP2C19 polymorphisms from whole blood using nanoparticle probes. EDTA-anticoagulated blood was loaded in Verigene test cartridges and then transferred to the Verigene processor, which is a fully automated system for genome extraction, preparation, and analysis.18,19 This system identified three of the potential allelic variants as follows: *2 (dbSNP: rs4244285), *3 (dbSNP: rs4986893), and *17 (dbSNP: rs12248560) of the CYP2C19 gene. The turnaround time was approximately 2 to 4 hours. All of the tests were performed by trained personnel and accuracy was expected to be higher than 99%. All of the equipment was regularly evaluated by qualified persons who used appropriate quality control measures.
Conventional genotyping
A volume of 3 ml of blood was drawn into EDTA tubes and sent for conventional genotyping. A commercial LightMix multiplex real-time polymerase chain reaction (PCR) and melting curve analysis kit (TIB MOLBOL, Germany) was carried out for analysis of the CYP2C19*2 (dbSNP: rs4244285) and CYP2C19*3 (dbSNP: rs4986893) alleles in the genomic DNA samples using the Roche LightCycler 480 instrument (LC480). Melting curves were acquired by measuring the fluorescence during the temperature transition from 40℃ to 85℃ with a ramp rate of 20℃/s after 20 seconds of the denaturation step at 95℃. High-resolution melting analysis of the CYP2C19*2 and CYP2C19*3 alleles was performed on the LC480 using a filter combination of 465–510 nm and 498–640 nm, respectively. All assays were conducted blindly without the knowledge of platelet reactivity. For the CYP2C19*17 (dbSNP: rs12248560) allele, commercial LightSNiP real-time PCR and the melting curve analysis kit (TIB MOLBOL, Germany) were used. Melting curves were acquired by measuring fluorescence during the temperature transition from 40℃ to 85℃ after 30 seconds of the denaturation step at 95℃. Positive DNA controls, including wild type, mutant, and heterozygous, as well as water as the negative control, were tested in each experiment.
We classified the CYP2C19 genotyping results based on guidelines from the Clinical Pharmacogenetics Implementation Consortium (CPIC)20 into extensive metabolizers (*1/*1), intermediate metabolizers (*1/*2, *1/*3), or poor metabolizers (*2/*2, *2/*3, *3/*3). Evidence concerning the effect of *17 on other LoF alleles is conflicting21,22 because of an inadequate compensatory effect of gain-of-function allele and linkage disequilibrium. Therefore, we classified the combination of the presence of *17 and LoF alleles into the IM group as in the CPIC guidelines.
Study endpoint
The primary endpoint of the study was platelet reactivity at 24 hours after the first loading dose of clopidogrel. We assessed patients’ on-treatment platelet reactivity by using the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA). The VerifyNow P2Y12 assay is a point-of-care, whole blood-based method used to measure the magnitude of ADP-induced platelet agglutination using 20 µmol/L ADP to induce platelet activation. The machine detects an optical signal and results are expressed as P2Y12 reaction units (PRU). The procedures were performed according to the manufacturer’s instructions by nursing staff who had no knowledge of the patients’ genotype status. If a glycoprotein IIbIIIa antagonist (eptifibatide in our institution) was used during the first 24 hours after randomization, the VerifyNow P2Y12 assay was performed 48 hours after cessation of drug infusion. We classified patients with PRU > 208 as having HTPR, which has been shown to correlate with worse clinical outcomes.23,24
Sample size calculation and statistical analysis
Based on our own experience and previous data,14,25 the percentages of high on-clopidogrel platelet reactivity in Chinese patients with and without the CYP2C19 LoF allele are approximately 70% and 30%, respectively. Additionally, the prevalence of Chinese patients possessing at least one CYP2C19 LoF allele is approximately 50%.14,16 For the GG group, the estimated percentage of HTPR was 10%, and for the SG group, it was 50%. Using a power of 0.9 and a two-sided 5% level of statistical significance, the calculated sample size was at least 26 patients per arm.
Continuous variables are reported as mean ± standard deviation (SD). Categorical values are presented as absolute values or percentages. Comparison of continuous variables was performed by one-way ANOVA, the Mann–Whitney U test, or the independent-samples Kruskal–Wallis test, depending on data distribution. The Chi-square and Fisher’s exact tests were used for comparison of categorical values and calculation of Hardy–Weinberg equilibrium. A two-sided P value of ≤0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows (version 19.0, SPSS Inc., Chicago, Illinois).
Results
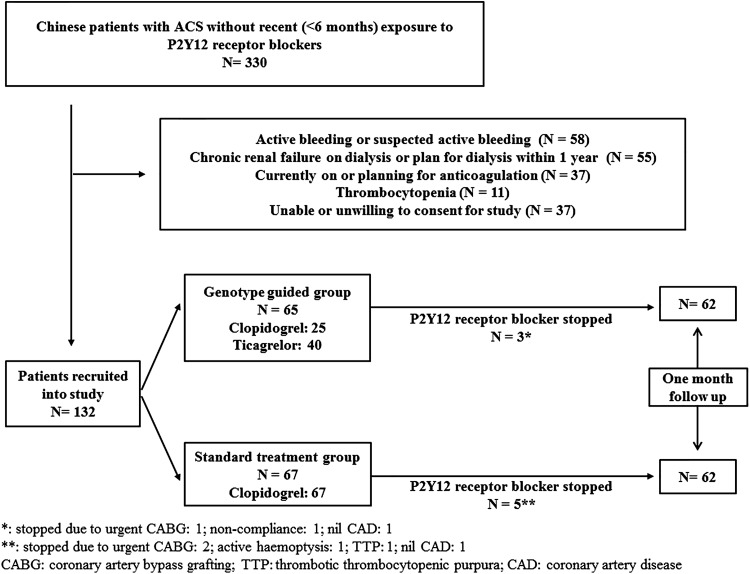
A total of 330 patients were screened and 132 patients were enrolled into the study (Figure 2). A total of 65 patients were randomized to the GG group. In this group, 40 patients possessed the CYP2C19 LoF allele and were switched to ticagrelor, while the remaining 25 continued on clopidogrel. A total of 67 patients were randomized to the SG group and all of them continued to receive clopidogrel. Eight patients stopped receiving P2Y12 receptor blockers (Figure 2) and were excluded from analysis at 1 month of follow-up. Baseline clinical characteristics are shown in Table 1 and they were comparable between both groups. Most patients underwent PCI for treatment of ACS, while some required coronary artery bypass grafting (CABG) or received medical treatment only.
Figure 2.
Study patients.
Table 1.
Baseline characteristics of the patients.
| Genotyping-guided N = 65 | Standard treatment N = 67 | P value | |
|---|---|---|---|
| Age, y (mean ± standard deviation) | 61.6 ± 11.8 | 60.3 ± 12.2 | 0.78 |
| Male sex (%) | 51 (78.5) | 55 (82.1) | 0.60 |
| Body weight in kg (mean) | 67.8 ± 9.2 | 69.6 ± 10.4 | 0.22 |
| Hypertension (%) | 38 (58.5) | 34 (50.7) | 0.37 |
| Diabetes mellitus (%) | 20 (30.8) | 16 (23.9) | 0.36 |
| Hyperlipidemia (%) | 15 (23.1) | 11 (16.4) | 0.34 |
| Diagnosis (%) | 0.78 | ||
| STEMI | 34 (52.3) | 36 (53.7) | |
| NSTEMI | 27 (41.5) | 25 (37.3) | |
| UA | 4 (6.2) | 6 (9.0) | |
| P2Y12 receptor blockers (%) | |||
| Clopidogrel | 25 (38.5) | 67 (100) | |
| Ticagrelor | 40 (61.5) | ||
| Aspirin (%) | 64 (98.5) | 66 (98.5) | 0.98 |
| ACEI/ARB (%) | 30 (46.2) | 31 (46.3) | 0.98 |
| Beta-blockers (%) | 37 (56.9) | 37 (55.2) | 0.59 |
| Ca channel blockers (%) | 6 (9.2) | 8 (11.9) | 0.61 |
| PPI (%) | 58 (89.2) | 59 (88.1) | 0.83 |
| Statin (%) | 62 (95.4) | 65 (97.0) | 0.62 |
| GPIIbIIIa (%) | 1 (1.5) | 6 (9.0) | 0.12 |
| PCI (%) | 52 (80) | 49 (73.1) | 0.35 |
| Infarct-related artery | 52 (80) | 49 (73.1) | 0.56 |
| LM (%) | 0 (0) | 1 (2.0) | |
| RCA (%) | 20 (38.5) | 14 (28.6) | |
| LAD (%) | 25 (48.1) | 27 (55.1) | |
| LCX (%) | 7 (13.5) | 7 (14.3) | |
| Time from clopidogrel loading to PCI in patients with STEMI and primary PCI (h) | 0.73 ± 1.24 | 0.24 ± 0.44 | 0.29 |
| CABG (%) | 2 (3.1) | 3 (4.5) | 0.36 |
STEMI, ST elevation myocardial infarction; NSTEMI, non-ST elevation myocardial infarction; UA, unstable angina; ACEI/ARB, Angiotensin converting enzyme inhibitor/Angiotensin receptor blocker; PPI, proton pump inhibitor; PCI, percutanous coronary intervention; LM, left main; RCA, right coronary artery; LAD, left anterior descending; LCX, left circumflex; CABG, coronary artery bypass grafting.
Table 2 shows the genotyping results of both groups of patients. There was 100% concordance between rapid genotyping by Verigene and conventional genotyping by real-time PCR. The prevalence of CYP2C19 LoF alleles *2 and *3 were comparable between both groups and they were in Hardy–Weinberg equilibrium (Table 3). Only one patient possessed the gain-of-function allele *17.
Table 2.
Genotyping results and metabolizer status.
| CYP2C19 Rapid genotyping (Verigene) | Genotype guided N = 65 | CYP2C19 genotyping (PCR) | Genotype guided N = 65 | Standard treatment N = 67 |
|---|---|---|---|---|
| CYP2C19*2 | CYP2C19*2 | |||
| GG (*1/*1) | 31 | GG (*1/*1) | 31 | 36 |
| GA (*1/*2) | 30 | GA (*1/*2) | 30 | 25 |
| AA (*2/*2) | 4 | AA (*2/*2) | 4 | 6 |
| CYP2C19*3 | CYP2C19*3 | |||
| GG (*1/*1) | 56 | GG (*1/*1) | 56 | 61 |
| GA (*1/*3) | 9 | GA (*1/*3) | 9 | 6 |
| AA (*3/*3) | 0 | AA (*3/*3) | 0 | 0 |
| CYP2C19*17 | CYP2C19*17 | |||
| CC (*1/*1) | 65 | CC (*1/*1) | 65 | 66 |
| CT (*1/*17) | 0 | CT (*1/*17) | 0 | 1 |
| TT (*17/*17) | 0 | TT (*17/*17) | 0 | 0 |
| Metabolizer status | ||||
| Ultra-rapid (UM)/Extensive (EM) | 25 (39%) | 32 (48%) | ||
| Intermediate (IM) | 33 (50%) | 27 (40%) | ||
| Poor (PM) | 7 (11%) | 8 (12%) | ||
Table 3.
Allelic frequency.
| Genotype of a single nucleotide polymorphism | N | Minor allelic frequency | Hardy–Weinberg equilibrium P value |
|---|---|---|---|
| CYP2C19*2 (rs 4244285) | |||
| GG (*1/*1) | 67 | 28% | 0.780 |
| GA (*1/*2) | 55 | ||
| AA (*2/*2) | 10 | ||
| CYP2C19*3 (rs 4986893) | |||
| GG (*1/*1) | 117 | 6% | 0.489 |
| GA (*1/*3) | 15 | ||
| AA (*3/*3) | 0 |
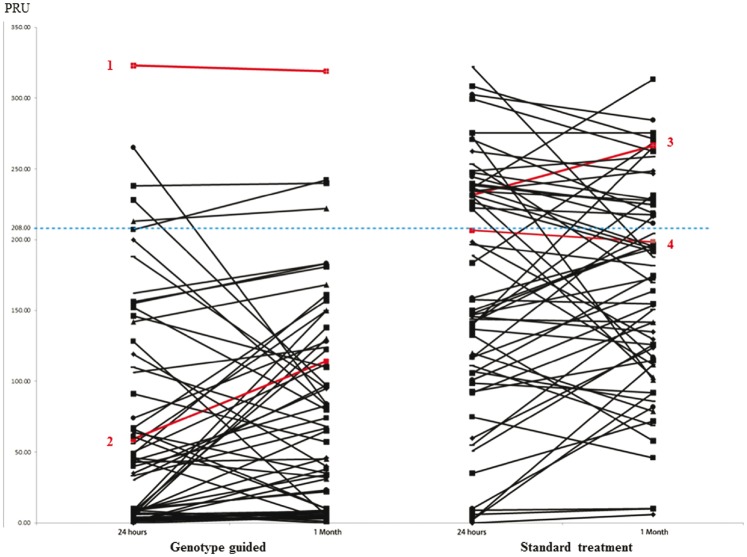
The prevalence of HTPR (PRU > 208) at 24 hours and 1 month in the GG group was significantly lower than that in the SG group (P < 0.001; Table 4, Figure 3). At 1 month of follow-up, one patient in the GG group had recurrent NSTEMI. In the SG group, one patient had recurrent NSTEMI and one patient suffered from acute ischemic stroke. With regard to major bleeding complications, one patient in the GG group suffered from acute subarachnoid haemorrhage and another patient suffered from acute gastrointestinal bleeding requiring blood transfusion. One patient in the SG group developed acute haemoptysis, which required early termination of clopidogrel.
Table 4.
On-treatment platelet reactivity.
| Genotyping-guided | Standard treatment | P value | |
|---|---|---|---|
| HTPR (PRU > 208) at 24 hours | 6/65 (9.2%) | 27/67 (40.3%) | <0.001 |
| HTPR (PRU > 208) at 1 month | 4/62 (6.5%) | 20/62 (32.3%) | <0.001 |
| Mean PRU at 24 hours | 72.4 ± 85.1 | 169.8 ± 87.5 | <0.001 |
| Mean PRU at 1 month | 80.0 ± 73.9 | 166.9 ± 74.4 | <0.001 |
| Metabolizer status | |||
| Ultra-rapid/extensive | |||
| HTPR (PRU > 208) at 24 hours | 6/25 (24%) | 10/32 (31.3%) | |
| HTPR (PRU > 208) at 1 month | 4/25 (16%) | 5/28 (17.9%) | |
| Mean PRU at 24 hours | 139.2 ± 95.5 | 148.63 ± 93.4 | |
| Mean PRU at 1 month | 129.4 ± 81.0 | 138.4 ± 80.4 | |
| Moderate | |||
| HTPR (PRU > 208) at 24 hours | 0/33 (0%) | 12/27 (44.4%) | |
| HTPR (PRU > 208) at 1 month | 0/31 (0%) | 10/27 (37%) | |
| Mean PRU at 24 hours | 25.1 ± 32.2 | 183.0 ± 82.4 | |
| Mean PRU at 1 month | 43.5 ± 43.5 | 180.9 ± 61.1 | |
| Slow | |||
| HTPR (PRU > 208) at 24 hours | 0/7 (0%) | 5/8 (62.5%) | |
| HTPR (PRU > 208) at 1 month | 0/6 (0%) | 5/7 (71.4%) | |
| Mean PRU at 24 hours | 43.8 ± 38.9 | 209.4 ± 62.0 | |
| Mean PRU at 1 month | 62.5 ± 52.4 | 226.7 ± 47.0 | |
Figure 3.
On-treatment platelet reactivity at 24 hours and 1 month.
The red line denotes patients with clinical events. Patient 1 suffered from subarachnoid haemorrhage. Patient 2 suffered from recurrent NSTEMI and acute upper gastrointestinal bleeding, which required intervention. Patient 3 had recurrent NSTEMI and patient 4 suffered from acute ischemic stroke.
Discussion
Our study showed that rapid genotyping-guided selection of a P2Y12 receptor blocker in Chinese patients with ACS was able to reduce HTPR. Rapid genotyping of CYP2C19 polymorphism by the Verigene system using whole blood was feasible at the bedside and 100% concordant with conventional genotyping by real-time PCR. This finding is consistent with a previous study17 that used another rapid genotyping platform (Spartan RX CYP2C19; Spartan Biosciences, Ottawa, ON, Canada). This previous study also showed that personalization of antiplatelet therapy by point-of-care genotyping device is efficacious for reducing HTPR. Our study is different in that all patients were Chinese who suffered from ACS and ticagrelor was used in patients with CYP2C19 LoF alleles instead of prasugrel.
Ticagrelor is a potent P2Y12 receptor blocker with superior anti-platelet effects over clopidogrel. In our study, all patients who were taking ticagrelor did not show HTPR. Indeed, the latest American and European guidelines5–7 recommend using ticagrelor over clopidogrel in patients with ACS. However, universal use of ticagrelor may increase the risk of non-procedural related major bleeding.4,26 A personalized approach for selecting appropriate drugs for appropriate patients may be of interest for selected populations, such as East Asian patients. Clinical experience suggests differences in thrombogenicity and haemostasis between races. East Asians are thought to have a higher risk of bleeding after exposure to anticoagulants and antiplatelet agents.9,27–29 These differences may partly explain the reluctance of physicians in East Asia to universally switch to novel P2Y12 receptor blockers for all East Asian patients suffering from ACS. Particularly for the current approved dose of ticagrelor in patients with ACS, the drug and exposure to its major active metabolite are significantly higher in East Asian patients than in White patients.30,31 This correlates with the higher level of platelet inhibition during ticagrelor treatment in East Asian individuals than in White individuals.31 The PHILO study32 showed that East Asian subjects taking ticagrelor have a higher rate of major bleeding than those taking clopidogrel (PLATO major bleeding: 10.3% vs 6.8%, non-CABG major bleeding: 8.3% vs 5.8%).4 This finding suggests that prescribing potent antiplatelet agents non-selectively to East Asians patients is potentially harmful. The prevalence of the CYP2C19 LoF allele in the East Asian population is greater than 50%, as shown in current study. A recent meta-analysis12 demonstrated that the correlation between the CYP2C19 LoF allele and major cardiovascular outcome in patients with ACS taking clopidogrel is stronger in East Asians than in other ethnic groups. Therefore, a pharmacogenetic approach of selecting P2Y12 receptor blockers in East Asians suffering from ACS appears to be attractive. Furthermore, clopidogrel is already available as a generic drug. Therefore, from a resource allocation perspective, using novel antiplatelet agents in selected individuals, especially in locations with financial constraints, appears to be reasonable.
CYP2C19 polymorphism is closely associated with a suboptimal response to clopidogrel and major cardiovascular outcomes in patients with ACS taking clopidogrel.10,11 However, a minority of patients without CYP2C19*2 and *3 may still have HTPR. There are other genetic determinants of clopidogrel metabolism, such as rare single nucleotide polymorphisms of the CYP2C19 gene,20 ABCB1,33,34 PON-1,35 and CES-1.36 However, their clinical significance has not been consistently replicated. Additionally, non-genetic factors, such as age, body mass index, renal function, and diabetes,11,37 also account for the clopidogrel response. Therefore, measuring the phenotype of platelet reactivity may be more comprehensive. Nevertheless, platelet reactivity in the setting of ACS has a large temporal fluctuation. Advantages of the pharmacogenetic approach are that genotype information is constant and individuals’ responses to clopidogrel can be predicted with reasonable confidence, even before patients take the drug. With the use of rapid genotyping technology, this is particularly beneficial during ACS because the patients can be stratified early to receive the appropriate drug. Adopting a global risk algorithm incorporating the CYP2C19 genotype and clinical profile, such as the PREDICT score38 (age, reduced ejection fraction, renal failure, diabetes, ACS), could be a useful approach of personalizing antiplatelet therapy. However, during the acute phase of STEMI, approximately 40% and 60% of patients demonstrate HTPR 2 hours after ticagrelor 180 mg loading39 and clopidogrel 600 mg loading,40 respectively. Therefore, our pharmacogenetic approach does not appear to be able to overcome the issue of delayed onset of action of antiplatelets in the first few hours of STEMI.
One major limitation of the current study is the use of a surrogate marker to assess outcome. Although HTPR, as measured by the VerifyNow P2Y12 assay, has been proven to correlate with major cardiovascular events,41 studies that aimed to reduce HTPR have failed to improve clinical outcomes.42,43 The limited sample size of our single-centre study precluded analysis of clinical endpoints, which are much more meaningful. However, the ongoing multi-centre Tailored Antiplatelet Therapy following PCI study will be able to provide more concrete evidence toward the clinical efficacy of a pharmacogenetic approach of selecting P2Y12 receptor blockers. Nonetheless, the current study suggests the feasibility of using a point-of-care rapid genotyping platform to guide the use of P2Y12 receptor blockers in patients of Chinese ethnicity with ACS who are thought to derive more benefit than universally using novel, potent antiplatelet agents. Another limitation is that we only used the VerifyNow P2Y12 assay. Therefore, our pharmacodynamics data may be less representative than using multiple platelet function assays.
Conclusion
In Chinese patients suffering from ACS, the rapid genotyping-guided approach of selecting P2Y12 receptor blockers is feasible and efficacious for reducing HTPR compared with the conventional approach. Further studies are required to investigate whether our approach can translate into a superior clinical outcome.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Gurbel PA, Bliden KP, Etherington A, et al. Assessment of clopidogrel responsiveness: measurements of maximum platelet aggregation, final platelet aggregation and their correlation with vasodilator-stimulated phosphoprotein in resistant patients. Thromb Res 2007; 121: 107–115. Epub 2007/04/03. [DOI] [PubMed] [Google Scholar]
- 2.Gurbel PA, Bliden KP, Hiatt BL, et al. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003; 107: 2908–2913. Epub 2003/06/11. [DOI] [PubMed] [Google Scholar]
- 3.Husted S, Emanuelsson H, Heptinstall S, et al. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. Eur Heart J 2006; 27: 1038–1047. Epub 2006/02/16. [DOI] [PubMed] [Google Scholar]
- 4.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361: 1045–1057. Epub 2009/09/01. [DOI] [PubMed] [Google Scholar]
- 5.Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 2999–3054. Epub 2011/08/30. [DOI] [PubMed] [Google Scholar]
- 6.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 2013; 127: e362–425. Epub 2012/12/19. [DOI] [PubMed] [Google Scholar]
- 7.Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33: 2569–2619. Epub 2012/08/28. [DOI] [PubMed] [Google Scholar]
- 8.Jeong YH. “East Asian paradox”: challenge for the current antiplatelet strategy of “one-guideline-fits-all races” in acute coronary syndrome. Curr Cardiol Rep 2014; 16: 485 Epub 2014/03/29. [DOI] [PubMed] [Google Scholar]
- 9.Levine GN, Jeong YH, Goto S, et al. Expert consensus document: world heart federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol 2014; 11: 597–606. Epub 2014/08/27. [DOI] [PubMed] [Google Scholar]
- 10.Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 2010; 304: 1821–1830. Epub 2010/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuldiner AR, O’Connell JR, Bliden KP, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009; 302: 849–857. Epub 2009/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorich MJ, Rowland A, McKinnon RA, et al. CYP2C19 genotype has a greater effect on adverse cardiovascular outcomes following percutaneous coronary intervention and in Asian populations treated with clopidogrel: a meta-analysis. Circ Cardiovasc Genet 2014; 7: 895–902. Epub 2014/09/27. [DOI] [PubMed] [Google Scholar]
- 13.Jang JS, Cho KI, Jin HY, et al. Meta-analysis of cytochrome P450 2C19 polymorphism and risk of adverse clinical outcomes among coronary artery disease patients of different ethnic groups treated with clopidogrel. Am J Cardiol 2012; 110: 502–508. Epub 2012/05/18. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Chen Y, Jin Y, et al. Genetic determinants of high on-treatment platelet reactivity in clopidogrel treated Chinese patients. Thromb Res 2013; 132: 81–87. Epub 2013/06/04. [DOI] [PubMed] [Google Scholar]
- 15.Tang XF, Wang J, Zhang JH, et al. Effect of the CYP2C19 2 and 3 genotypes, ABCB1 C3435T and PON1 Q192R alleles on the pharmacodynamics and adverse clinical events of clopidogrel in Chinese people after percutaneous coronary intervention. Eur J Clin Pharmacol 2013; 69: 1103–1112. Epub 2012/11/15. [DOI] [PubMed] [Google Scholar]
- 16.Xie X, Ma YT, Yang YN, et al. CYP2C19 phenotype, stent thrombosis, myocardial infarction, and mortality in patients with coronary stent placement in a Chinese population. PloS One 2013; 8: e59344 Epub 2013/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts JD, Wells GA, Le May MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet 2012; 379: 1705–1711. Epub 2012/04/03. [DOI] [PubMed] [Google Scholar]
- 18.Buchan BW, Peterson JF, Cogbill CH, et al. Evaluation of a microarray-based genotyping assay for the rapid detection of cytochrome P450 2C19 *2 and *3 polymorphisms from whole blood using nanoparticle probes. Am J Clin Pathol 2011; 136: 604–608. Epub 2011/09/16. [DOI] [PubMed] [Google Scholar]
- 19.Chae H, Kim M, Koh YS, et al. Feasibility of a microarray-based point-of-care CYP2C19 genotyping test for predicting clopidogrel on-treatment platelet reactivity. Biomed Res Int 2013. 2013: 154073. Epub 2013/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott SA, Sangkuhl K, Stein CM, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther 2013; 94: 317–323. Epub 2013/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frere C, Cuisset T, Gaborit B, et al. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. J Thromb Haemost 2009; 7: 1409–1411. Epub 2009/06/06. [DOI] [PubMed] [Google Scholar]
- 22.Tiroch KA, Sibbing D, Koch W, et al. Protective effect of the CYP2C19 *17 polymorphism with increased activation of clopidogrel on cardiovascular events. Am Heart J 2010; 160: 506–512. Epub 2010/09/10. [DOI] [PubMed] [Google Scholar]
- 23.Stone GW, Witzenbichler B, Weisz G, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet 2013; 382: 614–623. Epub 2013/07/31. [DOI] [PubMed] [Google Scholar]
- 24.Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013; 62: 2261–2273. Epub 2013/10/01. [DOI] [PubMed] [Google Scholar]
- 25.Nagashima Z, Tsukahara K, Morita S, et al. Platelet reactivity in the early and late phases of acute coronary syndromes according to cytochrome P450 2C19 phenotypes. J Cardiol 2013; 62: 158–164. Epub 2013/07/28. [DOI] [PubMed] [Google Scholar]
- 26.Becker RC, Bassand JP, Budaj A, et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J 2011; 32: 2933–2944. Epub 2011/11/18. [DOI] [PubMed] [Google Scholar]
- 27.Lutsey PL, Cushman M, Steffen LM, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA study. J Thromb Haemost 2006; 4: 2629–2635. Epub 2006/09/28. [DOI] [PubMed] [Google Scholar]
- 28.Mak KH, Bhatt DL, Shao M, et al. Ethnic variation in adverse cardiovascular outcomes and bleeding complications in the Clopidogrel for high Atherothrombotic risk and Ischemic stabilization, management, and avoidance (CHARISMA) study. Am Heart J 2009; 157: 658–665. Epub 2009/04/01. [DOI] [PubMed] [Google Scholar]
- 29.Shen AY, Yao JF, Brar SS, et al. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007; 50: 309–315. Epub 2007/07/31. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Butler K, Yang L, et al. Pharmacokinetics and tolerability of single and multiple doses of ticagrelor in healthy Chinese subjects: an open-label, sequential, two-cohort, single-centre study. Clin Drug Investig 2012; 32: 87–97. Epub 2011/12/16. [DOI] [PubMed] [Google Scholar]
- 31.Teng R, Butler K. Pharmacokinetics, pharmacodynamics, and tolerability of single and multiple doses of ticagrelor in Japanese and Caucasian volunteers. Int J Clin Pharmacol Ther 2014; 52: 478–491. Epub 2014/04/24. [DOI] [PubMed] [Google Scholar]
- 32.Goto S, Huang CH, Park SJ, et al. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome – randomized, double-blind, phase III PHILO study. Circulation journal: official journal of the Japanese Circulation Society 2015; 79: 2452–2460. Epub 2015/09/18. [DOI] [PubMed] [Google Scholar]
- 33.Luo M, Li J, Xu X, et al. ABCB1 C3435T polymorphism and risk of adverse clinical events in clopidogrel treated patients: a meta-analysis. Thromb Res 2012; 129: 754–759. Epub 2012/01/03. [DOI] [PubMed] [Google Scholar]
- 34.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360: 363–375. Epub 2008/12/25. [DOI] [PubMed] [Google Scholar]
- 35.Bouman HJ, Schomig E, van Werkum JW, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med 2011; 17: 110–116. Epub 2010/12/21. [DOI] [PubMed] [Google Scholar]
- 36.Lewis JP, Horenstein RB, Ryan K, et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenet Genomics 2013; 23: 1–8. Epub 2012/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochholzer W, Trenk D, Fromm MF, et al. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol 2010; 55: 2427–2434. Epub 2010/06/01. [DOI] [PubMed] [Google Scholar]
- 38.Geisler T, Grass D, Bigalke B, et al. The residual platelet aggregation after deployment of intracoronary stent (PREDICT) score. J Thromb Haemost 2008; 6: 54–61. Epub 2007/10/24. [DOI] [PubMed] [Google Scholar]
- 39.Alexopoulos D, Xanthopoulou I, Goudevenos J. Effects of P2Y12 receptor inhibition in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2014; 113: 2064–2069. Epub 2014/05/06. [DOI] [PubMed] [Google Scholar]
- 40.Alexopoulos D, Theodoropoulos KC, Stavrou EF, et al. Prasugrel versus high dose clopidogrel to overcome early high on clopidogrel platelet reactivity in patients with ST elevation myocardial infarction. Cardiovasc Drugs Ther 2012; 26: 393–400. Epub 2012/08/30. [DOI] [PubMed] [Google Scholar]
- 41.Breet NJ, van Werkum JW, Bouman HJ, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010; 303: 754–762. Epub 2010/02/25. [DOI] [PubMed] [Google Scholar]
- 42.Cayla G, Cuisset T, Silvain J, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet 2016. Epub 2016/09/02. [DOI] [PubMed] [Google Scholar]
- 43.Collet JP, Cuisset T, Range G, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012; 367: 2100–2109. Epub 2012/11/06. [DOI] [PubMed] [Google Scholar]