Abstract
Objective
To evaluate the association between plasma lipoprotein-associated phospholipase A2 (Lp-PLA2; known to release inflammatory mediators that promote atherosclerosis) and coronary heart disease (CHD) in Chinese patients.
Methods
This observational, cross-sectional study included a patient cohort who were assessed by coronary angiography and divided into patients with coronary heart disease and patients with normal coronary angiography (controls). Data for several biochemical indicators were collected. Plasma Lp-PLA2 concentrations were measured by enzyme-linked immunosorbent assay. Univariate and multivariate logistic regression were used to analyse the association between Lp-PLA2 concentration and CHD.
Results
A total of 531 patients were included, comprising 391 with CHD and 140 with normal coronary angiography (controls). Plasma Lp-PLA2 concentration was significantly higher in patients with CHD versus controls (median, 251 µg/l versus 219 µg/l, respectively), and particularly among patients with acute myocardial infarction and stable angina pectoris (249 µg/l and 266 µg/l, respectively). Multivariate analysis showed that Lp-PLA2 ≥ 292 µg/l (upper quartile of the whole cohort) was independently associated with CHD (odds ratio 2.814, 95% confidence interval 1.519, 5.214).
Conclusion
Plasma Lp-PLA2 concentration was independently associated with CHD in Chinese patients.
Keywords: Lipoprotein-associated phospholipase A2, coronary heart disease, atherosclerosis
Introduction
Coronary heart disease (CHD) has a significant impact on human health, with a lifetime risk of 67% in both males and females aged >55 years.1 In 2008, CHD was responsible for 12.7% of all deaths worldwide.2
Atherosclerosis is the pathological basis of CHD,3,4 and the formation, development, and rupture of an atherosclerotic plaque involves inflammatory factors.5–8 Epidemiological studies of traditional markers of inflammation confirmed that inflammatory processes are associated with the formation of coronary atherosclerotic plaques and the occurrence of acute cardiovascular events related to CHD.9–12 Vulnerable plaques display a thin fibrous cap and a sizeable, necrotic, lipid-rich core containing a large amount of inflammatory and thrombotic mediators, while stable plaques display a thick fibrous cap.13 Plaque remodelling is an ongoing process that involves many factors.14,15
Lipoprotein-associated phospholipase A2 (Lp-PLA2), a phospholipase enzyme encoded by the phospholipase A2 group VII (PLA2G7) gene, is a mediator of inflammatory reactions.16 Accumulating evidence suggests a role of Lp-PLA2 in promoting atherosclerosis. Lp-PLA2 was initially recognized for its action in hydrolysing a platelet-activating factor, and was first named platelet-activating factor acetylhydrolase. Secreted by monocytes, macrophages, and T cells, Lp-PLA2 is a member of the phospholipase A2 (PLA2) superfamily and comprises 441 amino acid residues with a relative molecular mass of 45.4 kD.16 Following secretion, Lp-PLA2 enters the blood circulation and binds to lipoprotein particles, mainly low-density lipoproteins (LDL; approximately 80%) and high-density lipoproteins (HDL).17 Lp-PLA2 can generate pro-inflammatory molecules such as lyso-phosphatidylcholine and oxidized free fatty acids,16 and these inflammatory factors promote atherosclerosis through several pathways.18 High levels of Lp-PLA2 have been associated with an increased risk of atherosclerosis.19–21
Although a relationship between the PLA2G7 gene and CHD has been demonstrated in the Chinese population,22–24 the relationship between serum Lp-PLA2 levels and CHD remains poorly understood in this population. The aim of the present study was to evaluate the association of Lp-PLA2 with CHD and coronary plaque stability in a Chinese population, in an attempt to provide novel clues regarding atherosclerosis development and eventual future therapeutic approaches.
Patients and methods
Study population
The present retrospective, observational cohort study included consecutively enrolled patients who underwent diagnostic coronary angiography for evaluation of CHD at the No. 2 Department of Cardiology, Tianjin Chest Hospital, Tianjin, China between February 2012 and July 2012. Patients diagnosed with CHD and patients with normal coronary angiography (control group) were included.
Diagnosis of CHD was based on vascular stenosis ≥ 50% in the left main artery, left anterior descending artery, left circumflex artery, and/or right coronary artery. The following clinical indicators of CHD were considered: (1) ischemic symptoms; (2) new ischemic electrocardiogram (ECG) changes (new ST-T wave changes or new left bundle branch block); (3) ECG pathological Q waves; (4) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality; and (5) coronary angiography or autopsy confirmation of thrombus in the coronary artery.25
For subgroup analyses, patients with CHD were further divided into those with stable angina pectoris (defined as angina during effort without evidence of recent worsening, or angina at rest in the preceding 3 months), unstable angina pectoris (defined as the presence of angina at rest that occurred during the preceding 48 hours with significant transient ischemic ST-segment and/or T-wave changes without a significant increase in serum creatine kinase level [Braunwald’s class III-B]), or acute myocardial infarction (defined as the presence of >30 min continuous chest pain, ST-segment elevation >2.0 mm on ≥ 2 contiguous electrocardiographic leads, and serum creatine kinase level >150 IU/dl).
Diabetes was diagnosed according to diagnostic criteria of the China Guideline for Type 2 Diabetes (2010 edition):26 (1) patients with diabetes symptoms (including typical symptoms such as polydipsia, polyuria, and unexplained weight loss) and (a) random blood glucose (without considering the last meal time, any time-of-day) blood glucose >11.1 mmol/, or (b) fasting blood glucose (fasting state at least 8 h without calorie consumption) >7 mmol/l, or (c) glucose 2 h following glucose load test >11.1 mmol/l; and (2) in patients without symptoms of diabetes, a repeated examination to obtain a clear diagnosis.
Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, and/or the use of anti-hypertensive drugs.
Patients meeting any of the following criteria were excluded: (1) primary myocardiopathy, endocarditis, or severe valvular heart disease; (2) coronary arteritis or diseases that may cause non-atherosclerotic coronary artery stenosis; (3) any autoimmune disease; (4) acute or chronic infectious disease within 2 weeks prior to study participation; (5) severe liver or renal insufficiency such as aminotransferase levels greater than twice the upper limit of normal, or creatinine clearance < 50 ml/min; or (6) malignant tumour.
The study was approved by the ethics committee of Tianjin Chest Hospital, and written informed consent was obtained from all patients.
Evaluation of coronary angiography and coronary stenosis
Coronary angiography was performed within 24 h of symptom onset using a LAUNCHER® coronary catheter (Medtronic, Minneapolis, MN, USA) and the standard Judkins technique.27 All patients were routinely injected with 2 000 U of sodium heparin using a standard transradial or femoral artery approach. The visual method was used with an angiography catheter as a reference (6 F angiography catheter, 1 F = 0.33 cm) to estimate the reference vessel diameter and pathological segment diameter stenosis at the following positions: left anterior oblique, 30°; left anterior oblique 30° + head position, 30°; left anterior oblique, 45° + foot position, 45°; front right oblique, 30° + head position, 30°; right anterior oblique, 30° + foot position, 30°; and other body positions.
Data collection and blood biochemistry
Data regarding smoking, alcohol consumption, hypertension, and diabetes were collected from all patients. Height, weight, and body mass index (BMI) were measured. Venous blood (10 ml) was collected prior to coronary angiography. Blood samples were allowed to stand at room temperature for 30 min to allow clotting, then serum was immediately collected and analysed for the following parameters: serum total bilirubin, total cholesterol, triglycerides, LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), lipoprotein(a), apolipoprotein A1, apolipoprotein B, C-reactive protein (CRP), and fibrinogen were determined. Biochemistry analyses were performed using a MODULAR P-800 autoanalyser and associated reagents (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instructions.
Lp-PLA2 measurement
Prior to coronary angiography (and within 24 h of symptom onset), a 2-ml venous blood sample was drawn from each patient into a tube containing 1.8 mg/ml ethylenediaminetetra-acetic acid, and stored at 4℃. Within 24 h of collection, blood samples were centrifuged at 15 000 g for 10 min at 4℃, then plasma was collected and stored at –80℃. Plasma Lp-PLA2 concentration was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Tianjin Kangerke Bioscience, Tianjin, China) according to the manufacturer’s instructions. ELISA results were measured using an iMark™ Microplate Absorbance Reader (Bio-Rad, CA, USA).
Statistical analyses
Kolmogorov–Smirnov test was used to analyse data normality. Continuous variables are presented as mean ± SD or median (interquartile range), as appropriate. Independent Student’s t-tests were used to compare between-group means, and three or more groups were compared using one-way analysis of variance with Bonferroni adjustment for multiple comparisons. Categorical variables are presented as n (%) prevalence and between-group differences were analysed using χ2-test. Univariate and multivariate logistic regression analyses were performed to determine the factors independently associated with the presence of CHD. All analyses were performed using SPSS software, version 19.0 (IBM, Armonk, NY, USA). Two-sided P values < 0.05 were considered statistically significant.
Results
Patient characteristics
A total of 531 patients were included (Table 1): 391 with CHD (median age, 62 years) and 140 with normal coronary angiography results (controls; median age, 59 years). Compared with controls, patients with CHD were older, showed a higher prevalence of male patients, diabetes, hypertension, and smoking (all P < 0.01), showed higher levels of triglycerides, fibrinogen, and CRP (all P < 0.01), and showed lower HDL-C levels (P = 0.002).
Table 1.
Demographic and clinical characteristics of 531 Chinese patients who underwent coronary angiography and were diagnosed with coronary heart disease (CHD) or had normal coronary angiography (controls)
| Patient group |
|||
|---|---|---|---|
| Parameter | CHD (n = 391) | Control (n = 140) | Statistical significance |
| Age, years | 62 (30–83) | 59 (31–81) | P = 0.001 |
| Sex, male | 271 (69.3%) | 58 (41.4%) | P < 0.001 |
| Diabetes | 122 (31.2%) | 21 (15.0%) | P < 0.001 |
| Hypertension | 266 (68.0%) | 80 (57.1%) | P = 0.008 |
| Smoking | 220 (56.3%) | 57 (40.7%) | P < 0.001 |
| BMI, kg/m2 | 25.9 (18.4–48.6) | 25.4 (16.9–34.1) | NS |
| Total cholesterol, mmol/l | 4.58 (0.86–8.07) | 4.59 (0.92–10.03) | NS |
| Triglycerides, mmol/l | 1.52 (0.48–11.69) | 1.25 (0.44–16.31) | P = 0.001 |
| HDL-C, mmol/l | 1.25 (0.33–3.33) | 1.38 (0.79–2.93) | P = 0.002 |
| LDL-C, mmol/l | 2.55 (0.24–5.34) | 2.49 (0.72–4.81) | NS |
| Lipoprotein(a), g/l | 0.20 (0.02–0.92) | 0.20 (0.05–0.97) | NS |
| Fibrinogen, g/l | 3.45 (1.63–8.72) | 3.28 (0.96–7.68) | P = 0.003 |
| CRP, ng/l | 0.82 (0–52.07) | 0.36 (0.02–11.30) | P < 0.001 |
| Lp-PLA2, µg/l | 250.6 (8.8–762.9) | 219.2 (1.6–620.0) | P = 0.001 |
| ≥292 µg/la | 127 (32.5%) | 30 (21.4%) | P = 0.015 |
| Probucol treatment | 49 (12.5%) | ||
Data presented as median (range) or n (%) prevalence.
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; Lp-PLA2, lipoprotein-associated phospholipase A2.
292 µg/l represents the upper quartile of Lp-PLA2 concentration in all patients.
NS, no statistically significant between-group difference (P > 0.05; Student’s independent t-test).
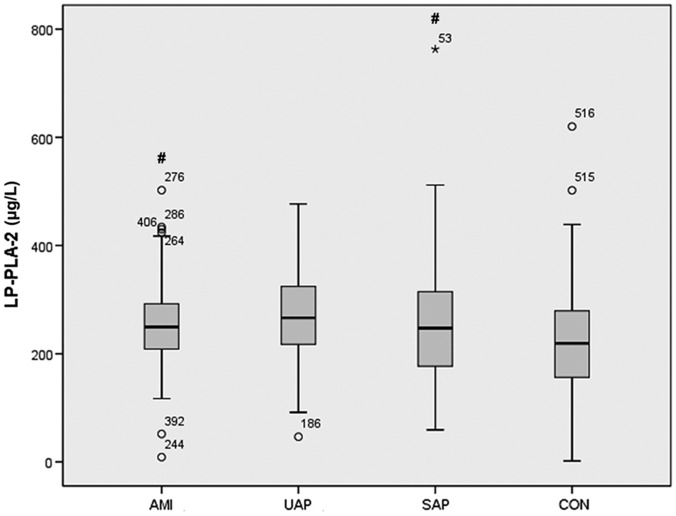
Plasma Lp-PLA2 levels were significantly higher in patients with CHD than in controls (median 250.6 versus 219.2 µg/l, respectively; P = 0.001). Among patients with CHD, subgroup analyses of patients with stable angina pectoris, unstable angina pectoris, or acute myocardial infarction revealed no statistically significant between-group differences in terms of age, sex, smoking, BMI, diabetes, or hypertension. A significantly higher proportion of patients with unstable angina pectoris had hypertension (P = 0.035), and patients with unstable angina pectoris had significantly higher total cholesterol and HDL-C levels, versus patients with stable angina pectoris or acute myocardial infarction (P < 0.05; Table 2). Patients with acute myocardial infarction had higher CRP levels versus patients with stable angina pectoris (P < 0.001; Table 2), and a higher proportion of patients with acute myocardial infarction and unstable angina pectoris were treated with probucol versus patients with stable angina pectoris (P = 0.001). In addition, compared with Lp-PLA2 concentrations in the control group (median, 219.2 µg/l), Lp-PLA2 concentrations in patients with acute myocardial infarction or stable angina pectoris were significantly higher (249.5 µg/l and 266.4 µg/l; P = 0.046 and P = 0.008, respectively; Figure 1).
Table 2.
Demographic and clinical characteristics of Chinese patients who underwent coronary angiography and were diagnosed with coronary heart disease, subdivided into patients with stable angina pectoris, unstable angina pectoris, or acute myocardial infarction
| Patient subgroup |
||||
|---|---|---|---|---|
| Parameter | Stable angina pectoris (n = 65) | Unstable angina pectoris (n = 254) | Acute myocardial infarction (n = 72) | Statistical significance |
| Age, years | 64 (42–82) | 62 (33–83) | 59 (30–83) | NS |
| Sex, male | 40 (61.5) | 171 (67.3) | 53 (73.6) | NS |
| Diabetes | 21 (32.3) | 77 (30.3) | 23 (31.9) | NS |
| Hypertension | 36 (55.4) | 180 (70.9) | 44 (61.1) | P = 0.035b |
| Smoking | 32 (49.2) | 138 (54.3) | 47 (65.3) | NS |
| BMI, kg/m2 | 27.2 (20.6–33.2) | 25.8 (18.4–33.5) | 26.0 (19.0–48.6) | NS |
| Total cholesterol, mmol/l | 4.27 (1.94–7.33) | 4.69 (1.92–8.07) | 4.43 (0.86–7.04) | P = 0.030b |
| Triglycerides, mmol/l | 1.36 (0.52–0.41) | 1.565 (0.48–11.69) | 1.55 (0.74–9.68) | NS |
| HDL-C, mmol/l | 1.13 (0.52–2.09) | 1.31 (0.33–3.33) | 1.13 (0.66–2.34) | P < 0.001b |
| LDL-C, mmol/l | 2.38 (0.75–4.07) | 2.55 (0.60–5.34) | 2.66 (0.24–4.53) | NS |
| Lipoprotein(a), g/l | 0.20 (0.05–0.74) | 0.2 (0.03–0.92) | 0.23 (0.02–0.79) | NS |
| Fibrinogen, g/l | 3.39 (2.44–4.97) | 3.46 (1.63–8.72) | 3.48 (2.18–7.51) | NS |
| CRP, ng/l | 0.4 (0.1–9.5) | 0.66 (0–26.5) | 1.6 (0.1–52.07) | P < 0.001c |
| Lp-PLA2, µg/l | 266.44 (46.43–476.83) | 250.58 (8.75–762.94) | 249.46 (8.75–502.28) | NS |
| ≥ 292 µg/la | 23 (35.4) | 82 (32.3) | 18 (25.0) | NS |
| Probucol treatment | 2 (3.1) | 30 (11.8) | 17 (23.6) | P = 0.001d |
Data presented as median (range) or n (%) prevalence.
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; Lp-PLA2, lipoprotein-associated phospholipase A2.
292 µg/l represents the upper quartile of Lp-PLA2 concentration in all patients.
Patients with unstable angina pectoris versus other groups; cpatients with acute myocardial infarction versus other groups;dpatients with unstable angina pectoris or acute myocardial infarction versus stable angina pectoris.
NS, no statistically significant between-group difference (P > 0.05; Student’s independent t-test, one-way analysis of variance or χ2-test, as appropriate).
Figure 1.
Box-whisker plots showing levels of Lp-PLA2 (µg/l) in Chinese patients who underwent coronary angiography and were diagnosed with coronary heart disease, divided into patients with acute myocardial infarction (AMI; n = 72), unstable angina pectoris (UAP; n = 254) or stable angina pectoris (SAP; n = 65), compared with a control group of patients with normal coronary angiography (CON; n = 140). #P < 0.05 versus controls. Central black horizontal line within the box, median; box extremities, upper and lower-quartiles; error bars, 1.5 times the interquartile range; ○, mild outlier; and *, extreme outlier
Lp-PLA2 concentration is independently associated with CHD
Risk factors for CHD were first assessed by univariate analyses of variables (Table 3). Variables with P values < 0.15 were then included in a multivariate regression model (Table 4). Among the cohort of 391 patients with CHD, following adjustment for age and sex, multiple regression analysis showed that age (odds ratio (OR) 1.06, 95% confidence interval (CI) 1.03, 1.09; P < 0.001), male sex (OR 4.98, 95% CI 2.76, 9.01; P < 0.001), diabetes (OR 3.59; 95% CI 1.89, 6.84; P < 0.001), CRP levels (OR 1.22, 95% CI 1.05, 1.43; P = 0.012), and Lp-PLA2 concentration ≥ 292 µg/l (upper quartile of the whole cohort; OR 2.81; 95% CI 1.52, 5.21; P = 0.001) were independently associated with CHD (Table 4).
Table 3.
Univariate regression analysis of factors associated with coronary heart disease (CHD) in 531 Chinese patients who underwent diagnostic coronary angiography for evaluation of CHD
| Characteristic | OR | 95% CI | Statistical significance |
|---|---|---|---|
| Age, years | 1.031 | 1.011, 1.052 | P = 0.002 |
| Sex, female | 0.291 | 0.194, 0.435 | P < 0.001 |
| Diabetes | 2.667 | 1.599, 4.45 | P < 0.001 |
| Hypertension | 1.721 | 1.152, 2.571 | P = 0.008 |
| Smoking | 2.029 | 1.366, 3.013 | P < 0.001 |
| BMI, kg/m2 | 1.045 | 0.982, 1.111 | NS |
| Total cholesterol, mmol/l | 1.026 | 0.856, 1.229 | NS |
| Triglycerides, mmol/l | 1.182 | 0.967, 1.444 | NS |
| HDL-C, mmol/l | 0.419 | 0.242, 0.726 | P = 0.002 |
| LDL-C, mmol/l | 1.079 | 0.864, 1.346 | NS |
| Lipoprotein(a), g/l | 0.683 | 0.235, 1.982 | NS |
| Fibrinogen, g/l | 1.41 | 1.096, 1.815 | P = 0.008 |
| CRP, ng/l | 1.236 | 1.084, 1.409 | P = 0.002 |
| Lp-PLA2 ≥ 292 µg/la | 1.752 | 1.109, 2.766 | P = 0.016 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; Lp-PLA2, lipoprotein-associated phospholipase A2.
292 µg/l represents the upper quartile of Lp-PLA2 concentration in all patients.
NS, no statistically significant association (P > 0.05).
Table 4.
Multivariate logistic regression analysis of risk factors for coronary heart disease (CHD) in 531 Chinese patients who underwent diagnostic coronary angiography for evaluation of CHD
| Adjusted |
Unadjusted |
|||||
|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | Statistical significance | OR | 95% CI | Statistical significance |
| Age, years | 1.056 | 1.025, 1.088 | P < 0.001 | |||
| Sex, female | 0.201 | 0.111, 0.362 | P < 0.001 | |||
| Diabetes | 3.592 | 1.887, 6.837 | P < 0.001 | 2.889 | 1.608, 5.191 | P < 0.001 |
| Hypertension | 1.029 | 0.598, 1.769 | NS | 1.407 | 0.861, 2.299 | NS |
| Smoking | 1.473 | 0.834, 2.601 | NS | 2.271 | 1.407, 3.667 | P = 0.001 |
| Triglycerides, mmol/l | 1.111 | 0.897, 1.376 | NS | 1.031 | 0.863, 1.233 | NS |
| HDL-C, mmol/l | 0.561 | 0.273, 1.154 | NS | 0.584 | 0.304, 1.124 | NS |
| Fibrinogen, g/l | 1.396 | 0.994, 1.962 | NS | 1.353 | 0.997, 1.836 | NS |
| CRP, ng/l | 1.224 | 1.046, 1.433 | P = 0.012 | 1.248 | 1.068, 1.458 | P = 0.005 |
| Lp-PLA2 ≥ 292 µg/la | 2.814 | 1.519, 5.214 | P = 0.001 | 2.391 | 1.349, 4.239 | P = 0.003 |
OR, odds ratio; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; CRP, C-reactive protein; Lp-PLA2, lipoprotein-associated phospholipase A2.
292 µg/l represents the upper quartile of Lp-PLA2 concentration in all patients.
NS, no statistically significant association (P > 0.05).
Discussion
In the present study, the association between CHD and Lp-PLA2, a novel inflammatory biomarker associated with atherosclerosis, was investigated. Lp-PLA2 concentration was found to be higher in patients with CHD versus control patients with normal coronary angiography. Multivariate analyses showed that Lp-PLA2 concentration was independently associated with CHD in the present population of Chinese patients undergoing coronary angiography.
The Lp-PLA2 phospholipase enzyme is an inflammatory marker associated with atherosclerosis, and is mainly produced by inflammatory cells.20,28–30 Lp-PLA2 concentration had been shown to alter considerably during the early phase of acute coronary syndrome;31 plasma Lp-PLA2 concentration decreased gradually in patients with acute coronary syndrome over the first 3 days following hospital admission and then remained stable. Long-term intensive therapy with statins decreases Lp-PLA2 concentration in addition to LDL-C levels, and change in Lp-PLA2 has been correlated with change in LDL-C.32–34 These studies suggest that Lp-PLA2 plays an active role in the pathogenesis of atherosclerosis and CHD.
Vulnerable plaques are associated with Lp-PLA2, and higher Lp-PLA2 concentration is associated with more severe atherosclerosis, higher cardiovascular risk, and more vulnerable plaques.35 By measuring activity of Lp-PLA2 and lysophosphatidylcholine in the left main coronary artery and coronary sinus,36 the role of Lp-PLA2 in local vascular inflammation and early atherosclerosis has been demonstrated; patients with CHD were found to have higher Lp-PLA2 activity and lysophosphatidylcholine levels than controls. Lp-PLA2 is likely to be an inflammatory biomarker in coronary arteries, and probably has an effect on atherosclerotic plaques and thus the development of CHD.
Activity of Lp-PLA2 has been associated with Framingham score.37 In addition to its role in inflammation, Lp-PLA2 might be directly or indirectly involved in plaque remodelling,33 but the exact role of Lp-PLA2 remains controversial. Specifically, two studies have indicated that Lp-PLA2 could be cardioprotective because it hydrolyses platelet-activating factor and oxidized phospholipids on LDL particles.38,39 In addition, a recent phase III trial using an Lp-PLA2 inhibitor reported no benefit in patients in terms of secondary prevention.40 A Japanese study showed that Lp-PLA2 activity was associated with carotid plaques, but a Mendelian randomization analysis suggested that Lp-PLA2 was not a causative factor for atherosclerosis.41 In the present study, and in accordance with other published studies,32–34,41,42 Lp-PLA2 concentration was independently associated with CHD.
The present study results may be limited by the following factors. The sample size was relatively small and all patients were from a single centre. In addition, the cross-sectional study design did not allow for determining a cause-and-effect relationship. The observational nature of the study and a number of uncontrolled factors could have influenced the results. Therapeutic drugs and natural supplements could also have influenced the associations being observed; unfortunately, data regarding patient medication and supplements were unavailable, due to the retrospective nature of the study. Finally, despite presenting with normal coronary angiography, the control patients had a medical condition that prompted the need for coronary angiography, which may have biased the results.
In conclusion, Lp-PLA2 concentration was independently associated with CHD in Chinese patients. Additional studies are necessary to validate these results across the spectrum of CHD.
Acknowledgments
The authors thank all the study collaborators, residents, and nurses of the Department of Cardiology, Tianjin Chest Hospital, and are especially grateful to all the study participants.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This study was supported by a grant from the Tianjin Health Bureau Scientific Research Foundation (Grant No. 2011kz63).
References
- 1.Leening MJ, Ferket BS, Steyerberg EW, et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ 2014; 349: g5992–g5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from world health organization and United Nations. Int J Cardiol 2013; 168: 934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature 2002; 420: 868–874. [DOI] [PubMed] [Google Scholar]
- 4.Hamm CW, Bassand JP, Agewall S, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 2999–3054. [DOI] [PubMed] [Google Scholar]
- 5.Lawton JS. Sex and gender differences in coronary artery disease. Semin Thorac Cardiovasc Surg 2011; 23: 126–130. [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines, and the American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation 2014; 130: 1749–1767. [DOI] [PubMed] [Google Scholar]
- 7.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American college of cardiology foundation/American heart association task force on practice guidelines, and the American college of physicians, American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol 2012; 60: e44–e164. [DOI] [PubMed] [Google Scholar]
- 8.Menees DS, Bates ER. Evaluation of patients with suspected coronary artery disease. Coron Artery Dis 2010; 21: 386–390. [DOI] [PubMed] [Google Scholar]
- 9.Insull W., Jr The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 2009; 122(1 Suppl): S3–S14. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011; 473: 317–325. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events (CARE) investigators. Circulation 1998; 98: 839–844. [DOI] [PubMed] [Google Scholar]
- 12.O’Donoghue M, Morrow DA, Sabatine MS, et al. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction) trial. Circulation 2006; 113: 1745–1752. [DOI] [PubMed] [Google Scholar]
- 13.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 2003; 108: 1664–1672. [DOI] [PubMed] [Google Scholar]
- 14.Dalager MG, Bøttcher M, Thygesen J, et al. Different plaque composition and progression in patients with stable and unstable coronary syndromes evaluated by cardiac CT. Biomed Res Int 2015; 2015: 401357–401357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo Y, Takumi T, Mathew V, et al. Plaque characteristics and arterial remodeling in coronary and peripheral arterial systems. Atherosclerosis 2012; 223: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epps KC, Wilensky RL. Lp-PLA2- a novel risk factor for high-risk coronary and carotid artery disease. J Intern Med 2011; 269: 94–106. [DOI] [PubMed] [Google Scholar]
- 17.Khakpour H, Frishman WH. Lipoprotein-associated phospholipase A2: an independent predictor of cardiovascular risk and a novel target for immunomodulation therapy. Cardiol Rev 2009; 17: 222–229. [DOI] [PubMed] [Google Scholar]
- 18.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol 2005; 25: 923–931. [DOI] [PubMed] [Google Scholar]
- 19.Packard CJ. Lipoprotein-associated phospholipase A2 as a biomarker of coronary heart disease and a therapeutic target. Curr Opin Cardiol 2009; 24: 358–363. [DOI] [PubMed] [Google Scholar]
- 20.Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the atherosclerosis risk in communities (ARIC) study. Circulation 2004; 109: 837–842. [DOI] [PubMed] [Google Scholar]
- 21.Koenig W, Khuseyinova N, Löwel H, et al. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation 2004; 110: 1903–1908. [DOI] [PubMed] [Google Scholar]
- 22.Jiang D, Zheng D, Wang L, et al. Elevated PLA2G7 gene promoter methylation as a gender-specific marker of aging increases the risk of coronary heart disease in females. PloS One 2013; 8: e59752–e59752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou L, Chen S, Yu H, et al. Associations of PLA2G7 gene polymorphisms with plasma lipoprotein-associated phospholipase A2 activity and coronary heart disease in a Chinese Han population: the Beijing atherosclerosis study. Hum Genet 2009; 125: 11–20. [DOI] [PubMed] [Google Scholar]
- 24.Hong M, Zhang M, Lu X. Nonsynonymous polymorphisms in PLA2G7 gene are associated with the risk of coronary heart disease in a southern Chinese population. Mamm Genome 2015; 26: 191–199. [DOI] [PubMed] [Google Scholar]
- 25.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J 2012; 33: 2551–2567. [DOI] [PubMed] [Google Scholar]
- 26.China Guideline for Type 2 Diabetes (2010 edition), http://www.diab.net.cn/uploadfile/ueditor/file/20160811/6360650768334000005174021.pdf (2010, accessed May 2011).
- 27.Osawa K, Miyoshi T, Koyama Y, et al. Additional diagnostic value of first-pass myocardial perfusion imaging without stress when combined with 64-row detector coronary CT angiography in patients with coronary artery disease. Heart 2014; 100: 1008–1015. [DOI] [PubMed] [Google Scholar]
- 28.Packard CJ, O’Reilly DS, Caslake MJ, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland coronary prevention study group. N Engl J Med 2000; 343: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 29.Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam study. Circulation 2005; 111: 570–575. [DOI] [PubMed] [Google Scholar]
- 30.Khuseyinova N, Koenig W. Predicting the risk of cardiovascular disease: where does lipoprotein-associated phospholipase A(2) fit in? Mol Diagn Ther 2007; 11: 203–217. [DOI] [PubMed] [Google Scholar]
- 31.Ostadal P, Vondrakova D, Kruger A, et al. Alteration in lipoprotein-associated phospholipase A2 levels during acute coronary syndrome and its relationship to standard biomarkers. Lipids Health Dis 2012; 11: 153–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stafforini DM, Tjoelker LW, McCormick SP, et al. Molecular basis of the interaction between plasma platelet-activating factor acetylhydrolase and low density lipoprotein. J Biol Chem 1999; 274: 7018–7024. [DOI] [PubMed] [Google Scholar]
- 33.Mannheim D, Herrmann J, Versari D, et al. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke 2008; 39: 1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolodgie FD, Burke AP, Skorija KS, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2006; 26: 2523–2529. [DOI] [PubMed] [Google Scholar]
- 35.Liu YS, Hu XB, Li HZ, et al. Association of lipoprotein-associated phospholipase A2 with characteristics of vulnerable coronary atherosclerotic plaques. Yonsei Med J 2011; 52: 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavi S, McConnell JP, Rihal CS, et al. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation 2007; 115: 2715–2721. [DOI] [PubMed] [Google Scholar]
- 37.Acevedo M, Varleta P, Kramer V, et al. Association of lipoprotein-associated phospholipase activity A2 with cardiovascular risk factors. Rev Med Chil 2013; 141: 1382–1388. [in Spanish, English abstract]. [DOI] [PubMed] [Google Scholar]
- 38.Steen DL, O'Donoghue ML. Lp-PLA2 Inhibitors for the reduction of cardiovascular events. Cardiol Ther 2013; 2: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bochkov VN, Kadl A, Huber J, et al. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature 2002; 419: 77–81. [DOI] [PubMed] [Google Scholar]
- 40.O’Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA 2014; 312: 1006–1015. [DOI] [PubMed] [Google Scholar]
- 41.Ueshima H, Kadowaki T, Hisamatsu T, et al. Lipoprotein-associated phospholipase A2 is related to risk of subclinical atherosclerosis but is not supported by Mendelian randomization analysis in a general Japanese population. Atherosclerosis 2016; 246: 141–147. [DOI] [PubMed] [Google Scholar]
- 42.Lind L, Simon T, Johansson L, et al. Circulating levels of secretory- and lipoprotein-associated phospholipase A2 activities: relation to atherosclerotic plaques and future all-cause mortality. Eur Heart J 2012; 33: 2946–2954. [DOI] [PubMed] [Google Scholar]