Abstract
Objective
To compare the efficacy and tolerability of celecoxib and ibuprofen for the treatment of knee osteoarthritis symptoms.
Method
In this 6-week, multicentre, double-blind, non-inferiority trial, patients were randomized to 200 mg celecoxib once daily, 800 mg ibuprofen three times daily or placebo. The primary outcome was non-inferiority of celecoxib to ibuprofen in Patient’s Assessment of Arthritis Pain (scored 0–100). Secondary outcomes included the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index, Pain Satisfaction Scale, and upper gastrointestinal tolerability.
Results
A total of 388 patients were treated (celecoxib n = 153; ibuprofen n = 156; placebo n = 79). Mean difference (95% confidence interval) between celecoxib and ibuprofen in the Patient’s Assessment of Arthritis Pain was 2.76 (−3.38, 8.90). As the lower bound was greater than −10, celecoxib was non-inferior to ibuprofen. The WOMAC total score was significantly improved with celecoxib and ibuprofen, versus placebo. Patients receiving celecoxib were significantly more satisfied (versus placebo) in 10 of 11 measures on the Pain Satisfaction Scale versus three measures with ibuprofen. Upper gastrointestinal events were less frequent with celecoxib (1.3%) than ibuprofen (5.1%) or placebo (2.5%).
Conclusion
Celecoxib was well tolerated and as effective as ibuprofen for symptoms associated with knee osteoarthritis.
ClinicalTrials.gov identifier
Keywords: COX-2 inhibitors, cyclooxygenase, osteoarthritis, non-steroidal anti-inflammatory drugs, celecoxib
Introduction
Osteoarthritis (OA) is the most common form of arthritis, and is a major cause of disability and chronic pain in adults.1,2 Even though OA can involve single and/or multiple peripheral joints, including the knee, hip and hand,1 the knee is the most common joint localization of symptomatic OA.2 Knee OA, affecting > 250 million people worldwide, has significant effects on patient function and considerable societal costs in terms of work loss, early retirement and joint replacement.3 The results of the OA process are cartilage degradation and bone remodelling; these features are associated with the development of symptoms of pain, stiffness and functional disability.1 In the current paradigm, the structural changes represent the disease, whereas the symptoms of aching, discomfort, pain and stiffness represent the illness for which patients seek medical care.4 Current treatment of OA is based on symptom management, primarily pain control, and relies on a combination of nonpharmacological and pharmacological approaches that are generally tailored to the patient’s needs and risk factors.5 Clinical guidelines recommend the use of oral nonsteroidal anti-inflammatory drugs (NSAIDs) in patients with persistent symptoms that have not responded adequately to paracetamol with or without topical NSAIDs.6 NSAIDs are the most frequently prescribed medicines for OA,4 yet they have significant toxicity, especially among the demographic groups in which the disorder is most prevalent.7 Therefore, care should be taken because NSAIDs are associated with upper8 and lower9,10 gastrointestinal harm, acute renal failure11,12 and increased cardiovascular risk.13,14
Celecoxib is a selective NSAID indicated for the treatment of the signs and symptoms associated with OA.15 Its efficacy in relieving pain and inflammation and improving physical function in patients with OA has been established,16–21 and it has a better gastrointestinal tolerability profile relative to nonselective NSAIDs.22 The relative effectiveness of celecoxib versus diclofenac and naproxen for the treatment of OA is well documented in individual clinical studies and meta-analyses.3,18,19 However, despite celecoxib and ibuprofen being commonly used NSAIDs globally, little comparative efficacy data from randomized controlled trials are available in the published literature. Although, there are limited indirect comparisons available, with recent meta-analyses suggesting comparable efficacy.3,23 More knowledge of the comparative efficacy of these compounds would be helpful for patients, physicians, payers and policymakers, in order to formulate rational treatment algorithms for OA. Therefore, the purpose of this study was to assess the efficacy of 200 mg celecoxib once daily compared with 800 mg ibuprofen three times daily, in patients with OA of the knee.
Patients and methods
Study design
This was a 6-week, multicentre, randomized, double-blind, placebo-controlled, active comparator, parallel-group study in patients with OA of the knee, conducted in 34 centres in Spain, Germany and the UK from February 2003 to January 2004. Patients diagnosed with OA in a flare state and with a Functional Capacity Classification of I to III were eligible for study participation.24 The study included four visits: screening, baseline, week 2 and week 6 or early termination. The screening visit occurred within 1 to 14 days prior to the first dose of study medication. Between screening and baseline, patients discontinued use of any NSAID and/or analgesic therapy. Acetaminophen/paracetamol (up to 2 g/day) was permitted as rescue analgesia for the treatment of arthritis symptoms during the pretreatment screening period. Patients were to discontinue use of acetaminophen/paracetamol at least 24 h prior to the baseline arthritis assessments. Patients were assigned to a treatment regimen according to a predetermined computer-generated randomization schedule to receive one of three regimens: 200 mg celecoxib once daily, 800 mg ibuprofen three times daily or placebo in a 2:2:1 ratio. Patients were randomized separately based on their pain status at baseline. Two separate randomization schedules were used for patients with baseline assessments of Arthritis Pain ≤ 69 mm and ≥ 70 mm on a 100-mm visual analogue scale (VAS). Use of analgesic medication for the treatment of arthritis symptoms was prohibited throughout the study period, but use of stable doses of aspirin (≤325 mg/day) for cardiovascular prophylaxis was allowed.
Efficacy evaluations were conducted in the per-protocol analysis (PPA) population and in the modified intent-to-treat (MITT) population; safety evaluations were conducted in the safety population. Both the MITT and safety populations included all patients who were randomized and received at least one dose of study drug. The PPA population was the subset of the MITT population who had no major protocol violations and completed all four visits, including at baseline.
The study was conducted in compliance with the ethical principles originating in, or derived from, the Declaration of Helsinki and in compliance with the Independent Review Boards at each participating centre, informed consent regulations and International Congress of Harmonisation Good Clinical Practice Guidelines. In addition, all local regulatory requirements were followed. Written informed consent was obtained prior to the patient entering the study (e.g. before initiation of protocol-specific procedures). The trial was registered with ClinicalTrials.gov at the US National Institutes of Health (ClinicalTrials.gov identifier: NCT00630929).
Patients
Patients who were ≥ 40 years of age with a clinical diagnosis of OA of the knee according to the American College of Rheumatology guidelines, in a flare state and with a Functional Capacity Class of I to III were eligible for participation.24 For patients who had been receiving NSAID or analgesic therapy, which was discontinued 48 h prior to the baseline visit, OA flare was defined as Patient’s Assessment of Arthritis Pain between 40 and 90 mm on a 100-mm VAS, and an increase of one or more grades in both the Patient’s and Physician’s Global Assessments of Arthritis (scored from 1, ‘very good’, to 5 ‘very poor’, based on impact of symptoms) between screening and baseline visits.25 In patients whose OA was not controlled and who had not been receiving treatment for their OA, OA flare was defined as Patient’s Assessment of Arthritis Pain between 40 and 90 mm on a 100-mm VAS, and a rating of ‘poor’ or ‘very poor’ in both Patient’s and Physician’s Global Assessments of Arthritis.
Patients were not eligible if they had inflammatory arthritis or gout/pseudogout or had experienced an acute flare within the past 2 years (patients with fibromyalgia were not excluded), previously had or anticipated a need for surgical or other invasive procedures on the joint with OA during the study or had received oral corticosteroids within 4 weeks or paracetamol within 24 h. Other exclusion criteria were malignancy or history of malignancy; active gastrointestinal disease; history of gastrointestinal perforations, obstructions or bleeding; cardiac, renal and/or hepatic disease; coagulation disorders or known hypersensitivity to cyclooxygenase (COX)-2 inhibitors, aspirin, NSAIDs or sulfonamide medication.
Study treatment
The three study treatment regimens consisted of 200 mg celecoxib once daily, 800 mg ibuprofen three times daily or placebo. Celecoxib capsules were administered orally as 200 mg capsules. Ibuprofen 800 mg tablets were administered orally. The double-dummy method of blinding was used where placebo capsules/tablets that were indistinguishable from celecoxib and ibuprofen were used to achieve double-blinding (both investigator and patient were blinded to the study medication). The treatment period was 6 weeks.
Assessments
The primary efficacy analysis was comparison of the change from baseline to week 6 in the Patient’s Assessment of Arthritis Pain measured on a 0–100-mm VAS in patients treated with 200 mg celecoxib once daily, 800 mg ibuprofen three times daily, or placebo, and analysed using the PPA population. In addition, the Patient’s Assessment of Arthritis (VAS) at week 6 was analysed for the MITT population. Secondary objectives, evaluated in the MITT population, were to compare the change in the Patient’s and Physician’s Global Assessments of Arthritis from baseline to week 6 between treatment groups. Other secondary objectives included change in Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index from baseline to week 6 and the Pain Satisfaction Scale (comprising 11 questions on information about pain and its treatment, and pain medication in general) at week 6.26 Assessment of safety measures, including upper gastrointestinal tolerability, was a secondary objective and was performed on the safety population.
Statistical analyses
A sample size of 100 patients per active treatment group was estimated to provide 80% power to detect a 95% confidence interval (CI) within the acceptable margins, assuming that the true treatment difference was zero and assuming a standard deviation of 25 mm and a type-1 error rate of 0.05. For placebo, a sample size of 60 patients was estimated to provide 80% power to detect a difference of 15 mm between the active treatment group and placebo (assuming a standard deviation of 25 mm).
The primary efficacy measure, change from baseline to week 6 in the Patient’s Assessment of Arthritis Pain score (VAS), was analysed using a general linear model with treatment and centre as fixed effects and baseline score as a covariate in the PPA population. All pairwise comparisons were conducted. Celecoxib was declared to be as effective as ibuprofen if the lower bound of the two-sided 95% CI of the treatment difference (ibuprofen–celecoxib) lay above −10 mm in the PPA population.26 All secondary efficacy analyses were assessed in the MITT population. The 24-item WOMAC scale and subscales were analysed using a general linear model with treatment and centre as fixed effects, and baseline WOMAC scores as a covariate. The Patient’s and Physician’s Global Assessments of Arthritis and Pain Satisfaction Scale scores were analysed using the Cochran–Mantel–Haenszel test (row-mean-score-test), stratified by centre. Safety analyses were evaluated in the safety population (patients who were randomized and received at least one dose of study medication). The incidence of upper gastrointestinal events was analysed using two-tailed Fisher’s exact test. A P-value < 0.05 was considered statistically significant.
Results
Patient disposition and baseline characteristics
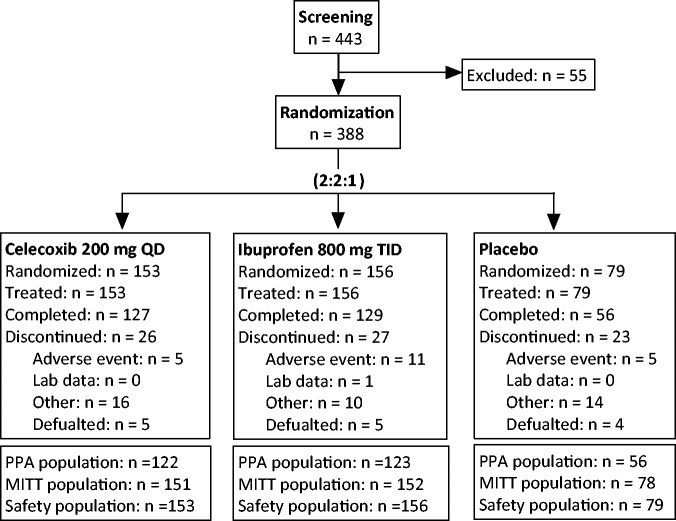
Of the 388 patients randomized, 153 received 200 mg celecoxib once daily, 156 received 800 mg ibuprofen three times daily and 79 received placebo. Demographic and baseline characteristics were similar across the three treatment groups (Table 1). The mean age was 62.2 to 64.5 years, the majority of patients were female (70%–74%) and the mean duration of OA was 5.1 to 6.4 years. A total of 381 patients received at least one dose of study medication and had at least one post-baseline OA pain assessment, and comprised the MITT population (Figure 1). From the MITT population, 80 patients (n = 29 celecoxib, n = 29 ibuprofen and n = 22 placebo) were excluded due to major protocol deviations, the remaining 301 patients were included in the PPA population. Of the 381 patients in the MITT population, a total of 312 patients completed the study (i.e. participated in the 6 weeks of the study): 127 (83.0%) in the celecoxib group, 129 (82.7%) in the ibuprofen group and 56 (70.9%) in the placebo group. A total of 76 patients discontinued from the study prematurely, 21 (n = 5 in the celecoxib, n = 11 in the ibuprofen and n = 5 in the placebo groups) as a result of adverse events (AEs). The remainder of the patients were either withdrawn for other reasons (i.e. protocol violations, did not meet entrance criteria) or defaulted (lost to follow-up or no longer willing to participate in the study). There were no patient deaths during the study.
Table 1.
Demographic and baseline osteoarthritis (OA) characteristics in patients with OA of the knee who participated in a 6-week, multicentre, randomized, double-blind, placebo-controlled, active comparator, parallel-group study to assess the efficacy of 200 mg celecoxib once daily compared with 800 mg ibuprofen three times daily.
| Characteristic | Celecoxib 200 mg once daily n = 153 | Ibuprofen 800 mg three times daily n = 156 | Placebo n = 79 |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 62.2 (9.5) | 62.9 (8.9) | 64.5 (11.2) |
| Range | 41–82 | 40–89 | 40–88 |
| Sex, n (%) | |||
| Female | 111 (72.5) | 116 (74.4) | 55 (69.6) |
| Male | 42 (27.5) | 40 (25.6) | 24 (30.4) |
| Race, n | |||
| White | 150 | 153 | 78 |
| Black | 1 | 1 | 0 |
| Hispanic | 0 | 0 | 0 |
| Asian | 1 | 2 | 1 |
| Other | 1 | 0 | 0 |
| Duration of OA, years | |||
| Mean (SD) | 6.4 (6.8) | 5.1 (5.1) | 5.6 (5.4) |
| Range | 0.1–33.0 | 0.1–28.0 | 0.1–28.0 |
| Patient’s Assessment of Arthritis Pain score, VAS, mm | |||
| Mean (SD) | 67.9 (13.0) | 68.4 (13.5) | 67.1 (15.4) |
| Range | 41.0–92.0 | 25.0–100.0 | 30.0–100.0 |
| Patient’s Global Assessment of Arthritis, n (%)a | |||
| Very Good | 0 | 0 | 0 |
| Good | 0 | 0 | 0 |
| Fair | 18 (11.8) | 14 (9.0) | 12 (15.2) |
| Poor | 108 (70.6) | 120 (77.4) | 55 (69.6) |
| Very Poor | 27 (17.6) | 21 (13.5) | 12 (15.2) |
| Physician’s Global Assessment of Arthritis, n (%)a | |||
| Very Good | 0 | 0 | 0 |
| Good | 0 | 0 | 0 |
| Fair | 17 (11.1) | 22 (14.2) | 11 (13.9) |
| Poor | 116 (75.8) | 125 (80.6) | 61 (77.2) |
| Very Poor | 20 (13.1) | 8 (5.2) | 7 (8.9) |
| WOMAC Total Domain scoreb | |||
| Mean (SD) | 48.0 (15.0) | 48.4 (15.8) | 47.8 (15.6) |
| Range | 17.0–88.0 | 12.0–84.0 | 16.0–91.0 |
| Functional Capacity Classification, n (%)a | |||
| I | 3 (2.0) | 1 (0.6) | 1 (1.3) |
| II | 82 (53.6) | 82 (52.9) | 45 (57.0) |
| III | 68 (44.4) | 72 (46.5) | 33 (41.8) |
| IV | 0 | 0 | 0 |
Percentages are calculated based on the number of randomized patients. One patient in the ibuprofen treatment group had no baseline efficacy data.
WOMAC Total Domain score is the sum of Pain, Stiffness and Physical Function Domain scores.
There were no statistically significant between-group differences (P ≥ 0.05) as assessed as follows: age and duration of OA, by general linear model with treatment and centre as factor; sex, by Cochran–Mantel–Haenszel (general association) test stratified by centre; Patient’s Assessment of Arthritis Pain VAS score and WOMAC Total Domain score, by general linear model with treatment and centre as fixed effects; and Patient’s and Physician’s Global Assessment of Arthritis and Functional Capacity Classification, by Cochran–Mantel–Haenszel (row mean scores differ) test stratified by centre.
VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Figure 1.
Flow diagram showing patient numbers in a 6-week, multicentre, randomized, double-blind, placebo-controlled, active comparator, parallel-group study to assess the efficacy of 200 mg celecoxib once daily compared with 800 mg ibuprofen three times daily in patients with osteoarthritis of the knee. MITT, modified intent-to-treat; PPA, per-protocol analysis; QD, once daily; TID, three times daily.
Treatment efficacy
Patient’s Assessment of Arthritis Pain (VAS) mean scores were similar among the three treatment groups at baseline (Table 1). At week 6, there was a decrease in mean OA pain scores in all three treatment groups in the PPA population, with the greatest mean (SE) decrease in the celecoxib group (−34.5 [2.23], compared with ibuprofen (−32.8 [2.28]) and placebo (−28.4 [3.41]) (Table 2). The least squares (LS) mean difference (95% CI) between celecoxib and ibuprofen was not statistically significant (2.76 [−3.38, 8.90]). As the lower bound of the two-sided 95% CI was greater than the pre-specified non-inferiority margin of −10, the primary endpoint (non-inferiority of celecoxib to ibuprofen on Patient’s Assessment of Arthritis Pain score at week 6 in the PPA population) was achieved and 200 mg celecoxib once daily was as effective as 800 mg ibuprofen three times daily in the reduction of OA pain. In the MITT population at week 6, there was also a decrease from baseline in mean (SE) OA pain VAS scores in all three treatment groups, with the greatest decrease in the celecoxib group (–31.6 [2.06]) compared with ibuprofen (−29.1 [2.12]) and placebo (−21.3 [3.06]) (Table 2). Pairwise comparisons of treatment differences in LS mean change from baseline showed that the LS mean decrease in the celecoxib group was significantly greater than in the placebo group (P = 0.0076). The LS mean decrease in the ibuprofen group was not significantly different from that seen in either the placebo or celecoxib group.
Table 2.
Primary efficacy assessment of the change in the Patient’s Assessment of Arthritis Pain scored using a visual analogue scale (VAS) from baseline to week 6 in patients with osteoarthritis of the knee who were treated with either 200 mg celecoxib once daily, 800 mg ibuprofen three times daily or placebo.
| Celecoxib 200 mg once daily | Ibuprofen 800 mg three times daily | Placebo | |
|---|---|---|---|
| Change from baseline in Patient’s Assessment of Arthritis Pain (VAS) – PPA Population | |||
| Change from baseline | |||
| n | 122 | 123 | 56 |
| Mean (unadjusted) | −34.5 | −32.8 | −28.4 |
| SE mean | 2.23 | 2.28 | 3.41 |
| Ibuprofen–Celecoxib | Ibuprofen–Placebo | Celecoxib–Placebo | |
| Difference in LS means | 2.76 | −2.50 | −5.26 |
| SE | 3.12 | 3.94 | 3.96 |
| 95% CI | −3.38, 8.90 | −10.25, 5.25 | −13.06, 2.54 |
| Statistical significancea | NS | NS | NS |
| Change from baseline in Patient’s Assessment of Arthritis Pain (VAS) – MITT Population | |||
| Change from baseline | |||
| n | 151 | 151 | 78 |
| Mean (unadjusted) | −31.6 | −29.1 | −21.3 |
| SE mean | 2.06 | 2.12 | 3.06 |
| Ibuprofen–Celecoxib | Ibuprofen–Placebo | Celecoxib–Placebo | |
| Difference in LS means | 3.72 | −5.69 | −9.41 |
| SE | 2.90 | 3.53 | 3.51 |
| 95% CI | −1.98, 9.43 | −12.63, 1.24 | −16.31, −2.52 |
| Statistical significancea | NS | NS | P = 0.0076 |
Analysed using a general linear model with treatment and centre as fixed effects and baseline score as a covariate.
PPA, per-protocol analysis; CI, confidence interval; MITT, modified intent-to-treat; LS, least squares; NS, no statistically significant between-group differences (P ≥ 0.05).
Patient’s global assessment of arthritis
At week 6, a greater proportion of patients in the celecoxib group (45.7%, 69 of 151) rated their OA condition as ‘good’ or ‘very good’ compared with the placebo (30.8%, 24 of 78) or ibuprofen (43.7%, 66 of 151) groups. The difference between celecoxib and placebo was significant (P = 0.0052), but differences between the other groups were not significant. Patients treated with celecoxib were significantly more likely to report that their OA condition ‘improved’ from baseline to week 6 (49.0%, 74 of 151) compared with those receiving placebo (35.9%, 28 of 78), who were more likely to report that their OA condition had ‘worsened’ or remained ‘unchanged’ (P = 0.0182). The proportion of patients whose condition ‘improved’ with ibuprofen (45.0%, 68 of 151) was not significantly different from the proportions in patients receiving celecoxib or placebo.
Physician’s global assessment of arthritis
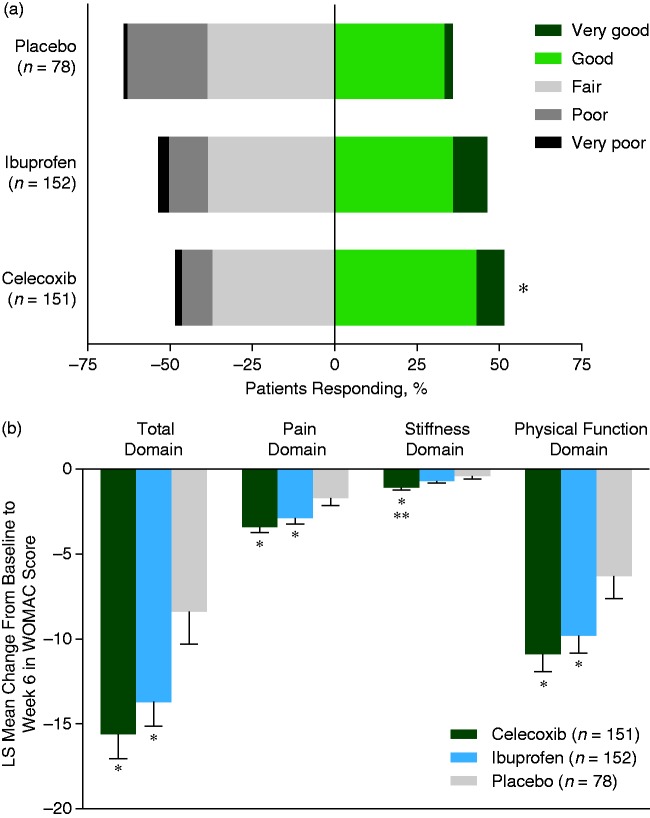
At week 6, a greater proportion of patients in the celecoxib (51.7%, 78 of 151) and ibuprofen (46.4%, 70 of 151) groups had their arthritis condition rated as ‘good’ or ‘very good’ compared with the placebo group (35.9%, 28 of 78) (Figure 2a). This difference was significant for celecoxib compared with placebo (P = 0.0027), but not for ibuprofen versus placebo or for celecoxib versus ibuprofen. The proportion of patients whose OA condition ‘improved’ from baseline to week 6 was greater in the celecoxib group (49.0%, 74 of 151) than in the ibuprofen (41.1%, 62 of 151) or placebo (37.2%, 29 of 78) groups, but these differences were not statistically significant.
Figure 2.
(a) Physician’s Global Assessment of Arthritis at week 6 (MITT). *P = 0.0027 versus placebo group. (b) LS mean change in WOMAC scores at week 6 (MITT). *P ≤ 0.03 versus placebo group; **P = 0.0220 versus ibuprofen group, for mean change in WOMAC scores. LS, least squares; MITT, modified intent-to-treat; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index. The colour version of this figure is available at: http://imr.sagepub.com.
WOMAC OA score
There were significant improvements in total WOMAC OA score and individual domain scores in the celecoxib and ibuprofen groups compared with placebo (Figure 2b). For example, both active treatment groups were significantly improved versus those in the placebo group in the measures of: total domain (P = 0.0014, celecoxib versus placebo; P = 0.0202, ibuprofen versus placebo); pain domain (P = 0.0012, celecoxib versus placebo; P = 0.0225, ibuprofen versus placebo); and physical function domain (P = 0.0040, celecoxib versus placebo; P = 0.0266, ibuprofen versus placebo). Patients treated with celecoxib had significantly greater improvement in the stiffness domain compared with both ibuprofen (P = 0.0220) and placebo (P = 0.0006) treated patients, whereas the improvement with ibuprofen was not significant versus placebo.
Pain satisfaction scale
A greater proportion of patients in the celecoxib and ibuprofen groups than in the placebo group agreed with the Pain Satisfaction Scale questions (Table 3). Patients treated with celecoxib were significantly more satisfied than those receiving placebo on 10 of the 11 Pain Satisfaction Scale questions, including those related to duration and onset of pain relief; effect on physical health; outlook on life; ease in performance of daily activities and leisure activities; improving independence, relationships with others and mood; and allowing easier movement. Although a greater proportion of celecoxib-treated patients, compared with placebo-treated patients, agreed that ‘My pain medication allows me to concentrate better’, this was not significant. Significantly more patients in the celecoxib group, compared with the ibuprofen group, agreed with the statements, ‘My pain medication allows me to perform daily activities more easily’ (70.9% versus 62.0%, respectively; P = 0.033) and ‘My pain medication allows me to participate in my leisure activities more often’ (70.9% versus 60.0%, respectively; P = 0.020). In the ibuprofen group, a significantly greater proportion of patients, compared with placebo, agreed with three of the 11 statements: ‘I am happy with the duration of pain relief provided by my pain medication’ (P = 0.003), ‘My pain medication relieves my pain quickly enough’ (P = 0.015) and ‘My pain medication improves my mood’ (P = 0.036).
Table 3.
Pain Satisfaction Scale at week 6 in patients with osteoarthritis of the knee who were treated with either 200 mg celecoxib once daily, 800 mg ibuprofen three times daily or placebo – pairwise comparison with placebo (MITT).
| Items | Celecoxib 200 mg once daily n = 148 | Ibuprofen 800 mg three times daily n = 150 | Placebo n = 77 | Statistical significancea |
||
|---|---|---|---|---|---|---|
| Celecoxib versus ibuprofen | Ibuprofen versus placebo | Celecoxib versus placebo | ||||
| 1. Happy with the duration of pain relief | 103 (69.6) | 99 (66.0) | 33 (42.9) | NS | P = 0.003 | P < 0.001 |
| 2. Relieves my pain quickly enough | 95 (64.2) | 89 (59.3) | 31 (40.3) | NS | P = 0.015 | P = 0.002 |
| 3. Has a positive effect on my physical health | 101 (68.2) | 93 (62.0) | 38 (49.4) | NS | NS | P = 0.018 |
| 4. Helps me have a better outlook on life | 92 (62.2) | 87 (58.4)b | 34 (44.2) | NS | NS | P = 0.025 |
| 5. Allows me to perform my daily activities more easily | 105 (70.9) | 93 (62.0) | 40 (51.9) | P = 0.033 | NS | P = 0.011 |
| 6. Allows me to perform my leisure activities more often | 105 (70.9) | 90 (60.0) | 35 (45.5) | P = 0.020 | NS | P < 0.001 |
| 7. Helps me do things independently | 99 (66.9) | 92 (61.7)b | 37 (48.1) | NS | NS | P = 0.010 |
| 8. Allows me to have better relationships with others | 88 (59.5) | 82 (55.0)b | 32 (41.6) | NS | NS | P = 0.026 |
| 9. Improves my mood | 95 (64.2) | 90 (60.0) | 34 (44.2) | NS | P = 0.036 | P = 0.010 |
| 10. Allows me to concentrate better | 80 (54.1) | 84 (56.0) | 35 (45.5) | NS | NS | NS |
| 11. Allows me to move around more easily | 108 (73.0) | 98 (65.8)b | 41 (53.2) | NS | NS | P = 0.007 |
Data presented as n of patients (%).
Significance assessed using a general linear model with treatment and centre as fixed effects, and baseline WOMAC scores as a covariate.
Data available for 149 patients.
MITT, modified intent-to-treat; NS, no statistically significant between-group differences (P ≥ 0.05).
Safety
A total of 100 patients reported 136 treatment-emergent AEs (Table 4). The incidence of treatment-emergent AEs was 20.3% (31 of 153) in the celecoxib group, 30.8% (48 of 156) in the ibuprofen group, and 26.6% (21 of 79) in the placebo group. A total of 20 patients discontinued the study because of treatment-emergent AEs. Of these, 16 discontinued as a result of AEs considered treatment-related (n = 3 [2.0%] with celecoxib, n = 9 [5.8%] with ibuprofen and n = 4 [5.1%] with placebo). Nine patients experienced severe AEs (n = 1 [0.7%] with celecoxib, n = 5 [3.2%] with ibuprofen and n = 3 [3.8%] with placebo). Of these AEs, five (n = 1 [0.7%] with celecoxib, n = 3 [1.9%] with ibuprofen and n = 1 [1.3%] with placebo) were considered treatment-related. There were no deaths, but one patient (a 79-year-old female receiving concomitant aspirin and with a history of cardiac arrhythmia) in the ibuprofen group experienced two serious AEs (aggravated hypertension and congestive heart failure), considered by the investigator to be unrelated to study medication. There were no serious AEs in the celecoxib or placebo groups.
Table 4.
Summary of treatment-emergent adverse events (AEs) in patients with osteoarthritis of the knee who were treated with either 200 mg celecoxib once daily, 800 mg ibuprofen three times daily or placebo for 6 weeks.
| Celecoxib 200 mg once daily n = 153 | Ibuprofen 800 mg three times daily n = 156 | Placebo n = 79 | |
|---|---|---|---|
| Number of AEs | 43 | 66 | 27 |
| Patients with AEs, n (%) | 31 (20.3) | 48 (30.8) | 21 (26.6) |
| Patients with serious AEs, n (%) | 0 | 1 (0.6) | 0 |
| Patients with severe AEs, n (%) | 1 (0.7) | 5 (3.2) | 3 (3.8) |
| Patients discontinued due to AEs, n (%) | 5 (3.3) | 10 (6.4) | 5 (6.3) |
| Patients with dose reduced or temporary discontinuation due AEs, n (%) | 0 | 5 (3.2) | 1 (1.3) |
| UGI eventa | 2 (1.3) | 8 (5.1) | 2 (2.5) |
| Common treatment-related AEs,b n (%) | |||
| Diarrhoea | 4 (2.6) | 1 (0.6) | 1 (1.3) |
| Dyspepsia | 4 (2.6) | 7 (4.5) | 2 (2.5) |
| Abdominal pain | 1 (0.7) | 8 (5.1) | 1 (1.3) |
| Headache | 0 | 1 (0.6) | 2 (2.5) |
Data presented as n of patients (%), which are based on the number of patients evaluable for AEs.
Defined as a moderate or severe instance of one or more of abdominal pain, dyspepsia, and/or nausea. There were no statistically significant between-group differences in the number of UGI events with each treatment (P ≥ 0.05).
Reported by ≥2% of patients in any treatment group.
UGI, upper gastrointestinal.
Upper gastrointestinal events (sum of moderate or severe abdominal pain, dyspepsia and/or nausea) were reported less frequently with celecoxib (1.3%, 2 of 153) than in the ibuprofen (5.1%, 8 of 156) or placebo (2.5%, 2 of 79) groups, although these differences were not significant (Table 4). Treatment-related AEs reported by ≥ 2% of patients in any treatment group are presented in Table 4. Dyspepsia, abdominal pain and upper gastrointestinal events were reported by more ibuprofen-treated patients, whereas diarrhoea was reported by more celecoxib-treated patients.
Discussion
In this 6-week non-inferiority study, celecoxib (at 200 mg/day) was as effective as high-dose ibuprofen (2400 mg/day) for the treatment of pain associated with OA. Although the efficacy of celecoxib compared with diclofenac and naproxen in relieving pain and improving physical function in patients with OA has previously been established,16–21 to date there has been little direct evidence comparing celecoxib with ibuprofen, despite ibuprofen being one of the most commonly used NSAIDs.
In this present study, both active treatments resulted in significant improvements, compared with placebo, in the pain, physical function and total domains of the WOMAC OA Index. This is consistent with previous indirect comparisons between celecoxib and ibuprofen that have suggested they have comparable efficacy.3,23,27 However, in this present study, only celecoxib resulted in significant improvements in the stiffness domain when compared with ibuprofen or placebo. In a chronic disease such as OA, it is particularly important to assess the degree of patient satisfaction with treatment. Given the changes in symptom severity over time, together with the occasional need to switch treatments, based on reduced efficacy or tolerability, patient satisfaction may be a more accurate measure of the overall effectiveness of a treatment.28–30 Patients in the present study were significantly more satisfied with celecoxib treatment than placebo on all but one of the 11 measures of the Pain Satisfaction Scale. This measure, ‘allows me to concentrate better,’ was also the sole measure with a nominally higher proportion of satisfied patients receiving ibuprofen than celecoxib (54.1% celecoxib versus 56.0% ibuprofen and 45.5% placebo). Patients receiving ibuprofen were significantly more satisfied than those receiving placebo on only three of the 11 measures.
Patient satisfaction in the present study was also assessed by the Patient’s Global Assessment of Arthritis, in which patients receiving celecoxib were significantly more likely than those receiving placebo to rate their condition as ‘good’ or ‘very good’ at endpoint. However, the proportion of patients receiving ibuprofen who rated their condition as ‘good’ or ‘very good’ at endpoint was not significantly different from the proportion receiving placebo. These results suggest that, in general, patients receiving celecoxib were more likely to be satisfied with their treatment than patients receiving ibuprofen.
It has been suggested that, because patient perception of the effectiveness of a treatment can be an indication of its suitability, measures of patient satisfaction should be included in clinical trials and in the overall evaluation of any treatment.31 This is supported by studies demonstrating that patient satisfaction with treatment improves with switching to a more effective treatment option.30,31 Because the duration of this present study (6 weeks) was shorter than that of several previous studies,16–18,21 it could be argued that the results may not extrapolate to longer treatment periods. However, a previous trial demonstrated that improvements in pain and patient and physician perceptions that were apparent after 6 weeks were maintained for up to 12 weeks of celecoxib treatment.18
Celecoxib was well tolerated compared with placebo and ibuprofen. The proportion of patients with an upper gastrointestinal event was higher with ibuprofen than with celecoxib. Given the low number of events in this study, the lack of a significant difference between active treatment groups is not surprising. However, this trend is consistent with the findings from pooled and meta-analyses, which have suggested that there is a lower risk of gastrointestinal events with celecoxib than with ibuprofen or other NSAIDs.17,22,32
There were two serious AEs in this study, aggravated hypertension and congestive heart failure, both of which occurred in a single patient treated with ibuprofen. This patient, a 79-year-old female, was receiving concomitant aspirin during the study and had a history of cardiac arrhythmia. Although these serious AEs were not considered to be related to study treatment, the potential for drug–drug interactions between aspirin and nonselective NSAIDs, such as ibuprofen, should be noted. Evidence, particularly for ibuprofen among traditional NSAIDs, suggests that these treatments may interfere through competitive interaction with COX-1,33 and that regular use of ibuprofen, particularly when ingested before aspirin, may abrogate the well-established cardio-protective benefits of the latter medicine.34,35 Celecoxib, as a COX-2-specific NSAID, does not appear to have this effect on these benefits of aspirin.36
It should be noted that the dose of ibuprofen used in this study, 800 mg three times daily, is the maximum daily dose permitted in Europe and the US for the treatment of OA. In many clinical situations, either over-the-counter or with a prescription, a lower dose would likely be used.37 Recent European guidance offered by the Pharmacovigilance Risk Assessment Committee suggests that although the over-the-counter doses (up to 1200 mg/day) of ibuprofen carry no increased risk of cardiovascular toxicity, the maximum licensed dose should be avoided in patients with existing cardiovascular disease because of similar levels of cardiovascular toxicity to COX-2 inhibitors (including the recently reclassified diclofenac).38 While this study was not designed, or powered, to evaluate cardiovascular safety, there was no notable difference in cardiovascular toxicity between ibuprofen and celecoxib. The present findings are largely consistent with the most recent, and largest, meta-analysis that demonstrated that while some COX-selective inhibitors, including supra-therapeutic doses of celecoxib (800 mg/day), are associated with an increased risk of cardiovascular toxicity versus placebo, celecoxib and ibuprofen pose similar levels of cardiovascular risk.39 Further information on cardiovascular safety, in a population of arthritis patients with higher cardiovascular risk, will be provided by the ongoing Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen Or Naproxen (PRECISION) trial.40
In summary, the results from this present study show that celecoxib is as effective as, and in some measures more effective than, high doses of ibuprofen for the treatment of symptoms associated with OA of the knee. Celecoxib improved measures of pain, OA symptoms and patient satisfaction, and was well tolerated compared with placebo and ibuprofen. These data may be useful for physicians when making treatment decisions in patients with OA.
Acknowledgements
Medical writing support was provided by Joshua Fink, PhD, of Engage Scientific Solutions and funded by Pfizer.
Declaration of conflicting interests
Ana Carla Gordo, Chris Walker, Beatriz Armada and Duo Zhou are full-time employees of Pfizer and hold stock options with Pfizer.
Funding
This work was supported by Pfizer Inc.
References
- 1.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011; 377: 2115–2126. [DOI] [PubMed] [Google Scholar]
- 2.Pereira D, Peleteiro B, Araújo J, et al. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011; 19: 1270–1285. [DOI] [PubMed] [Google Scholar]
- 3.Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015; 162: 46–54. [DOI] [PubMed] [Google Scholar]
- 4.Smelter E, Hochberg MC. New treatments for osteoarthritis. Curr Opin Rheumatol 2013; 25: 310–316. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 2008; 16: 137–162. [DOI] [PubMed] [Google Scholar]
- 6.Cutolo M, Berenbaum F, Hochberg M, et al. Commentary on recent therapeutic guidelines for osteoarthritis. Semin Arthritis Rheum 2014; 44: 611–617. [DOI] [PubMed] [Google Scholar]
- 7.McGettigan P, Henry D. Use of non-steroidal anti-inflammatory drugs that elevate cardiovascular risk: an examination of sales and essential medicines lists in low-, middle-, and high-income countries. PLoS Med 2013; 10: e1001388–e1001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez-Diaz S, Rodriguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med 2000; 160: 2093–2099. [DOI] [PubMed] [Google Scholar]
- 9.Allison MC, Howatson AG, Torrance CJ, et al. Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med 1992; 327: 749–754. [DOI] [PubMed] [Google Scholar]
- 10.Lanas A, Serrano P, Bajador E, et al. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology 1997; 112: 683–689. [DOI] [PubMed] [Google Scholar]
- 11.Henry D, Page J, Whyte I, et al. Consumption of non-steroidal anti-inflammatory drugs and the development of functional renal impairment in elderly subjects. Results of a case-control study. Br J Clin Pharmacol 1997; 44: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin MR, Yared A, Ray WA. Nonsteroidal antiinflammatory drugs and acute renal failure in elderly persons. Am J Epidemiol 2000; 151: 488–496. [DOI] [PubMed] [Google Scholar]
- 13.Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med 2000; 160: 777–784. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Rodriguez LA, Hernandez-Diaz S. Nonsteroidal antiinflammatory drugs as a trigger of clinical heart failure. Epidemiology 2003; 14: 240–246. [DOI] [PubMed] [Google Scholar]
- 15.Pfizer Inc. CELEBREX® US prescribing information. http://labeling.pfizer.com/ShowLabeling.aspx?id=793 (1998, last update May 2016, accessed 20 May 2016).
- 16.Bensen WG, Fiechtner JJ, McMillen JI, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999; 74: 1095–1105. [DOI] [PubMed] [Google Scholar]
- 17.Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002; 325: 619–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kivitz AJ, Moskowitz RW, Woods E, et al. Comparative efficacy and safety of celecoxib and naproxen in the treatment of osteoarthritis of the hip. J Int Med Res 2001; 29: 467–479. [DOI] [PubMed] [Google Scholar]
- 19.McKenna F, Borenstein D, Wendt H, et al. Celecoxib versus diclofenac in the management of osteoarthritis of the knee. Scand J Rheumatol 2001; 30: 11–18. [DOI] [PubMed] [Google Scholar]
- 20.Pincus T, Koch G, Lei H, et al. Patient preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann Rheum Dis 2004; 63: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh G, Fort JG, Goldstein JL, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I study. Am J Med 2006; 119: 255–266. [DOI] [PubMed] [Google Scholar]
- 22.Niculescu L, Li C, Huang J, et al. Pooled analysis of GI tolerability of 21 randomized controlled trials of celecoxib and nonselective NSAIDs. Curr Med Res Opin 2009; 25: 729–740. [DOI] [PubMed] [Google Scholar]
- 23.van Walsem A, Pandhi S, Nixon RM, et al. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta-analysis. Arthritis Res Ther 2015; 17: 66–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hochberg MC, Chang RW, Dwosh I, et al. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum 1992; 35: 498–502. [DOI] [PubMed] [Google Scholar]
- 25.Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum 1993; 36: 729–740. [DOI] [PubMed] [Google Scholar]
- 26.Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 2000; 27: 2635–2641. [PubMed] [Google Scholar]
- 27.Chen YF, Jobanputra P, Barton P, et al. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess 2008; 12: 1–278. [DOI] [PubMed] [Google Scholar]
- 28.Weaver M, Patrick DL, Markson LE, et al. Issues in the measurement of satisfaction with treatment. Am J Manag Care 1997; 3: 579–594. [PubMed] [Google Scholar]
- 29.Nett RB, Tiseo PJ, Almas M, et al. Patient satisfaction with eletriptan in the acute treatment of migraine in primary care. Int J Clin Pract 2007; 61: 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gimenez S, Armada B, Iturralde Iriso J, et al. Clinical management of patients with hip and knee osteoarthritis: patient satisfaction with treatment switch. Rheumatol Int 2014; 34: 823–832. [DOI] [PubMed] [Google Scholar]
- 31.Oteo-Alvaro A, Marin MT, Ruiz-Iban MA, et al. Treatment satisfaction after switching to another therapy in Spanish orthopaedic clinic outpatients with knee or hip osteoarthritis previously refractory to paracetamol. Clin Drug Investig 2012; 32: 685–695. [DOI] [PubMed] [Google Scholar]
- 32.Moore RA, Derry S, McQuay HJ, et al. What do we know about communicating risk? A brief review and suggestion for contextualising serious, but rare, risk, and the example of cox-2 selective and non-selective NSAIDs. Arthritis Res Ther 2008; 10: R20–R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaziano JM, Gibson CM. Potential for drug-drug interactions in patients taking analgesics for mild-to-moderate pain and low-dose aspirin for cardioprotection. Am J Cardiol 2006; 97: 23–29. [DOI] [PubMed] [Google Scholar]
- 34.Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 2001; 345: 1809–1817. [DOI] [PubMed] [Google Scholar]
- 35.Hudson M, Baron M, Rahme E, et al. Ibuprofen may abrogate the benefits of aspirin when used for secondary prevention of myocardial infarction. J Rheumatol 2005; 32: 1589–1593. [PubMed] [Google Scholar]
- 36.Wilner KD, Rushing M, Walden C, et al. Celecoxib does not affect the antiplatelet activity of aspirin in healthy volunteers. J Clin Pharmacol 2002; 42: 1027–1030. [PubMed] [Google Scholar]
- 37.Schmidt M, Hallas J, Friis S. Potential of prescription registries to capture individual-level use of aspirin and other nonsteroidal anti-inflammatory drugs in Denmark: trends in utilization 1999–2012. Clin Epidemiol 2014; 6: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.European Medicines Agency. PRAC recommends updating advice on use of high-dose ibuprofen. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/04/WC500185426.pdf (2015, accessed 22 May 2015).
- 39.Bhala N, Emberson J, et al. Coxib and traditional NSAID Trialists' (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013; 382: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker MC, Wang TH, Wisniewski L, et al. Rationale, design, and governance of Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen Or Naproxen (PRECISION), a cardiovascular end point trial of nonsteroidal antiinflammatory agents in patients with arthritis. Am Heart J 2009; 157: 606–612. [DOI] [PubMed] [Google Scholar]