Abstract
Objective
To investigate the expression and clinicopathological significance of the oestrogen receptor (ER) in non-small cell lung cancer (NSCLC).
Methods
ER expression was examined by immunohistochemical staining of tumour tissue and adjacent normal lung tissue from 67 NSCLC patients. The relationships between ER expression and clinicopathological features were analysed.
Results
A higher percentage of NSCLC tissues (28/67, 41.79%) than adjacent normal lung tissues (10/55, 18.18%) were ER positive. ER expression correlated with tumour differentiation but not with gender, age, tumour histological type, tumour size, lymph node metastasis, or clinical TNM staging. The median survival times of patients with ER-positive (n = 28) and -negative (n = 39) tumours were 36 and 27 months, respectively. The 1-, 3-, and 5-year survival rates were higher for patients with ER-positive tumours than for patients with ER-negative tumours.
Conclusion
ER expression could be a useful prognostic biomarker and therapeutic target for patients with NSCLC.
Keywords: Clinical, estrogen receptor, immunohistochemistry, non-small cell lung cancer, pathology
Introduction
According to the World Health Organization, lung cancer is the most common cancer worldwide and has the highest mortality. Although an integrated multidisciplinary approach to the treatment of lung cancer has improved patient outcomes, there have been no breakthrough treatments with long-term efficacy. Our understanding of the pathogenesis of lung cancer is still incomplete, and fundamental and effective treatment approaches are lacking. There is thus an urgent need to identify novel therapeutic strategies for lung cancer. Using tumour endocrinology and molecular biology techniques, many studies have shown that hormones play an important role in the initiation and development of tumours. Moreover, treatments targeting hormones and their receptors have achieved good outcomes in multiple cancers, such as breast, prostate, and thyroid cancers. Recent work showing that oestrogen receptor (ER) expression is linked to the incidence and progression of lung cancer has increased interest in this area of research. In the present study, we performed immunohistochemical (IHC) staining to investigate ER expression in tumour samples and adjacent normal lung tissue samples from patients with non-small cell lung cancer (NSCLC) and to determine its clinicopathological significance.
Patients and methods
Patients
A total of 67 patients with NSCLC who had undergone complete resection at Fujian Medical University Union Hospital between January 2001 and December 2005 and for whom there were complete clinical data were randomly selected for the study. Patients did not have chronic liver, kidney, or endocrine disorders; had not received preoperative radiotherapy or chemotherapy; and had not been treated with hormone drugs for 1 month before surgery. Ethical approval for the study was provided by the Ethics Committee of Fujian Medical University (Ref. No. 2013KY019). Written informed consent for the use of tissue samples in this study was provided by the patient or by a relative if the patient was too ill to consent.
IHC analysis of ER expression in lung tissues
Sixty-seven samples of NSCLC tissue and 55 samples of adjacent normal lung tissue (∼2 – 3 cm from the tumours) were obtained from 67 patients. Tissues were fixed with 10% formaldehyde, embedded in paraffin, and serially sectioned (2–3 µm). Sections were transferred to slides and incubated at 60℃ for 4 h. Sections were dewaxed and hydrated by conventional methods, and antigen retrieval was performed by incubation in citrate buffer under high pressure for 1.5 min. Sections were incubated with 3% hydrogen peroxide at room temperature for 10 min and then with normal goat serum at room temperature for 10 min. Sections were incubated with primary antibody (rabbit anti-human oestrogen receptor monoclonal antibody1) at room temperature for 60 min and then with secondary antibody (KIT-5910 MaxVision HRP-Polymer anti-rabbit IHC Kit; Fuzhou Maixin Biotechnology Development) at room temperature for 15 min. 3,3′-Diaminobenzidine tetrahydrochloride (DAB) substrate was added for 5 min, and the sections were counterstained with haematoxylin and differentiated with acid alcohol. Finally, samples were dehydrated through a graded series of alcohol, cleared in xylene, coverslipped, and observed under a microscope.
Scoring of ER expression
Slides were examined under a light microscope by two experienced specialist physicians who were blinded to the tissue origin. Positive staining appeared as brown or yellow granules in the nucleus. Five fields (×400) of each section were randomly selected and 200 cells/field were counted. Tissue samples with > 20% positively stained cells were considered positive for ER expression.
Statistical analyses
Data were analysed with SPSS software (version 19.0; IBM, Armonk, NY, USA). Group differences were analysed by χ2-test and Fisher’s exact test. Survival analysis was performed by the Kaplan–Meier method with log rank test. A P value of <0.05 was considered statistically significant.
Results
Patients
Patient clinicopathological characteristics are shown in Table 1. The 67 patients comprised 45 men and 22 women with a median age of 57 years (range 36 – 78). Forty-one patients were aged < 60 years. TNM staging was performed using the Union for International Cancer Control 1997 guidelines2: 8 patients had stage I disease, 24 had stage II, 32 had stage III, and 3 had stage IV. Forty-four patients had lymph node metastasis. According to the World Health Organization 1999 criteria3 for lung cancer histological types, 26 patients had squamous cell carcinoma, 38 had adenocarcinoma, and 3 had large cell carcinoma. Patients were followed for 3 – 97 months after surgery. The median survival time was 31 months (range 3 – 97 months) and the 1-, 3-, and 5-year survival rates were 91.05% (61/67), 40.30% (27/67), and 20.90% (14/67), respectively.
Table 1.
ER expression in NSCLC tissues and adjacent normal lung tissues.
| Group | n | ER expression |
χ2 | P | |
|---|---|---|---|---|---|
| Positive (n) | Positive rate (%) | ||||
| NSCLC | 67 | 28 | 41.79 | 7.851 | 0.005 |
| Controla | 55 | 10 | 18.18 | ||
Adjacent normal lung tissue.
ER, oestrogen receptor; NSCLC non-small cell lung cancer.
ER expression in NSCLC and normal lung tissues
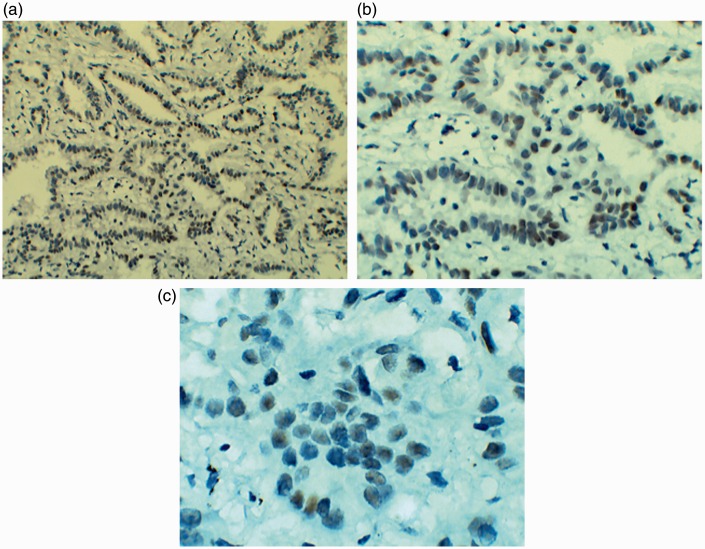
Positive ER staining was detected in the nucleus as yellow and brown granules (Figure 1). ER expression was observed in a significantly higher proportion of NSCLC tissues (28/67, 41.79%) than adjacent normal lung tissues (10/55, 18.18%; P < 0.01) (Table 1).
Figure 1.
ER expression in lung adenocarcinoma tissues: (a) 100×, (b) 200× and (c) 400×.
Relationship between ER expression and clinicopathological features
ER expression in the 67 NSCLC tissue samples was not related to the gender, age, histological types, tumour size, lymph node metastasis, or clinical TNM stage. However, ER expression was correlated with the degree of tumour differentiation (P = 0.002, Table 2).
Table 2.
Relationship between ER expression in NSCLC tissues and clinicopathological features.
| Clinicopathological feature | ER expression |
||||
|---|---|---|---|---|---|
| n | Positive (n) | Positive rate (%) | χ2 | P | |
| Histological type | |||||
| Squamous carcinoma | 26 | 12 | 46.15 | ||
| Adenocarcinoma | 38 | 15 | 39.47 | 0.834a | |
| Large cell lung cancer | 3 | 1 | 33.33 | ||
| Tumour differentiation | |||||
| None or poor | 17 | 2 | 11.77 | 12.267 | 0.002 |
| Moderate | 25 | 9 | 36.00 | ||
| High | 19 | 13 | 68.42 | ||
| TNM stage | |||||
| I | 8 | 4 | 50.00 | ||
| II | 24 | 11 | 45.83 | 0.881a | |
| III | 32 | 12 | 37.50 | ||
| IV | 3 | 1 | 33.33 | ||
| Tumour volume | |||||
| T1 | 8 | 4 | 50.00 | ||
| T2 | 39 | 19 | 48.72 | 0.205a | |
| T3–T4 | 20 | 5 | 25.00 | ||
| Lymph node metastasis | |||||
| + | 44 | 17 | 38.64 | 0.524 | 0.469 |
| − | 23 | 11 | 47.83 | ||
| Gender | |||||
| Males | 45 | 20 | 44.44 | 0.397 | 0.529 |
| Females | 22 | 8 | 36.36 | ||
| Age (years) | |||||
| ≥60 | 26 | 12 | 46.15 | 0.332 | 0.564 |
| <60 | 41 | 16 | 39.02 | ||
P value determined by Fisher’s exact test.
ER, oestrogen receptor; NSCLC non-small cell lung cancer.
Relationship between ER expression and patient survival
Of the 67 NSCLC patients, 28 had ER-positive and 39 had ER-negative tumours. Tumour ER expression was significantly related to the median survival time, which was 36 months for the ER-positive group and 27 months for the ER-negative group (P < 0.05, Figure 2 and Table 3). The 1-, 3-, and 5-year survival rates of the ER-positive group were also significantly higher than the rates of the ER-negative group (P < 0.05, Table 4).
Figure 2.
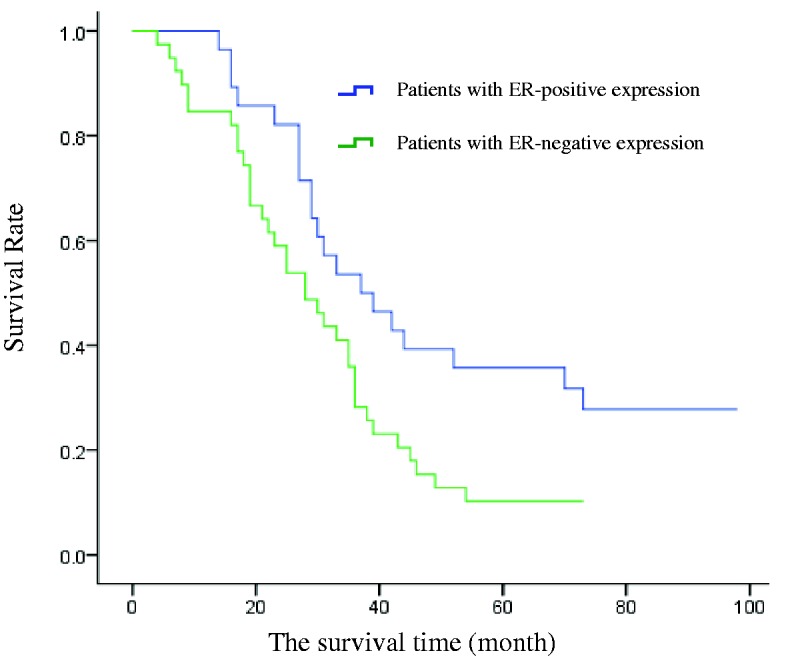
Kaplan–Meier survival curves for patients with ER-positive and ER-negative NSCLC.
Table 3.
Comparison of survival time of patients with ER-positive and ER-negative NSCLC.
| Group | n | Median survival time (months) | χ2 | P |
|---|---|---|---|---|
| ER + | 28 | 36 | 5.169 | 0.023 |
| ER− | 39 | 27 |
ER, oestrogen receptor; NSCLC non-small cell lung cancer.
Table 4.
Comparison of survival rates of patients with ER-positive and ER-negative NSCLC.
| Group | N | 1-year survival | 3-year survival | 5-year survival |
|---|---|---|---|---|
| ER + | 28 | 28 (100.00) | 15 (53.57) | 10 (35.71) |
| ER− | 39 | 33 (84.62) | 11 (28.21) | 4 (10.26) |
| χ2 | 4.416 | 6.391 | ||
| P | 0.036a | 0.036 | 0.011 |
Data are presented as n (%) of patients.
P value determined by Fisher’s exact test.
ER, oestrogen receptor; NSCLC non-small cell lung cancer.
Discussion
In 1955, Olson et al.4 first proposed that hormone-like factors were present in lung cancer tissues, and they raised the possibility that lung cancer patients might be treated with steroids. ER expression was first examined in tumour tissues by Chaudhuri et al.5 in 1982; they tested 25 samples of lung cancer and found that 4 of the 7 samples of adenocarcinoma were ER positive, but all 14 samples of squamous cell carcinoma and 4 samples of small cell lung cancer were ER negative. Further studies conducted by Beattie et al.6 in 1985 showed that the ER was expressed in the lungs of both healthy subjects and lung cancer patients but expression was higher in the cancer patients, suggesting that lung cancer is a hormone-dependent tumour. Moreover, Zhang et al.7, Yan et al.8, and Omoto Y et al.9 investigated ER expression in NSCLC and other lung cancer tissues using a method or IHC; they found ER-positive rates of 49.7%, 14.3%, and 67%, respectively. In the present study, 41.79% of NSCLC tumours were ER positive, which is a slightly lower percentage than the findings reported previously. This difference may be due to variations in the staining methods, such as the epitope retrieval procedure and monoclonal antibody clones, or in the criteria for positive expression.
To date, most studies have shown that tumour ER expression is not related to gender, age, TNM stage, tumour size, or lymph node metastasis. This is consistent with the findings of the present study. However, whether ER expression is related to the tumour histological type or differentiation status remains controversial. Some studies have shown that ER expression in lung adenocarcinoma is higher than in lung squamous cell carcinoma,7,9–11 but other studies have found no significant difference between them.12 Similarly, ER expression has been shown in some studies to positively correlate with the degree of lung cancer differentiation,6,10,13 whereas other studies find no such relationship.8 In the present study, there was no significant difference in ER expression in lung adenocarcinoma and squamous cell cancer, but a positive correlation with cancer differentiation was detected. This suggests that ER expression may to some extent reflect the degree of malignancy and thus might be useful for assessing the prognosis of lung cancer patients.
The relationship between ER expression and cancer prognosis is controversial. For example, Zhang et al.7 and Canver et al.14 reported that ER expression is unrelated to survival in lung cancer patients. However, Vargas et al.15 found that female patients with ER-negative NSCLC had significantly higher survival rates than patients with ER-positive tumours. Kawai et al.11 showed that NSCLC patients with ERα+/ERβ− tumours had a significantly worse prognosis than those with ERα−/ERβ+ tumours (P = 0.00007). The reasons for these discrepancies are unclear, but they are thought to be related to factors such as cancer staging, histological type, and inter-individual differences. In the current study, the postoperative 1-, 3- and 5-year survival rates of NSCLC patients were higher in patients with ER-positive tumours than in patients with ER-negative tumours. Moreover, the ER-positive group also had significantly longer survival times than the ER-negative group patients. These findings suggest that ER expression is closely related to prognosis and might function as a biological prognostic marker for NSCLC.
In summary, the results of the present study reveal an important role for the ER in the incidence and progression of NSCLC and suggest that ER expression might be a useful prognostic marker for NSCLC. These results also suggest that endocrine therapy targeting the ER could be a novel strategy for the prevention and treatment of NSCLC. Animal experiments and clinical studies have both confirmed that ER antagonists can inhibit cancer cell growth16–19 and reverse chemotherapeutic drug resistance.20 For example, combination therapy with an ER antagonist and cisplatin had good outcomes in chemotherapy-resistant lung cancer and did not induce resistance,21 suggesting the potential for endocrine therapy in lung cancer treatment. Further studies could examine the role of the ER in lung cancer by targeting its function and expression with monoclonal antibodies and RNA interference, respectively. Targeted drug development targeting ER signaling pathway may provide a new directions, such as the combination of ER with DNA and the recruitment of coactivator. We believe that these findings may lead to new advances in lung cancer prevention and treatment in the near future.
Declaration of conflicting interest
The Authors declare that there is no conflict of interest.
Funding
This study was supported by Key Specialty Discipline Construction Program of Fujian and the Nation, P.R. China, Fujian Provincial Department of Health’s Key Middle-aged and Young Talents Program (No. 2013-ZQN-JC-14) and Project of Natural Science Foundation of Fujian Province (No. 2015J01466).
References
- 1.Novel rabbit monoclonal antibody to estrogen receptor (Clone SP1): No heat pretreatment but effective on paraffin-embedded tissue. Huang Z., et al. (Submitted).
- 2.Naruke T. Results of resected lung cancer based on the new UICC TNM classification, 5th edition. Gan To Kagaku Ryoho 1997; 24: 2289–2295. [PubMed]
- 3.Travis WD, Brambilla E, Muller-Hermelink HK and Harris CC (eds) World Health Organization Classification of Tumours Pathology and Genetics of Tumours of the Lung, Pleura and Heart. Lyon: IARC Press, 2004.
- 4.Olson KB. A study of certain sex factors and hormone treatment in bronchogenic carcinoma. Am J Med Sci 1955; 230: 157–160. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri PK, Thoms PA, Walker MJ, et al. Steroid receptors in human lung cancer cytosols. Cancer Lett 1982; 16: 327–332. [DOI] [PubMed] [Google Scholar]
- 6.Beattie CW, Hansen NW, Thomas PA. Steroid receptors in human lung cancer. Cancer Res 1985; 45: 4206–4214. [PubMed] [Google Scholar]
- 7.Zhang H, Lu F, Deng L, et al. Expression and clinical significance of p53 protein, estrogen and progesterone receptors in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2000; 3: 276–279. [in Chinese, Abstract English]. [DOI] [PubMed] [Google Scholar]
- 8.Yan M, Chen X, Wang S, Li Y, et al. Expression of ER and AR in lung cancer. Zhongguo Fei Ai Za Zhi 2008; 11: 126–129. [DOI] [PubMed] [Google Scholar]
- 9.Omoto Y, Kobayashi Y, Nishida K, et al. Expression, function, and clinical implications of the estrogen receptor beta in human lung cancers. Biochem Biophys Res Commun 2001; 285: 340–347. [DOI] [PubMed] [Google Scholar]
- 10.Mollerup S, Jorgensen K, Berge G, et al. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer 2002; 37: 153–159. [DOI] [PubMed] [Google Scholar]
- 11.Kawai H, Ishii A, Washiya K, et al. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res 2005; 11: 5084–5089. [DOI] [PubMed] [Google Scholar]
- 12.Su JM, Hsu HK, Chang H, et al. Expression of estrogen and progesterone receptors in non- small-cell lung cancer: immunohistochemical study. Anticancer Res 1996; 16: 3803–3806. [PubMed] [Google Scholar]
- 13.Wu CT, Chang YL, Lee YC. Expression of the estrogen receptor beta in 37 surgically treated pulmonary sclerosing hemangiomas in comparison with non-small cell lung carcinomas. Hum Pathol 2005; 36: 1108–1112. [DOI] [PubMed] [Google Scholar]
- 14.Canver CC, Memoli VA, Vanderveer PL, et al. Sex hormone receptors in non-small-cell lung cancer in human beings. J Thorac Cardiovasc Surg 1994; 108: 153–157. [PubMed] [Google Scholar]
- 15.Vargas SO, Leslie KO, Vacek PM, et al. Estrogen-receptor-related protein p29 in primary nonsmall cell lung carcinoma: pathologic and prognostic correlations. Cancer 1998; 82: 1495–1500. [PubMed] [Google Scholar]
- 16.Zhang X, Li Q, Han Y, et al. Synergistic effect of toremifene and cisplatin on human lung cancer cell line A549. Zhonghua Zhong Liu Za Zhi 2002; 24: 537–539. [in Chinese, Abstract English]. [PubMed] [Google Scholar]
- 17.Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res 2002; 62: 2141–2150. [PubMed] [Google Scholar]
- 18.Hershberger PA, Vasquez AC, Kanterewicz B, et al. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res 2005; 65: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 19.Márquez-Garbán DC, Chen HW, Fishbein MC, et al. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 2007; 72: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez EA, Gandara DR, Edelman MJ, et al. Phase I trial of high-dose tamoxifen in combination with cisplatin in patients with lung cancer and other advanced malignancies. Cancer Invest 2003; 21: 1–6. [DOI] [PubMed] [Google Scholar]
- 21.Lara PN, Jr, Gandara DR, Longmate J, et al. Activity of high-dose toremifene plus cisplatin in platinum-treated non-small-cell lung cancer: a phase II California cancer consortium trial. Cancer Chemother Pharmacol 2001; 48: 22–28. [DOI] [PubMed] [Google Scholar]