Abstract
Asthma is a heterogeneous disease characterized by symptoms of chronic inflammation and airway structural and functional changes. It affects about 300 million people worldwide and causes 250 000 deaths annually, but its symptoms can be greatly relieved by regular use of inhaled glucocorticoids (GCs). GCs exert their function through interacting with glucocorticoid receptors (GRs). Diosgenin is a naturally occurring steroidal saponin abundantly present in many medicinal plants, including Dioscorea nipponica, which shares a similar steroidal structure with GC. In this study, ovalbumin (OVA)-induced asthmatic mice and primary tracheal epithelial cells (TECs) were used as research models. ELISAs were applied to measure the secretion of TNF-α, IL-1β, and IL-6, while quantitative PCR and western blotting were applied to evaluate expression of GRs SLPI, TTP, GILZ, MKP-1, and NF-κB. Our data demonstrated that diosgenin suppressed the secretion of TNF-α, IL-1β, and IL-6 by enhancing the expression of GRs, SLPI, GILZ, and MKP-1, and inhibiting the expression of HSP70. These data provide some evidence on the molecular mechanism of diosgenin, which might facilitate its clinical applications.
Keywords: Diosgenin, glucocorticoid, glucocorticoid receptor, asthma
Introduction
Asthma is a heterogeneous disease with symptoms of chronic inflammation and airway structural and functional changes.1,2 It affects about 300 million people worldwide and causes 250 000 deaths annually, but its symptoms can be greatly relieved by regular use of inhaled glucocorticoids (GCs).3
GCs are important chemicals widely used in the therapy of inflammatory diseases. Furthermore, they are involved in many cellular activities such as cell survival, proliferation, and differentiation through a variety of signalling cascades in many cell types and tissues.4 GCs exert their effects through interacting with glucocorticoid receptors (GRs).5 After the interaction with GCs, GRs activate and translocate into the nucleus to function as transcription factors via three main mechanisms6: (1) directly binding to glucocorticoid response elements to promote transcription of anti-inflammatory genes including secretory leukocyte protease inhibitor (SLPI),7 mitogen-activated protein kinase phosphatase-1 (MKP-1),8 and glucocorticoid-induced leucine zipper (GILZ)9,10; (2) directly binding to cAMP response element binding protein-binding protein (CBP) to repress the functions of proinflammatory transcription factors such as nuclear factor- κB (NF-κB)11,12; (3) increasing the expression of tristetraprolin (TTP) that represses the expression of some inflammatory cytokines such tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 by reducing the stability of their mRNAs.13,14 Unactivated GRs reside predominantly in the cytoplasm together with a chaperone complex consisting of heat shock protein (Hsp) 70 and Hsp90. While Hsp90 protects GRs from aggregation and enhances their ligand affinity, HSP70 facilitates GR aggregation and reduces their ligand affinity.15
Diosgenin is a naturally occurring steroidal saponin abundantly present in many medicinal plants including Dioscorea nipponica. It was found to attenuate allergen-induced intestinal inflammation and treat asthma.16,17 However, the underling molecular mechanisms are still unclear. Considering that its structure is similar to GCs,18 we hypothesized that diosgenin might function through affecting GRs involved in anti-inflammatory pathways. Our results indicated that diosgenin suppresses the secretion of TNF-α, IL-1β, and IL-6 through enhancing the expression of GRs in ovalbumin (OVA)-induced asthmatic mice and primary airway epithelial cells. Our data also demonstrated that diosgenin enhanced the expression of GRs SLPI, TTP, GILZ, and MKP-1, while reducing the expression of NF-κB in primary airway epithelial cells.
Materials and methods
Reagents and antibodies
Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Rabbit anti-mouse GR, HSP70, SLPI, MKP-1, GILZ, NF-κb, TTP, and β-actin antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA. Goat anti-Rabbit IgG/horseradish peroxidase (HRP) was obtained from KPL, Inc (Gaithersburg, MD, USA). All primers were synthesized by Genepharma (Shanghai, China). BALB/c mice were provided by Slaccas (Shanghai, China). Enzyme-linked immunosorbent assay (ELISA) kits for mouse IL-6, IL-1β, and TNF-α were purchased from Abnova (Taipei, Taiwan).
Animals
Specific-pathogen-free female BALB/c mice were used in this study. All animal experiments were approved by Animal Care and Use Committee of Zhejiang Chinese Medicine University. Animals were divided into groups as follows: (1) normal control group; (2) OVA-induced asthma group; (3) asthma group with diosgenin treatment; (4) asthma group with prednisone acetate treatment; (5) asthma group with diosgenin and prednisone acetate treatment; (6) asthma group with RU486 treatment; (7) asthma group with RU486 plus diosgenin treatment; (8) asthma group with RU486 plus prednisone acetate treatment.
The asthmatic mouse model was established by OVA sensitization. On days 1 and 7, mice were injected intraperitoneally (i.p.) at 200 µl/mouse with 50 µg of alum-precipitated chicken egg OVA. Following the injections and beginning on day 15, mice were exposed to 5 mg/ml aerosolized OVA in a 0.85% NaCl solution for 30 min/day over 14 consecutive days. Mice in the normal control group were injected i.p. and exposed to the aerosolized 0.85% NaCl solution alone. Diosgenin (100 mg/kg/day)19–21 and 5 mg/kg/day prednisone acetate22 were intragastrically administered starting on day 15 over 14 consecutive days. RU486 (10 mg/kg) was injected i.p. starting at day 15 for 14 consecutive days.
Mice in each group were anaesthetized with 3 ml/kg of 3% pentobarbital at 24 h after the last treatment. Bronchoalveolar lavage fluid (BALF) from the left mouse lung was collected for ELISA analysis. The right mouse lung was collected for haematoxylin and eosin (HE) staining, quantitative PCR, and western blotting.
Isolation and culture of primary tracheal epithelial cells (TECs)
TECs were isolated from the tracheas of normal and OVA-induced asthmatic BALB/c mice, and analysed as passage 0 cells.23 The cells were maintained in DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 50 µg/ml streptomycin. Cells from asthmatic mice were treated with various drugs including: (1) diosgenin (0.5, 1, 5, and 10 µM; (2) prednisone acetate (10 nM, 50 nM and 100 nM); (3) 10 µM diosgenin plus 100 nM prednisone acetate; (4) 100 nM RU486; (5) 100 nM RU486 plus 10 µM diosgenin; (6) 100 nM RU486 plus 100 nM prednisone acetate. All treatments were applied for 1 h, and then culture media and cells were collected separately for different analyses. Culture media and cells from normal and asthmatic mice without any treatment were used as controls. Culture media were applied to ELISAs. Cells were analysed by quantitative PCR and western blotting.
HE staining
Right lung tissues of mice in each group were excised, fixed in 4% formaldehyde for 24 h, embedded in paraffin, sectioned at of 5 µm in thickness, and prepared for HE staining. Nuclei were stained with alum haematoxylin and cytoplasm was stained with eosin. Images of sections from each group were obtained under an inverted microscope for comparison of pathomorphological changes.
ELISAs
Concentrations of IL-6, IL-1β, and TNF-α in culture media and mouse BALF were measured by ELISAs. Cells were seeded in a 6-well culture plate. At 1 h after each treatment, culture supernatants were collected and stored at −80℃ before analysis. The mouse left lung was lavaged with PBS at 24 h after the last treatment, and BALF was stored at −80℃ before analysis. All ELISA steps were performed according to the manufacturer’s instructions.
Quantitative PCR
Total RNA was extracted from tissues and cells using TRIzol Reagent and reverse transcribed with a Superscript Reverse Transcription kit (Thermo Fisher). Quantitative real-time PCR was performed using SYBR Green Master Mix (Bio-rad) in a Bio-Rad C1000 Thermal Cycler. The following primers were used: GRα forward: 5′-ACACAGGCTTCAGGTATCTT-3′, GRα reverse: 5′-ACTGCTTCTGTTGCCAAG-3′; HSP70 forward: 5′-CTGACAAG AAGAAGGTGCTGG-3′, HSP70 reverse: 5′-AGCAGCCATCAAGAGTCTGTC-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward: 5′-CCAGGTGGTCTCCTCTGA-3′, GAPDH reverse: 5′-GCT GTAGCCAAATCGTTGT-3′. mRNA expression was normalized to GAPDH mRNA levels.
Western blotting
Animal tissues were snap frozen in liquid nitrogen and ground with a mortar. Proteins were extracted with protein lysis solution (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% SDS, 0.5% sodium deoxycholate, and 0.5% Triton X-100) on ice. Whole cell proteins were also extracted with protein lysis solution. Protease inhibitors were added to the lysates. Protein concentrations were measured with a bicinchoninic acid assay. Western blot analysis was performed using the following antibodies: rabbit anti-mouse GR, HSP70, SLPI, MKP-1, GILZ, NF-κb, TTP, and β-actin. Briefly, equal amounts of protein (50 ng) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. HRP-conjugated goat-anti-rabbit IgG was used as the secondary antibody. Bound antibodies were detected with an ECL chemiluminescent kit (Pierce) and exposed on x-ray film.
Statistical analysis
The Student’s t test was applied to analyse statistical differences between groups. p < 0.05 was considered to be statistically significant. Each experiment was repeated at least three times.
Results
Diosgenin reduces inflammatory cell infiltration and secretion of TNF-α, IL-1β, and IL-6 in asthmatic BABL/c mice
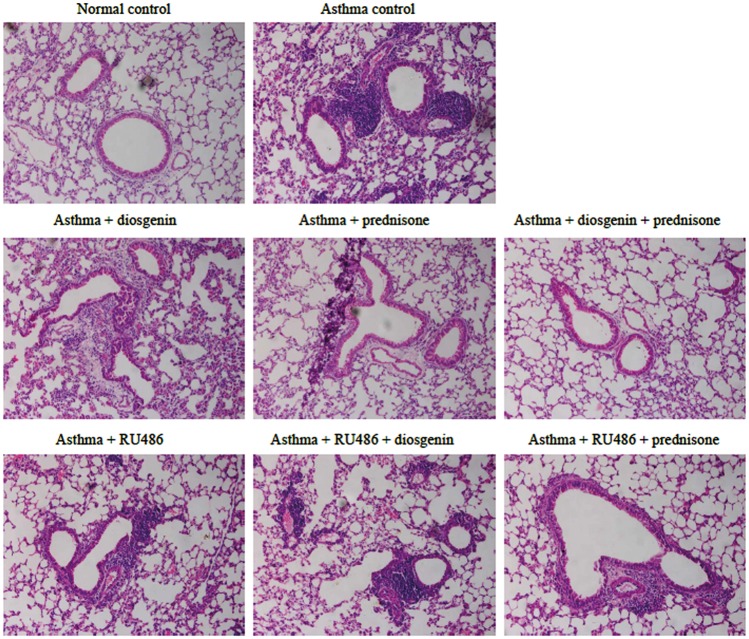
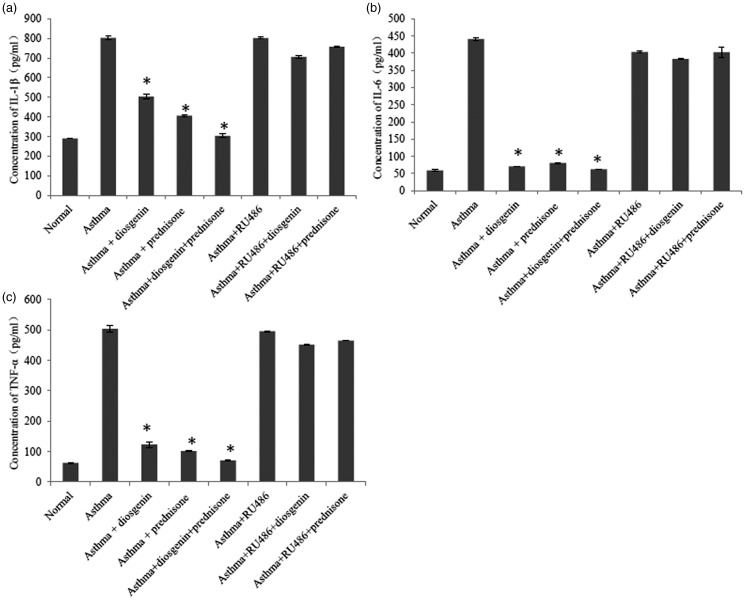
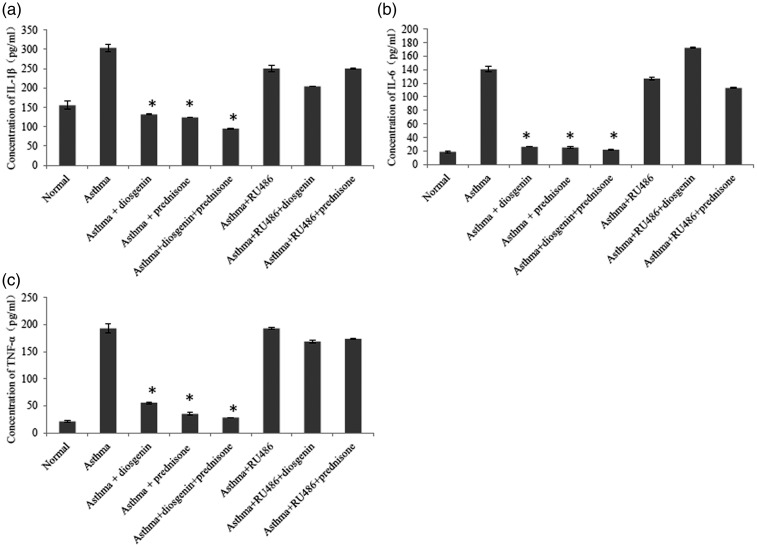
To evaluate the anti-inflammatory effects of diosgenin, we established asthmatic BABL/c mice by OVA induction. Subsequently, asthmatic mice were treated with 100 mg/kg/day diosgenin and/or 5 mg/kg/day prednisone acetate. Prednisone acetate is commonly used to control inflammation during clinical treatments through interactions with GRs. HE staining of the lung bronchus was applied to assess inflammatory cell infiltration under various treatments. In the asthma group, inflammatory cells had infiltrated around airways in clusters (Figure 1). In addition, the tracheal epithelium and smooth muscle layer were thickened compared with the control group. These symptoms indicated that the asthma models were established successfully. After treatments with diosgenin and/or prednisone acetate, inflammatory cell infiltration was hardly seen. Inflammatory cell infiltration was found again around airways after administration of RU486 that functions as a GR antagonist.24 Furthermore, we measured the concentrations of TNF-α, IL-1β,and IL-6 in BALB/c mouse BALF. The secretion of these cytokines was significantly higher in the asthma group than the normal group. As expected, the amounts of the cytokines were reduced obviously in asthmatic mouse BALF after treatment with 100 mg/kg/day diosgenin and/or 5 mg/kg/day prednisone acetate. Treatment with RU486 blocked the effects of diosgenin and prednisone acetate. As shown in Figure 2, cytokine secretion returned to levels similar to those the untreated asthmatic mouse group. Taken together, diosgenin reduces inflammatory cell infiltration and secretion of TNF-α, IL-1β, and IL-6 in asthmatic BALB/c mice, possibly through interactions with GRs because RU486 suppressed its anti-inflammatory effects.
Effects of diosgenin on expression of GRs and HSP70 in asthmatic BALB/c mice
Figure 1.
Comparisons of HE staining in the left lung bronchus (200×) and lung inflammatory cell infiltration in BALB/c mice. There were eight groups including normal control, asthma, asthma + diosgenin, asthma + prednisone, asthma + diosgenin + prednisone, asthma + RU486, asthma + RU486 + diosgenin, and asthma + RU486+ prednisone. Purple-blue-stained cells around the airway are infiltrated inflammatory cells. Their numbers indicate the severity of asthma.
Figure 2.
ELISA analyses of IL-1β (a), IL-6 (b), and TNF-α (c) in BALB/c mouse BALF of various groups. There were eight groups including normal control, asthma, asthma + diosgenin, asthma + prednisone, asthma + diosgenin + prednisone, asthma + RU486, asthma + RU486+ diosgenin, and asthma + RU486 + prednisone. Values are means ± SD (n = 3). * P < 0.05, significant differences between drug-treated groups and asthmatic groups.
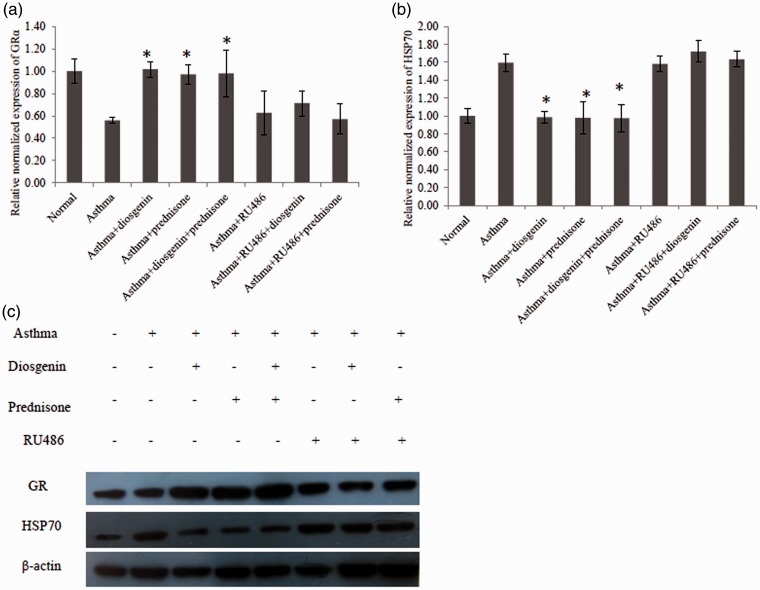
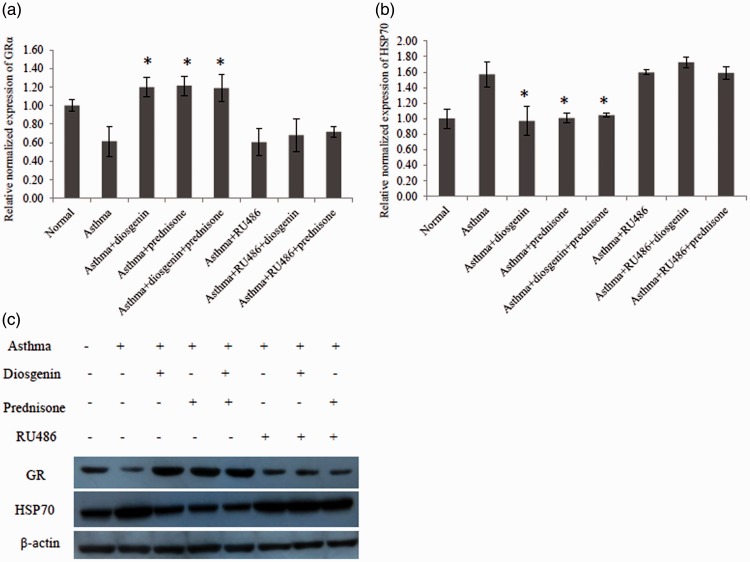
To investigate whether diosgenin functioned through interacting with GRs, quantitative PCR and western blotting were employed to measure the mRNA and protein levels of GRs and their chaperones in mouse lung tissues. As shown in Figure 3(a), the mRNA level of GRα was greatly reduced in lung tissues of asthmatic mice compared with normal mice. Interestingly, diosgenin and/or prednisone acetate treatment recovered the mRNA level of GRα. Furthermore, we found that diosgenin and/or prednisone acetate treatment reduced the mRNA level of HSP70 that blocks the activation of GRs (Figure 3(b)). As demonstrated in Figure 3(c), diosgenin and/or prednisone acetate treatment enhanced the protein level of GRs while attenuating the protein level of HSP70, which was consistent with our quantitative PCR results. Taken together, diosgenin exerts its anti-inflammatory effect by upregulating the expression of GRs and downregulating the expression of HSP70 in asthmatic BALB/c mice.
Diosgenin reduces the secretion of TNF-α, IL-1β, and IL-6 in primary TECs
Figure 3.
Quantitative PCR and western blot analyses of GRα and HSP70 expression levels in BALB/c mouse lung tissues of various groups. There were eight groups including normal control, asthma, asthma + diosgenin, asthma + prednisone, asthma+ diosgenin+ prednisone, asthma + RU486, asthma + RU486 + diosgenin, and asthma + RU486+ prednisone. (a) Quantitative PCR data of changes in GRα mRNA levels among groups. Values are means ± SD (n = 3). * P < 0.05, significant differences between drug-treated groups and asthmatic groups. (b) Quantitative PCR data of changes in HSP70 mRNA levels among groups. Values are means ± SD (n = 3). * P < 0.05,significant differences between drug-treated groups and asthmatic groups. (c) Western blot data of changes in GR and HSP70 protein levels among groups.
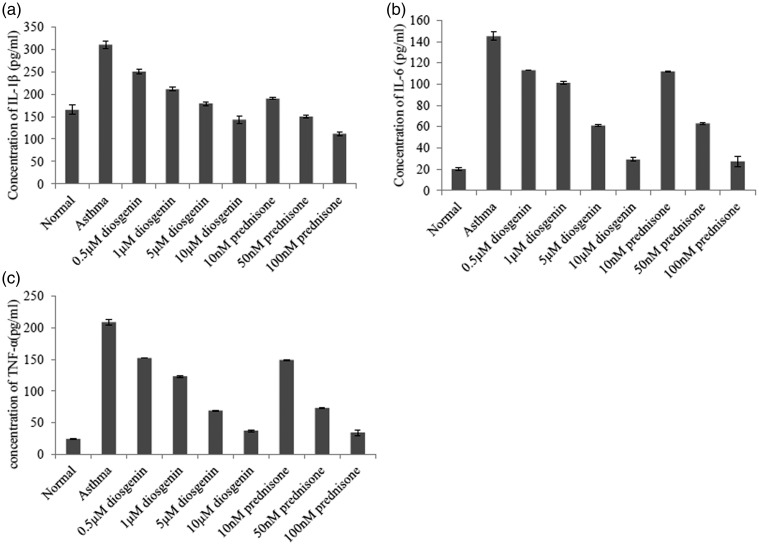
To further reveal the anti-inflammatory effects of diosgenin, we used primary TECs isolated from normal and asthmatic mice. TECs are the main cell type that forms the barrier against allergens, pollutants, and infectious agents.3 These cells also highly express GRs and perform a critical role in inflammation-related signalling.25 As shown in Figure 4, the concentrations of TNF-α, IL-1β, and IL-6 in the culture medium decreased with the addition of diosgenin and prednisone acetate in a dose-dependent manner. Thus, 10 µM diosgenin and 100 nM prednisone acetate were selected for the following experiments. As indicated in Figure 5, 10 µM diosgenin and/or 100 nM prednisone acetate treatments significantly reduced the secretion of TNF-α, IL-1β, and IL-6 from TECs of asthmatic mice, which was suppressed by RU486 treatment. Taken together, diosgenin reduces the secretion of TNF-α, IL-1β, and IL-6 from TECs of asthmatic mice.
Effects of diosgenin on the expression of GRs, HSP70, and downstream molecules in primary airway epithelial cells
Figure 4.
ELISA analyses of IL-1β (a), IL-6 (b), and TNF-α (c) in BALB/c mouse TECs of various groups. There were nine groups including normal control, asthma, asthma + diosgenin (0.5, 1, 5, and 10 µM), asthma + prednisone (10 M, 50 , and 100 nM). Values are means ± SD (n = 3). * P < 0.05, significant differences between drug-treated groups and asthmatic groups.
Figure 5.
ELISA analyses of IL-1β (a), IL-6 (b), and TNF-α (c) in BALB/c mouse TECs of various groups. There were eight groups incusing normal control, asthma, asthma + diosgenin, asthma + prednisone, asthma + diosgenin + prednisone, asthma + RU486, asthma + RU486+ diosgenin, and asthma + RU486 + prednisone. The concentrations of diosgenin, prednisone and RU486 were 10 µM, 100 nM and 100 nM, respectively. Values are means ± SD (n = 3). * P < 0.05, significant differences between drug-treated groups and asthmatic groups.
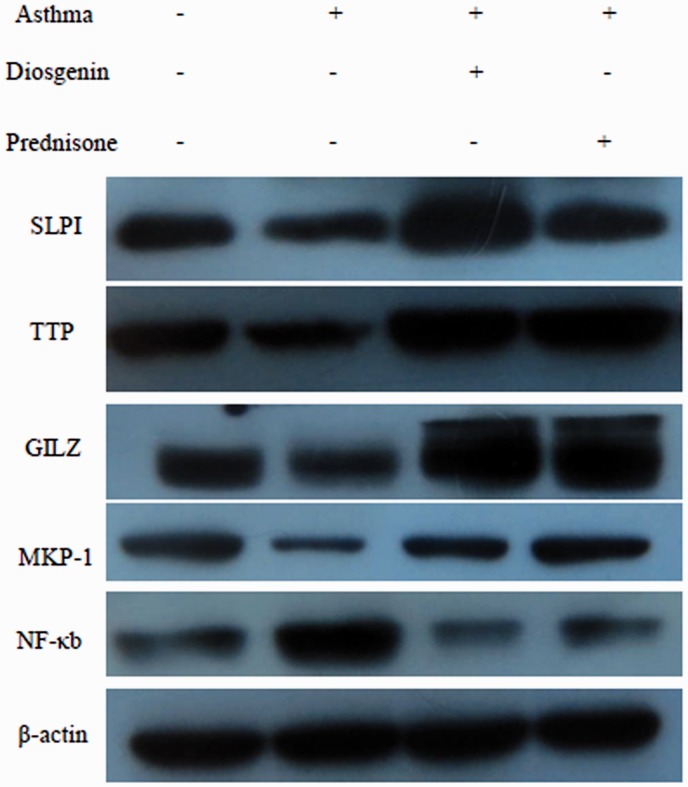
To confirm that diosgenin functions through affecting GRs and HSP70, quantitative PCR and western blotting were used to measure the changes in mRNA and protein levels of TECs after various treatments. As demonstrated in Figure 6, the changes in mRNA and protein levels of GR and HSP70 in TECs isolated from asthmatic mice were similar to those in asthma mouse lung tissues. Treatment with 10 µM diosgenin and/or 100 nM prednisone acetate significantly increased the expression of GR and decreased the expression of HSP70. These results were consistent with data obtained from mouse lung tissues. Furthermore, downstream signalling molecules, including SLPI, TTP, GILZ, MKP-1, and NF-κB, were measured by western blotting. As shown in Figure 7, the protein levels of SLPI, TTP, GILZ, and MKP-1 were reduced in TECs from asthmatic mice compared with those from normal mice. Treatment with 10 µM diosgenin or 100 nM prednisone increased the expression of SLPI, TTP, GILZ, and MKP-1. NF-κB expression was enhanced in TECs from asthmatic mice compared with those from normal mice, whereas 10 µM diosgenin or 100 nM prednisone treatment obviously decreased its protein level. Taken together, diosgenin not only enhances the expression of GRs and downstream molecules such as SLPI, TTP, GILZ and MKP-1 in primary airway epithelial cells, but also suppresses the expression of HSP70 and NF-κB.
Figure 6.
Quantitative PCR and western blot analyses of GRα and HSP70 expression levels in BALB/c mouse TECs of various groups. There were eight groups including normal control, asthma, asthma + diosgenin, asthma + prednisone, asthma + diosgenin + prednisone, asthma + RU486, asthma + RU486 + diosgenin, and asthma + RU486+ prednisone. The concentrations of diosgenin, prednisone and RU486 were 10 µM, 100 nM and 100 nM, respectively. (a) Quantitative PCR data of changes in GRα mRNA levels among groups. Values are means ± SD (n = 3). * P < 0.05, significant differences between drug-treated groups and asthmatic groups. (b) Quantitative PCR data of changes in HSP70 mRNA levels among groups. Values are means ± SD (n = 3). * P < 0.05, significant differences between drug-treated groups and asthma groups. (c) Western blot data of changes in GR and HSP70 protein levels among groups.
Figure 7.
Western blot analysis of changes in SLPI, TTP, GILZ, MKP-1 and NF-κB protein levels of TECs from normal and asthmatic mice treated with 10 µM diosgenin or 100 nM prednisone.
Discussion
Bronchial inflammation is the central feature of asthma and involves increased expression of multiple inflammatory genes.26 Diosgenin is effective for asthma treatment because it plays an important role in immune modulation and anti-inflammation. However, diosgenin-affected molecular signalling cascades are still uncertain. In this study, we found that diosgenin enhanced the expression of GRs and triggered GR-mediated anti-inflammatory signalling pathways. Consequently, the expression of SLPI, TTP, GILZ, MKP-1, and NF-κB was affected by diosgenin. Data were consistent with previously reported studies.
In this present study, OVA-induced asthmatic BALB/c mice were used for animal experiments and primary TEC isolation. Through in vivo and in vitro experiments, we found that diosgenin inhibited the secretion of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. The secretion of these cytokines is regulated by NF-κB and GRs. Because both diosgenin and GCs have a similar steroidal structure, diosgenin enhanced the expression and activation of GRs. Activated GRs are reported to bind directly to CBP to suppress the expression level of NF-κB,11,12 which leads to reduced secretion of proinflammatory cytokines. This is the first report showing that diosgenin functions through the GR/NF-κB signalling pathway.
We also found that diosgenin induced the expression of SLPI, GILZ, and MKP-1 in TECs through GR activation. These genes are up-regulated by corticosteroids such as dexamethasone7–9 that also shares a similar structure. GILZ inhibits T cell activation and the release of TNF-α and IL-1β. MKP-1 contributes to inhibition of IL-6 secretion. Thus, the inhibitive effects of diosgenin on proinflammatory cytokine secretion was partially due to up-regulation of SLPI, GILZ, and MKP-1. Diosgenin treatment also up-regulated the expression of TTP through activation of GRs, which facilitates down-regulation of TNF-α, IL-1β, and IL-6 mRNAs by targeting the AU-rich element.27 Interestingly, we found that the expression of HSP70 was suppressed by diosgenin treatment, which led to HSP90 binding to GRs. As a result, GR functions were enhanced and the symptoms of inflammation were relieved. More studies are needed to reveal specific mechanisms in the future.
Conclusion
Our data demonstrate that diosgenin suppresses the secretion of TNF-α, IL-1β, and IL-6 by enhancing the expression of GRs SLPI, GILZ, and MKP-1, and inhibiting the expression of HSP70. These data provide some evidence on the molecular mechanism of diosgenin, which might facilitate its clinical applications.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by National Natural Science Foundation of China (No. 81273679).
References
- 1.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy 2004; 59: 469–478. doi:10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST, Arshad HS, Roberts GC, et al. A new look at the pathogenesis of asthma. Clin Sci (Lond) 2009; 118: 439–450. doi:10.1042/CS20090474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olin JT, Wechsler ME. Asthma: pathogenesis and novel drugs for treatment. BMJ 2014; 349: g5517–g5517. doi:10.1136/bmj.g5517. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Q, Morand E, Yang YH. Development of novel treatment strategies for inflammatory diseases-similarities and divergence between glucocorticoids and GILZ. Front Pharmacol 2014; 5: 169–169. doi:10.3389/fphar.2014.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pujolsa L, Mullol J, Picado C. Glucocorticoid receptor in human respiratory epithelial cells. Neuroimmunomodulation 2009; 16: 290–299. doi:10.1159/000216187. [DOI] [PubMed] [Google Scholar]
- 6.Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther 2012; 134: 54–67. doi:10.1016/j.pharmthera.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Zani ML, Tanga A, Saidi A, et al. SLPI and trappin-2 as therapeutic agents to target airway serine proteases in inflammatory lung diseases: current and future directions. Biochem Soc Trans 2011; 39: 1441–1446. doi:10.1042/BST0391441. [DOI] [PubMed] [Google Scholar]
- 8.Quante T, Ng YC, Ramsay EE, et al. Corticosteroids reduce IL-6 in ASM cells via up-regulation of MKP-1. Am J Respir Cell Mol Biol 2008; 39: 208–217. doi:10.1165/rcmb.2007-0014OC. [DOI] [PubMed] [Google Scholar]
- 9.Bruscoli S, Donato V, Velardi E, et al. Glucocorticoid-induced leucine zipper (GILZ) and long GILZ inhibit myogenic differentiation and mediate anti-myogenic effects of glucocorticoids. J Biol Chem 2010; 285: 10385–10396. doi:10.1074/jbc.M109.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riccardi C, Bruscoli S, Ayroldi E, et al. GILZ, a glucocorticoid hormone induced gene, modulates T lymphocytes activation and death through interaction with NF-kB. Adv Exp Med Biol 2001; 495: 31–39. [DOI] [PubMed] [Google Scholar]
- 11.Imanifooladi AA, Yazdani S, Nourani MR. The role of nuclear factor-kappaB in inflammatory lung disease. Inflamm Allergy Drug Targets 2010; 9: 197–205. [DOI] [PubMed] [Google Scholar]
- 12.Surjit M, Ganti KP, Mukherji A, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 2011; 145: 224–241. doi:10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Kratochvill F, Machacek C, Vogl C, et al. Tristetraprolin-driven regulatory circuit controls quality and timing of mRNA decay in inflammation. Mol Syst Biol 2011; 7: 560–560. doi:10.1038/msb.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabhala P, Ammit AJ. Tristetraprolin and its role in regulation of airway inflammation. Mol Pharmacol 2015; 87: 629–638. doi:10.1124/mol.114.095984. [DOI] [PubMed] [Google Scholar]
- 15.Kirschke E, Goswami D, Southworth D, et al. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell 2014; 157: 1685–1697. doi:10.1016/j.cell.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CH, Ku CY, Jan TR. Diosgenin attenuates allergen-induced intestinal inflammation and IgE production in a murine model of food allergy. Planta Med 2009; 75: 1300–1305. doi:10.1055/s-0029-1185578. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Tian Y, Zhang X, et al. Anti-tumour and immunomodulating activities of diosgenin, a naturally occurring steroidal saponin. Nat Prod Res 2012; 26: 2243–2246. doi:10.1080/14786419.2011.648192. [DOI] [PubMed] [Google Scholar]
- 18.Lu D, Liu JP, Li HJ, et al. Phenanthrene derivatives from the stems and leaves of Dioscorea nipponica Makino. J Asian Nat Prod Res 2010; 12: 1–6. doi:10.1080/10286020903403626. [DOI] [PubMed] [Google Scholar]
- 19.Patil L, Balaraman R. Effect of green tea extract on Doxorubicin induced cardiovascular abnormalities: antioxidant action. Iran J Pharm Res 2011; 10: 89–96. [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CT, Wang ZH, Hsu CC, et al. In vivo protective effects of diosgenin against doxorubicin-induced cardiotoxicity. Nutrients 2015; 7: 4938–4954. doi:10.3390/nu7064938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z, Song C, Fu X, et al. High-dose diosgenin reduces bone loss in ovariectomized rats via attenuation of the RANKL/OPG ratio. Int J Mol Sci 2014; 15: 17130–17147. doi:10.3390/ijms150917130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol 2007; 120(5 Suppl): S94–S138. doi:10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 23.You Y, Richer EJ, Huang T, et al. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 2002; 283: L1315–L1321. doi:10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 24.Pastva A, Estell K, Schoeb TR, et al. RU486 blocks the anti-inflammatory effects of exercise in a murine model of allergen-induced pulmonary inflammation. Brain Behav Immun 2005; 19: 413–422. doi:10.1016/j.bbi.2005.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 2012; 18: 684–692. doi:10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 26.Chiappara G, Chanez P, Bruno A, et al. Variable p-CREB expression depicts different asthma phenotypes. Allergy 2007; 62: 787–794. doi:10.1111/j.1398-9995.2007.01417.x. [DOI] [PubMed] [Google Scholar]
- 27.Lai WS, Parker JS, Grissom SF, et al. Novel mRNA targets for tristetraprolin (TTP) identified by global analysis of stabilized transcripts in TTP-deficient fibroblasts. Mol Cell Biol 2006; 26: 9196–9208. doi:10.1128/MCB.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]