Abstract
Objective
To investigate associations between running speeds and contraction times in 8- to 13-year-old children.
Method
This longitudinal study analyzed tensiomyographic measurements of vastus lateralis and biceps femoris muscles’ contraction times and maximum running speeds in 107 children (53 boys, 54 girls). Data were evaluated using multiple correspondence analysis.
Results
A gender difference existed between the vastus lateralis contraction times and running speeds. The running speed was less dependent on vastus lateralis contraction times in boys than in girls. Analysis of biceps femoris contraction times and running speeds revealed that running speeds of boys were much more structurally associated with contraction times than those of girls, for whom the association seemed chaotic.
Conclusion
Joint category plots showed that contraction times of biceps femoris were associated much more closely with running speed than those of the vastus lateralis muscle. These results provide insight into a new dimension of children’s development.
Keywords: Children, tensiomyography, running speed, contraction time, skeletal muscle, motor development
Introduction
The study of motor development is undoubtedly one of the most interesting fields of research into integral children’s development. Shore described the influence of the neurophysical background on integral children’s development and the impact of underdeveloped and differentiated levels of management of movement on this developmental path. He highlighted particularly the role and impact of early experiences, approaches, and processes.1 The transition from a lower to a higher stage of development depends on a number of factors, the most significant being physical growth, environmental influences, and self-activity. Among environmental influences, the presence of stimuli provided by physical/sports activities is ranked specifically. According to the theory of integral development, there is a close relation between a child’s physical, motor, intellectual, emotional, and social development.2 Children’s muscle composition has attracted increased interest as it could help us to understand better their motor and physical development. The invasiveness of the approaches used in this field, however, is the main barrier to obtaining data on muscle composition. The most commonly investigated muscles are the vastus lateralis (VL)1 and biceps femoris (BF).3 To date, most studies have been cross-sectional. Longitudinal studies are rare, and their sample sizes are mostly small.
An important milestone in the research of skeletal muscle was achieved by Dahmane,4 who, via histochemical and biomechanical analyses, demonstrated that each muscle of the human body is unique and that the skeletal muscles of the lower and upper limbs have varying numbers and volume percentages of slow (type 1) and fast (type 2) twitch fibers. She also showed that it is possible to demonstrate a difference in the number and volume percentages of slow and fast twitch fibers via tensiomyography (TMG), whereby several contractile parameters may be defined.4 The TMG method, proposed by Valenčič in 19905 for the study of muscles, has the advantages of being noninvasive and selective. Hence, TMG is a suitable method for determining the proportion of slow and fast twitch muscle fibers in skeletal muscles.6
According to Valenčič and Knez,7 TMG can be used to assess skeletal muscle contractile properties, which are related to muscle composition.4,6 Contraction time is correlated with the proportion of slow twitch skeletal muscle,8–10 and it has been shown that not only the contraction time, but also the delay time and relaxation half-time, should be known for a more valid identification of muscle composition.
Sprinting speed—one of the distinguishing indicators of successful motoric performance in children—develops throughout childhood and adolescence as children grow and mature.11 Running speed during childhood and adolescence develops in a non-linear manner owing to the large maturational influences associated with increased limb length and muscle mass and the changing intrinsic muscle–tendon properties. Therefore, failure to master sprinting may be an enormous barrier, preventing children from gaining more complex physical activity skills.11
Surprisingly, little is known about the association between muscle contraction times and sprinting speeds. The search in Web of Science (Thomas Reuters, USA) and PubMed (National Center for Biotechnology Information, U.S. National Library of Medicine, USA) bibliographic database using the string “contraction time” and muscle and speed and (sprint* or run*) at the time of designing the study revealed no articles. Therefore, one of the aims when analyzing the data collected during the 5-year longitudinal study presented here was to research the associations between various running speeds and muscle contraction time category values in 8- to 13-year-old children using multiple correspondence analysis (MCA). The following research questions were posed:
What kinds of associations, if any, exist between running speed and VL and BF muscle contraction times?
What is the difference in running speed and VL and BF muscle contraction time associations between boys and girls?
What is the difference in running speed and muscle contraction time association between the VL and BF muscles?
Participants, materials, and methods
Participants were recruited from three Slovenian regions in September 2001: Central Slovenia region, Drava region, and Coastal-Karst region. Three primary schools in each region, were selected randomly. Participants came from five Slovenian towns (Figure 1): two major cities in central and northeast Slovenia and three small towns in the coastal region. Thus, we ensured that the sample covered much of Slovenia.
Figure 1.
Backgrounds of the participants.
The researchers then organized workshops for the schools’ head teachers, physical education teachers, children, and parents with the aim of presenting the purpose of the study and inviting potential participants. In total, 300 children aged 8 years at the beginning of this study and 13 at the end of this study were recruited, 100 from each region. Among them, 265 children participated in the baseline study: 138 male (M) and 127 female (F). The selection process was performed in a random manner to prevent any possible bias (e.g., gender, geographic distribution, anthropometric characteristics). The most important inclusion criterion was good health, although the researchers confirmed that none of the children had a history of neuromuscular disorders or muscle disease. Before carrying out the research study (baseline or follow-up), the children and their parents were informed that participation in the study was on a voluntary basis and that the participants could drop out from the study at any time and for any reason. Additionally, the researchers pointed out that the results of the longitudinal study would be used only for research purposes. On this basis, the parents of the participants gave their written consent for the children to participate. All procedures conformed to the 1964 Declaration of Helsinki and were approved by the National Medical Ethics Committee of the Republic of Slovenia.
For the first follow-up study, 263 children (125 M, 138 F) participated; in the second, 252 children (127 M,125 F); in the third, 179 children (98 M, 81 F); in the fourth, 175 children (98 M, 77 F); and in the fifth (last), 176 children (96 M, 80 F). In total, 107 children (53 M, 54 F) (initial average age 9.1 ± 0.5 years) completed all six longitudinal measurements and were selected for the analysis. The study participants were in the third grade at primary school at the baseline and in the eighth grade at the last follow-up.
Research design and measures
The 5-year longitudinal research study was performed once per school year, from 2001 to 2006. Each session included cross-sectional (single point), quantitative measurements of anthropometric characteristics, contractile properties of skeletal muscles, and running speed.
During the study, six repeat measurements were performed from the third to eighth grades of the children’s primary school education. The same procedure was used at each measurement. Because all major physical or sport activities were discouraged before each measurement (approximately 2 days), the research team informed each school of the exact date on which each stage of the study was to be implemented at least 1 week before it occurred.
Measurements of skeletal muscle contractile properties
TMG measuring method
TMG is a method for measuring skeletal muscles’ contractile properties.10 It determines the muscle’s mechanical responses based on radial muscle belly displacement induced by a single electrical stimulus.12 TMG is implemented mostly by a TMG S1 system (TMG Science for Body Evaluation). The method was first introduced as a measuring technique and was then proposed as a method for defining parameters. Later, the link between the amplitude of the mechanical TMG response and the relative muscle force was introduced—which is a linear relation.7 The TMG method was then evaluated for these uses, and its validity was proven in several studies, including long-term stability.13–16
Two muscles, VL and BF (leg-dominant sites), were chosen for the measurements, which are shown in Table 1. We used a single 1-msec pulse, applied through the cathode and anode, to elicit a twitch contraction. The stimulation current at the start was just above the contraction threshold and was increased gradually until the response amplitude ceased to increase. Two maximum twitch responses were recorded and saved.
Table 1.
Measurements of vastus lateralis and biceps femoris muscles.
| Position of measurement |
| Vastus lateralis: Measurements on the VL were performed in the supine position at 30° knee flexion, where 0° represents the extended joint. |
| Biceps femoris: Measurements on the BF were performed in the prone position at 5° knee flexion. |
| Procedure |
| 1. Place the muscle to be measured in a relaxed, predefined position and evoke muscle contraction by applying a brief electrical stimulus. |
| 2. Prepare a pair of self-adhesive stimulation Pals electrodes (50 mm diameter) (AxelGaard, Fallbrook, CA, USA). |
| 3. Measure the muscle in a bipolar way in that the negative electrode (cathode) is installed 5 cm distal and the positive electrode (anode) 5 cm proximal to the measurement point. |
| 4. Select the measurement point where the belly muscle is largest while ensuring that the selected place is between the two electrodes. Start the experimental measurements (with palpation and re-installation of the electrodes and sensors if needed). Because each muscle has its own specific anatomical structure, some adjustments may be needed regarding the locations of the measuring equipment. |
| a. Vastus lateralis: Set the sensor perpendicular to the skin overlying the muscle belly at 30% of the femur length above the patella on the lateral side. |
| b. Biceps femoris: Set the sensor perpendicular to the skin overlying the muscle belly of the BF at the midpoint of the line between the fibular head and the ischial tuberosity. |
VL = vastus lateralis; BF = biceps femoris
The maximum displacement amplitude and contraction times were calculated from each twitch response.5,7 Maximum displacement amplitude (in millimeters) was defined as the peak amplitude on the displacement−time curve of the TMG twitch response. The contraction time (in milliseconds) was the time from 10% to 90% of the maximum displacement amplitude.17 The average value of these parameters extracted from two twitch responses was used for further analysis. Various experts from the fields of electrical engineering and computer science, physical education teachers, and undergraduate sport sciences students performed the measurements. A medical doctor supervised all measurements.
Measurement of running speed
Before measuring maximum running speed, all participants were “warmed up” appropriately for approximately 20 minutes.
The warm-up process was composed of the following.
– Running (5 minutes)
– Stretching (5 minutes)
– Warm-up running practice (10 minutes)
Each participant’s warm-up followed the same procedure and was led by the same person. Each participant completed two experimental sprints. We measured maximum running speed at a distance of 7 m from a flying start that was 10 m long. The participants performed preliminary runs during the measurement process, followed by the first photocell checkpoint, and after 7 m the second photocell checkpoint. We divided the running distance into 7-m segments and identified a position from which we could calculate the sprint speed. Each participant repeated the sprint twice, and the better result was used for further analysis. Maximum running speed was measured using a wireless Brower measurement system (Brower Timing Systems, Draper, UT, USA).
Data analysis
Six sets of measurements were obtained for each participant. A set consisted of the muscle contraction times for the VL and BF together with the running speed. For the analysis performed in the present study, we were not interested in absolute changes in muscle contraction times or running speed during childhood development. We were interested in the changes in the contraction times and speed categories and the association between these two entities. We used the MCA18 to analyze the changes and associations. MCA is a multivariate statistical technique used to analyze multi-way contingency tables that unveils some correspondence between columns (variables) and rows (category values). Its aims are similar to those of principal component analysis, but it uses categorical, rather than continuous, data. MCA enables the user to associate variables and their category values graphically. The MCA results are presented as “bi-plots.” The proximity of category values in a bi-plot presents the degree of association between them (the closer the values, the greater is the association). Each bi-plot can be characterized by naming the dimensions, which adds a qualitative viewpoint to the analysis—one of the advantages of MCA. The other notable advantage is the visualization of many associations on a single bi-plot, including associations between variables, associations between category values of a single variable, and combinations of the two. Thus, MCA might reveal more information than, for example, correlation analysis or analysis of variance. A possible weakness might be that the associations are presented graphically and their magnitude cannot be quantified exactly. We used IBM SPSS Statistics 23 software (SPSS, Chicago, IL, USA) to perform the MCA.
Each measured variable was discretized prior to analysis. For the sake of the readability of bi-plots, only four category values were calculated for each transformed variable. For running speed, we used very slow, slow, fast, and very fast. For contraction times, we used below average minus the standard deviation (−SD), below average (−Avg), above average (+Avg), and above average plus the standard deviation (+SD). Category values were obtained using SPSS Visual Binning Automatic Transformation (SPSS, Chicago, IL, USA).
Results
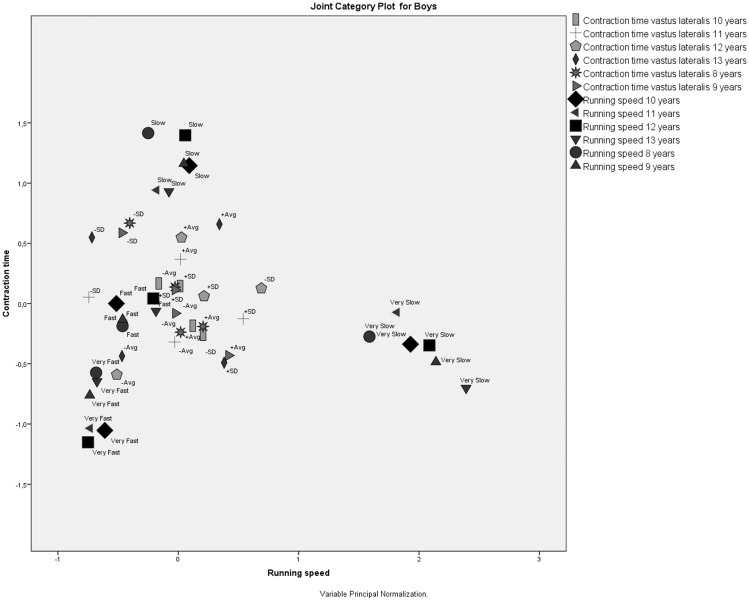
The MCA of the VL contraction time and running speed for boys (Figure 2) at six ages showed that all running speed category values formed their own clusters (i.e., very slow, slow, fast, very fast), regardless of how far away they were located from each other or from both slow contraction time category values. This clustering revealed the following assumptions.
– All running speed category values were very different from each other, the most different being the very slow speed category value.
– Neither of the slow running speed category values was associated with VL contraction times.
– Boys’ running speed category values remained closely associated with each other throughout the study, meaning that boys who were very slow stayed very slow, boys who were very fast stayed very fast, and so on.
Figure 2.
Correspondence analysis of the vastus lateralis contraction time and running speed for boys at six ages.
No structured associations could be established between the fast speed running category value and VL contraction times. Additionally, the joint category plot shows that the very fast speed category in 13-year-old children is associated with below-average VL contraction times at the ages of 12 and 13 years (Figure 2).
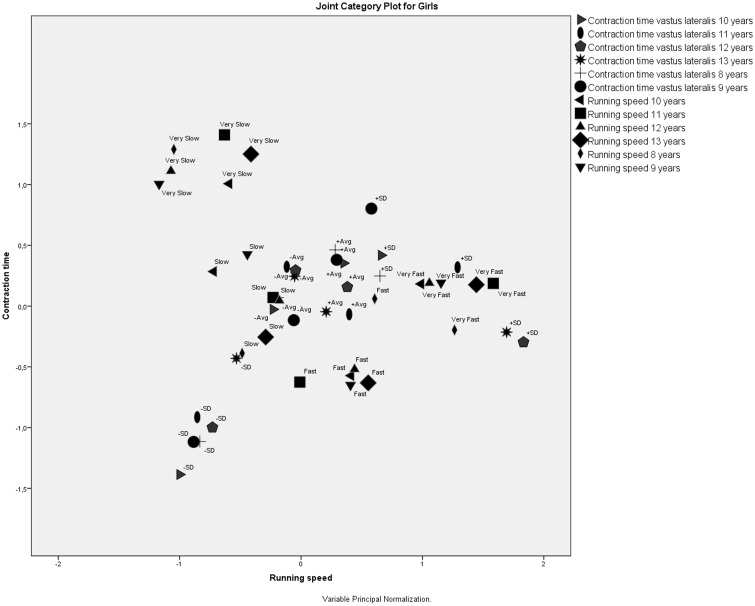
A joint category plot for VL contraction time and running speed for girls at six ages (Figure 3) showed that all four running speed category values formed their own clusters that were located far away from each other. Additionally, the very slow and fast category values were located far away from the VL contraction time category values. This clustering revealed three assumptions.
– All running speed categories were very different.
– Running speed category values very slow and fast were independent of VL contraction times.
– Girls’ running speed category values stayed closely associated with each other throughout the study, meaning that girls who were very slow stayed very slow, girls who were very fast stayed very fast, and so on.
Figure 3.
Correspondence analysis of vastus lateralis and running speed for girls at six ages.
It is interesting to note that contraction times labeled –SD (contraction times: –Avg –SD) were also grouped in an isolated cluster, meaning that extremely low contraction times did not have any effect on running speed and that extremely low VL contraction times were categorically constant throughout the study period. Low running speed for girls was associated with under-average VL contraction times and very fast running speed with extremely high VL contraction times (+SD).
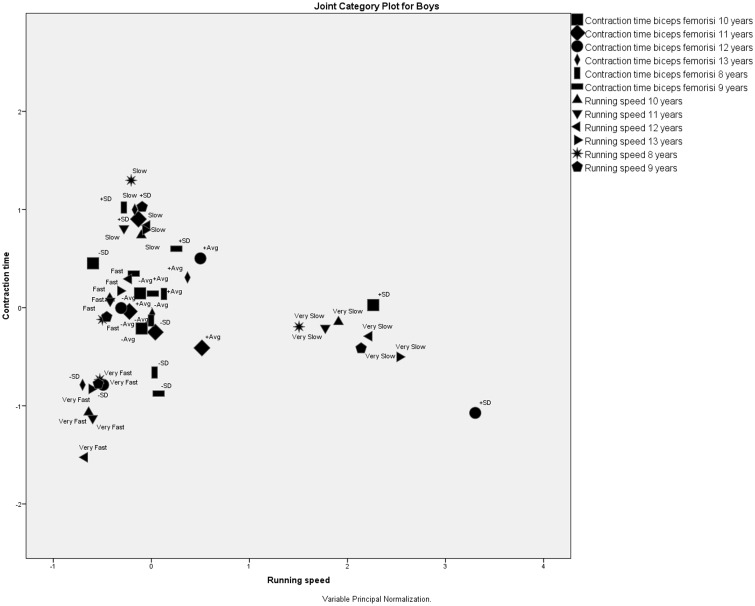
The MCA of BF contraction time and running speed for boys at six ages showed that all four running speed category values formed clusters located far away from each other, meaning that the four running speed category values were not associated. As above, the running speed category values stayed constant: slow boys stayed slow and fast boys stayed fast (Figure 4).
Figure 4.
Correspondence analysis of biceps femoris contraction time and running speed for boys at six ages.
In contrast to the VL muscle, running speed categories were associated with the BF contraction time category values. These associations can lead to the following conclusions.
– Very slow and slow running speeds were associated with very slow contraction times, which were more than 1 SD from the average BF contraction time.
– Very fast running speeds were associated with very fast contraction times, which were less than 1 SD from the average BF contraction time.
– Fast running speed was associated with below-average contraction times
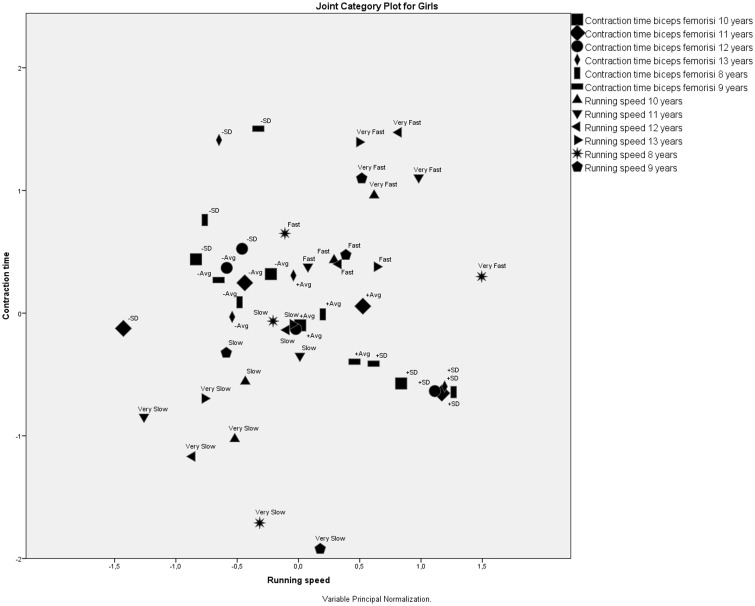
The MCA of BF contraction time and running speed for girls at six ages showed that the extreme running speed category values—very slow and very fast—formed their own clusters located far away from each other and far away from the BF contraction time category values (Figure 5). This clustering revealed three assumptions.
– The extreme running speed category values—very slow and very fast—were very different from each other.
– Neither extreme running speed category value was associated with the BF contraction time.
– Girls who were very slow or very fast remained very slow or very fast during all 6 years of the study.
– The +SD muscle contraction times were grouped in one cluster, meaning that the highest contraction time category value stayed constant throughout the study.
Figure 5.
Correspondence analysis of biceps femoris contraction time and running speed for girls at six ages.
Additionally, contraction times labeled +SD (contraction times above average + 1 SD) were grouped in an isolated cluster, meaning that extremely high BF contraction times had no effect on running speed. In contrast, slow and fast running speed category values were associated with different BF contraction time categories. Nevertheless, no clear association could be established between running speeds and BF contraction times.
The associations between contraction time category values and running speed category values in regard to gender and muscle are summarized in Table 2. There are some clear associations at specific category values, in contrast to other category values, where there seem to be no association between speed and contraction times.
Table 2.
Associations between contraction time and running speed in regard to gender and muscle.
| Muscle and gender | Very slow* | Slow* | Fast* | Very fast* |
|---|---|---|---|---|
| Vastus lateralis | ||||
| Boys | Unstructured association | −SD | Unstructured association | No association |
| Girls | No association | −Avg | Unstructured association | +SD |
| Biceps femoris | ||||
| Boys | +SD | +SD, +Avg | Unstructured association | −SD |
| Girls | No association | Unstructured association | Unstructured association | No association |
Running speeds
A comparison of the clustering of muscle contraction time category values and that of running speed category values shows that the same running speed category values are always grouped in one coherent cluster. Thus, participants’ categorical running speed did not changed with age. In contrast, muscle contraction time category values did not form coherent clusters, meaning that there were some categorical changes in muscle contraction times associated with the participants’ ages.
Regarding gender, the running speed of boys was less dependent on VL contraction times than that of girls. This finding is in contrast to that of the BF muscle, where girls’ BF contraction times had either no or unstructured associations with running speeds compared with those of boys, in whom there were some clear associations.
Joint category plots showed that BF contraction times were associated more closely with running speeds than were those of the VL muscle. It is interesting that extremely high contraction times (above average + 1 SD) were not associated with running speeds for the BF muscle, in contrast to the VL muscle, where extremely low contraction times (below average − 1SD) were not associated with running speeds.
The dimensions
After examining the muscle contraction speed category values and running speed category values, all four bi-plots’ dimensions were named in an equal manner. The x-axis represented the “Running speed,” and the y-axis represented the “Contraction time.” The following general observations about associations between contraction times and running speed could be derived based on these dimensions.
VL muscle: The association is proportional (higher contraction times result in higher running speeds).
BF muscle: The association is inversely proportional (higher contraction times result in slower running speeds).
Discussion
The analysis just described contributes a new insight into children’s skeletal muscle development. We can summarize the results by taking into consideration the research questions posed earlier. In general, the association of muscle contraction time and running speed was established, although clear associations exist only for specific category values of the two variables. There are gender differences in the association of running speed and muscle contraction times. It seems, however, that the associations in boys have more structure that those in girls. The structure of associations differs between muscles as well as the type of association (proportional for VL and inversely proportional for BF).
Associations between muscle contraction times and running speed
The aim of this longitudinal study was to gain insight into the development of children’s skeletal muscles, focusing on the associations between running speed and muscle contraction times, and comparing age- and gender-related differences. Our analysis revealed the presence of an association between muscle contraction times and running speed. Loturco et al.19 discovered similar associations in adults. Their study confirmed that the TMG-derived velocity of BF contraction—calculated from the ratio between maximum radial displacement and the sum of contraction time and delay time—is associated with 20 -m and change-of-direction sprint performances of elite soccer players. Bell et al.20 showed that the distribution patterns and ultrastructure of skeletal muscles in children did not differ from those in normal adult tissues. Hence, the combined results of these two studies support the outcomes of our research.
The period from ages 7–10 for girls and 7–12 for boys—called “late childhood”—is characterized by rapid linear growth of the limbs. The next growth surge occurs during adolescence and lasts for about 2 years, from around ages 11–13 in girls and 12–14 in boys. At this stage, some of the body’s dimensions rapidly increase. Our study, however, showed that the running speed category values generally did not change during that period. Unfortunately, no other similar published studies could be found. The lack of comparable studies could be because skeletal muscle composition is difficult to measure in healthy children. Some studies have investigated VL composition in adults, but there have been few studies on children20 or adolescents.21
Gender differences
Our study showed that there were gender differences in the association of running speed and muscle contraction times, which had been confirmed previously with correlation analysis and published elsewhere.22 The analysis showed some gender differences in the VL muscle: (1) the contraction time changes with age (faster in boys than in girls); (2) maximum running speed of the boys over the whole measurement period increased, whereas for the girls it increased only up to the seventh grade; (3) the contraction times of VL first declined until the fourth grade and later increased (significantly more in boys than in girls). These accomplishments could be attributed to the fact that the early school period is the most important for the development of children’s motor potentials and for learning movement patterns. Usually, children learn new movement techniques quickly and with little effort.23,24 As children develop coordination, they usually change the way they run. From a technical point of view, boys run more correctly, including their use of the two-headed muscle of the thigh (BF).
Praprotnik et al.25 studied the correlation between the BF muscle and the maximum speeds of male sprinters. They found that optimal running resulted from using a proper technique and taking advantage of the BF. It was also found that boys perform better than girls in most tests of coordination.26 The myelination of the nervous system is crucial for muscle responsiveness. Additionally, significant differences in the muscle contraction speed coincide with “blast growth” during middle and late childhood and adolescence.27 Šimunič et al.28 showed that overall muscle mass is 13–17% greater in boys than in girls between the ages of 8 and 13 years, which could be an additional source of gender-related differences in running speed. According to Always et al.,29 women have a smaller cross section of muscle fibers than men, which could be why they have a shorter contraction time. Contrary to the above studies, some researchers claim that there are no significant differences in the percentage of fiber types between genders.30,31
Muscle differences
We confirmed that the structure of associations, as well as the type of association (proportional in VL and inversely proportional in BF), differs between these muscles. The VL muscle is an anti-gravitational muscle and so receives many mechanical stimuli for its adaptation (i.e., hypertrophy). The BF is not an anti-gravitational muscle and therefore has few everyday mechanical stimuli. The two-headed thigh muscle (BF) can be trained to a greater extent because it contains the largest number of type IIc, or satellite-based, fibers, which, depending on the functional requirements of the body, can be transformed into type I or type II fibers.
Limitations
Our study has some limitations. First is the selection of the research environment: only three of twelve Slovenian regions were selected for the study. Second is the use of a non-standardized measurement tool. Because other studies on children’s physical activities already exist, it might have been better to use some previously developed measurement tools. Nonetheless, the methods used in the present study were quite similar to those of other studies, so comparison may still be possible.
Conclusion
By using MCA of VL contraction time and running speed for girls and boys, we were able to show that running speed categories are different in boys and girls. For boys, the very slow speed category was the most different. Also in boys, the slow and very slow running speed categories were independent of VL contraction times, in contrast to the situation in girls, for whom very slow and fast running speed categories were independent of VL contraction times. Interestingly, boys’ running speed categories stayed constant throughout the study, whereas only two categories remained constant for girls (very slow and fast). Our study also revealed that boys’ running speed was less dependent on VL contraction times than that of girls.
The MCA of BF contraction time and running speed for boys and girls revealed that, in boys, contrary to what was observed with the VL muscle, running speed categories were associated with BF contraction time categories. In boys, fast and very fast running speeds were associated with contraction times below −SD from the average BF contraction time. In contrast, girls’ fast and very fast running speed categories were very different and independent of BF contraction times. Our study also showed that boys’ running speed categories were much more structurally associated with BF contraction times than those of girls.
Comparing the MCA results for the VL and BF contraction times and running speeds for boys and girls shows that the BF contraction times were associated much more closely with running speed than were the VL contraction times. It was also found out that extremely long contraction times of the BF are not associated with running speed, in contrast to the VL whose extremely short contraction times are not associated with running speed.
The present results provide insight into a whole new dimension of a child’s development. We are confident that our assessment of the contractile parameters in two skeletal muscles, VL and BF, will contribute to the general understanding of children’s skeletal muscle development. It is important to note also the longitudinal nature of this study as such studies are rare in our field of interest.
Acknowledgements
The authors thank the research groups of the TMG-BMC Company and the Department of Muscle Biomechanics at the Faculty of Electrical Engineering, University of Ljubljana. Finally, we are thankful to all who have contributed in any way to the execution of the research study.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This study was funded by the Ministry of Education, Science, and Sport of the Republic of Slovenia, via the Slovenian Research Agency, through two 3-year projects undertaken at the Institute for Kinesiology Research, Science, and Research Centre of Koper, University of Primorska, in cooperation with the Dr. Adolf Drolc Healthcare Center, Maribor.
References
- 1.Shore R. New insight into early development: rethinking the brain, New York: Families and Work Institute, 2000. [Google Scholar]
- 2.Pišot R. The analysis of the structure of six-and-a-half years old children s motor space in the light of its developement as a whole, Kinanthropologica; Prague: Charles University, 2000. [Google Scholar]
- 3.Child R, Brown S, Day S, et al. Changes in indices of antioxidant status, lipid peroxidation and inflammation in human skeletal muscle after eccentric muscle actions. Clin Sci (Lond) 1999; 96: 105–115. [PubMed] [Google Scholar]
- 4.Dahmane R. Histokemična, morfometrična in biomehanska primerjava mišic zgornjega in spodnjega uda. Ljubljana; Univerza v Ljubljani, 2002.
- 5.Valenčič V. Direct measurement of the skeletal muscle tonus. In: Popovič D. (ed). Advances in external control of human extremities, Beograd: Nauka, 1990, pp. 102–108. [Google Scholar]
- 6.Simunič B. Between-day reliability of a method for non-invasive estimation of muscle composition. J Electromyogr Kinesiol 2012; 22: 527–530. [DOI] [PubMed] [Google Scholar]
- 7.Valencic V, Knez N. Measuring of skeletal muscles’ dynamic properties. Artif Organs 1997; 21: 240–242. [DOI] [PubMed] [Google Scholar]
- 8.Šimunič B, Pišot R and Rittweger J. The effect of ageing on contraction time of postural and non-postural skeletal muscles in master athletes. Novi Sad: Faculty of Sport and Physical Education, University of Novi Sad; 2009.
- 9.Dahmane R, Djordjevic S, Simunic B, et al. Spatial fiber type distribution in normal human muscle: histochemical and tensiomyographical evaluation. J Biomech 2005; 38: 2451–2459. [DOI] [PubMed] [Google Scholar]
- 10.Dahmane R, Valenčič V, Knez N, et al. Evaluation of the ability to make non-invasive estimation of muscle contractile properties on the basis of the muscle belly response. Med Biol Eng Comput 2001; 39: 51–55. [DOI] [PubMed] [Google Scholar]
- 11.Oliver J, Lloyd R, Rumpf M. Developing speed throughout childhood and adolescence: the role of growth, maturation and training. Strength Cond J 2013; 35: 42–48. [Google Scholar]
- 12.Tous-Fajardo J, Moras G, Rodríguez-Jiménez S, et al. Inter-rater reliability of muscle contractile property measurements using non-invasive tensiomyography. J Electromyogr Kinesiol 2010; 20: 761–766. [DOI] [PubMed] [Google Scholar]
- 13.Kersevan K, Valencic V, Djordjevic S, et al. The muscle adaptation process as a result of pathological changes or specific training procedures. Cell Mol Biol Lett 2002; 7: 367–269. [PubMed] [Google Scholar]
- 14.Martín-Rodríguez S, Estupiñán-Henríquez M, Rodríguez-Matoso D, et al. Neuromuscular interlimb coordination responses to a high volume resistance exercise on biceps brachii using tensiomiography. International conference 2014 - Human performance development through strength and conditioning; Murcia, 2014.
- 15.Alvarez-Diaz P, Alentorn-Geli E, Ramon S, et al. Comparison of tensiomyographic neuromuscular characteristics between muscles of the dominant and non-dominant lower extremity in male soccer players. Knee Surg Sports Traumatol Arthrosc 2016; 24: 2259–2263. [DOI] [PubMed] [Google Scholar]
- 16.de Paula Simolaa RÁ, Raedera C, Wiewelhovea T, et al. Muscle mechanical properties of strength and endurance athletes and changes after one week of intensive training. J Electromyogr Kinesiol 2016; 30: 73–80. [DOI] [PubMed] [Google Scholar]
- 17.Jemec J and Đorđevič S. Do We Have Enough Information. http://ipsitransactions.org/journals/papers/tir/2015jan/p6.pdf, 2015.
- 18.de Rooij M and van der Heijden P. Correspondence analysis of longitudinal data. Wiley StatsRef: Statistics Reference Online, 2014, p.e10.
- 19.Loturco I, Pereira LA, Kobal R, et al. Muscle contraction velocity: a suitable approach to analyze the functional adaptations in elite soccer players. J Sports Sci Med 2016; 15: 483–491. [PMC free article] [PubMed] [Google Scholar]
- 20.Bell RD, MacDougal JD, Billeter R, et al. Muscle fiber types and morphometric analysis of skeletal muscle in six-year-old children. Med Sci Sports Exerc 1980; 12: 28–31. [PubMed] [Google Scholar]
- 21.Glenmark B, Hedberg G, Jansson E. Changes in muscle fiber type from adolescence to adulthood in women and men. Acta Physiol Scand 1992; 146: 251–259. [DOI] [PubMed] [Google Scholar]
- 22.Završnik J. Empirical analysis of changes of the biomechanical properties of chosen skeletal muscles in motoric development of children with bibliometric analysis of the literature, Slovenj Gradec: University College of Health Sciences Slovenj Gradec, 2016. [Google Scholar]
- 23.Shaffer D, Kipp K. Developmental psychology: childhood and adolescence, 8th ed UK: Cengage Learning, 2009, pp. 208–220. [Google Scholar]
- 24.Koffka K. The growth of mind, Milton Park, UK: Routledge, 2002, pp. 49–59. [Google Scholar]
- 25.Praprotnik U, Valenčič V, Čoh M, et al. Povezanost maksimalne hitrosti teka s kontraktilnimi lastnostmi mišic. In: Zajc B. (ed). Zbornik enajste mednarodne Elektrotehniške in računalniške konference ERK 2002 in IEEE Region 8 Slovenska sekcija, Portorož: IEEE, 2002. [Google Scholar]
- 26.Dolenec M and Pistotnik B. Primerjava nekaterih motoričnih razsežnosti otrok, starih 7 do 11 let. 14. strokovni posvet športnih pedagogov; Kranjska Gora; 2001.
- 27.Tomazo Ravnik T. Biološka rast človeka. In: Marjanovič Umek L, Zupančič M. (eds). Razvojna psihologija, Ljubljana: Rokus, 2004, pp. 119–124. [Google Scholar]
- 28.Šimunič B, Volmut T, Pišot R. Pomen optimizacije gibanja otrok: znanstveno podprta moderna izhodišča = The significance of optimization of physical activity in children: scientifically - supported current baseline. In: Dolinšek J. (ed). Otrok in šport. Obravnava otrok z drisko. Šokovna stanja v otroškem obdobju, Maribor: Univerzitetni klinični center, Klinika za pediatrijo, 2010, pp. 43–47. [Google Scholar]
- 29.Always SE, Grumbt WH, Gonyea WJ, et al. Contrasts in muscle and myofibers of elite male and female bodybuilders. J Appl Physiol (1985) 1989; 67: 24–31. [DOI] [PubMed] [Google Scholar]
- 30.Drinkwater BL. Women and exercise: physiological aspects. Exerc Sport Sci Rev 1984; 12: 21–51. [PubMed] [Google Scholar]
- 31.Staron RS, Hagerman FC, Hikida RS, et al. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 2000; 48: 623–629. [DOI] [PubMed] [Google Scholar]