Abstract
Objective
To investigate the synergistic effects of cryptotanshinone (CPT) and doxorubicin (DOXO) on induction of apoptosis in human gastric cancer cells and the mechanisms.
Methods
Cell proliferation and apoptosis were detected using the CCK8 assay and AnnexinV/PI staining, respectively. Western blotting was used to determine the levels and phosphorylation of proteins encoded by STAT3-regulated genes and the cleaved forms of caspases and PARP.
Results
CPT significantly potentiated the antiproliferative effect of DOXO in gastric cancer cell lines. CPT combined with DOXO induced apoptosis and cleavage of caspases-3,-7,-9 as well as PARP. CPT or a STAT3 siRNA significantly suppressed constitutive and IL-6-induced phosphorylation of STAT3 Tyr705, decreasing the levels of proteins encoded by STAT3-target genes (Bcl-xL, Mcl-1, survivin, and XIAP).
Conclusions
CPT enhanced the anticancer activity of DOXO in gastric cancer cells via STAT3 inactivation and suppression STAT3-regulated antiapoptotic gene expression, indicating that DOXO combined with CPT may serve as effective therapy for gastric cancer.
Keywords: Human gastric cancer, cryptotanshinone, doxorubicin, STAT3
Introduction
Gastric cancer is one of the most common and lethal malignancies worldwide.1–3 Chemotherapy has been used to treat patients with unresectable advanced or metastatic gastric cancer; however, resistance to chemotherapy remains a major obstacle that reduces efficacy.4,5 Doxorubicin (DOXO) is an anthracycline antibiotic that is widely used to treat gastric cancer. During the early 1980s, a regimen comprising fluorouracil, doxorubicin, and mitomycin was accepted as the gold standard for patients with advanced gastric cancer.6 However, treatment with DOXO is associated with severe side effects such as cardiotoxicity and bone marrow suppression. Drug resistance and toxicity are common causes of treatment failure.7,8 Therefore, combination treatment strategies are required to enhance the efficiency of DOXO and reduce its toxicity.
Constitutive activation of signal transducer and activator of transcription 3 (STAT3) often correlates with poor prognosis and metastasis in gastric cancer, breast cancer, lung cancer, and myeloma.9–11 STAT3 exerts its antiapoptotic, prosurvival, and proliferative effects through transcriptional regulation of target genes in cancer cells, including those encoding antiapoptotic proteins such as Bcl-xL, Bcl-2, Mcl-1, survivin and XIAP as well as proteins that regulate proliferation, such as cyclin D1 and c-Myc. Accumulating evidence suggests that STAT3 confers resistance upon cells to chemotherapeutic agents such as DOXO, cisplatin, and docetaxel through its antiapoptotic effects.12 Moreover, inhibiting STAT3 activity increases the sensitivity to chemotherapeutic drugs of cancer cells lines in vitro and in xenograft models in vivo.13–16 Thus, this evidence indicates that abrogation of STAT3 activation renders tumor cells more susceptible to chemotherapeutic drugs.
Cryptotanshinone (CPT) Figure 1(a)), a diterpene quinone isolated from the root of Salvia miltiorrhiza, possesses anti-inflammatory, anticancer, antioxidative, and antiangiogenic activities.17–26 CPT exerts its anticancer activities via targeting the STAT3 signaling pathway,27 decreasing the expression levels of mammalian target of rapamycin and the phosphorylation of Rb,28 suppressing VEGFR-3 mediated ERK1/2 phosphorylation, and inhibiting signaling through small GTPase-mediated pathways.29 Moreover, CPT sensitizes cancer cells to anticancer agents, including TNF-α, TRAIL, Fas, and imatinib.30–33
Figure 1.
Cryptotanshinone (CPT) sensitizes human gastric cancer cells to doxorubicin (DOXO)-induced apoptosis. (a) Structure of CPT. (b) Human gastric cancer cells were treated with 15 μM CPT, 0.5 µg/ml DOXO, or in combination, for 24 h, and cell proliferation was evaluated using the CCK8 assay. (c) SGC7901 cells were treated with 15 μM CPT, 0.5 µg/ml DOXO, or in combination, for 24 h. Total proteins were analyzed using western blotting to detect the activation of PARP, caspase-3, caspase-7 and caspase-9, using ACTB as the internal reference. The gray ratio of each group was calculated, and the data are expressed as the mean ± S.E.M. of at least three independent experiments. **p < 0.01.
We demonstrated that the combination of CPT and As2O3 exerted a synergistic effect on the induction of apoptosis in multiple myeloma U266 cells via activation of the JNK signaling pathway.34 Furthermore, CPT induces cell cycle arrest and apoptosis in human leukemia cells by decreasing the expression levels of cyclin D1 and Bcl-2 as well as enhancing caspase activity.35 These results support the development of CPT as an inducer of apoptosis or chemosensitizer in combination cancer therapy.
Here, we demonstrate that CPT synergistically potentiated the antitumorigenic activity of DOXO against human gastric cancer cells via inactivation of STAT3, suggesting that the combination of CPT and DOXO may be useful for treating human gastric cancer.
Materials and methods
Antibodies and reagents
Penicillin, streptomycin, RPMI 1640, and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA, USA). Antibodies against STAT3, p-STAT3Tyr705, p-STAT3Ser727, p-JAK2, PARP, pro-caspase 3, survivin, Bcl-xL, Mcl-1 and β-actin (ACTB) were purchased from Cell Signaling Technology (Beverly, MA, USA). HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (CA, USA). CPT was purchased from Chengdu Must Biotechnology (Chengdu, China) and prepared as a 30 mmol/L stock solution in DMSO. DOXO hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA). A small interfering RNA (siRNA) targeting STAT3 were purchased from Shanghai GenePharma (Shanghai, China) (sense/anti-sense: 5′- CCCGGAAAUUUAACAUUCUTT-3′, 5′- AGAAUGUUAAAUUUCCGGGTT-3′). A scrambled siRNA (sense/anti-sense: 5′-UUCUCCGAACGUGUCACGUTT-3′, 5′- ACGUGACACGUUCGGAGAATT-3′) served as the negative control.
Cell culture
The human gastric cancer cell lines SGC-7901 and HGC-27 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), and the human gastric cancer cell line MKN45 was obtained from the American Type Culture Collection (Manassas, VA, USA). The three cell lines were maintained in RPMI 1640 medium supplemented with 10% FBS, 100 units/ml penicillin, and 100 µg/ml streptomycin. Cells were incubated at 37℃ in a humidified atmosphere containing 5% CO2.
Cell proliferation assay
The human gastric cancer cell lines SGC7901, MKN45, or HGC27 were seeded in triplicate in 96-well plates (5,000 cells per well) and incubated for 24 h in fresh complete medium containing test compounds or DMSO. Cell viability was determined using a cell counting kit-8 (CCK8, Dojindo, Japan), and absorbance at 450 nm was measured using an ELISA plate reader according to the manufacturer’s instructions.
Western blot analysis
Western blot analysis was performed as previously described.34 Briefly, gastric cancer cells were washed in phosphate-buffered saline, lysed for 30 min using RAPI extraction buffer (Beyotime Institute of Biotechnology, Jiangsu, China) containing freshly prepared protease inhibitors (Merck, Germany) and then centrifuged for 5 min at 14,000 rpm at 4℃. The protein concentration of the supernatant was determined using the BCA protein assay (Pierce, Rockford, IL, USA). Proteins (40 µg) were separated using polyacrylamide gel (10%) electrophoresis, electrophoretically transferred to a polyvinylidene fluoride (PVDF) membrane (BioRad, Richmond, CA, USA), and blocked for 2 h with TBS containing 5% dried milk and 0.1% Tween-20. Membranes were incubated with primary antibodies overnight at 4℃, washed, and then incubated with HRP-conjugated secondary antibodies. Immune complexes were detected using an ECL Plus kit (BioRad, Hercules, CA, USA).
Transient transfection and RNA interference
SGC7901 cells were transfected with the scrambled or STAT3 siRNA using Lipofectamine RNAiMAX (Invitrogen, CA, USA) following the manufacturer’s instructions. After 24 h, proteins were extracted, and the expression levels of p-STAT3, STAT3, Bcl-xL, survivin, Mcl-1, XIAP and ACTB were analyzed using western blotting. Simultaneously, cells were incubated with different concentrations of DOXO for 24 h, and apoptosis was detected using AnnexinV/propidium iodide (PI) and a FACScan flow cytometer (Becton Dickinson).
Apoptosis analysis
Apoptosis was measured using a BD Pharmingen™ FITC Annexin V Apoptosis Detection Kit and a FACScan flow cytometer (Becton Dickinson) according to the manufacturer’s. Briefly, DOXO-treated cells were counted, resuspended in binding buffer, and stained with 5 µg/mL FITC-conjugated Annexin V and PI for 15 min in the dark at room temperature. The FL-1 and FL-2 channels were used simultaneously to gate FITC-positive cells and PI-positive cells, respectively.
Statistical analysis
Data are expressed as the mean ± SD. We applied the Student t test using SPSS 16.0 software. The Student t test (one-tailed) was used to analyze the differences in drug-response data acquired from at least three independent experiments. P < 0.01 indicates a significant difference.
Results
CPT enhances DOXO-induced apoptosis in human gastric cancer cells
The gastric cancer cell lines SGC7901, MKN45, and HGC27 were treated with DOXO, CPT, or in combination for 24 h. As shown in Figure 1(b), cell viability was not significantly affected by DOXO or CPT (0.5 µg/ml or 15 μM, respectively) (Figure 1(b)), whereas viability was reduced dramatically by CPT combined with DOXO. Cleavage of caspase 3, caspase 7, caspase 9, and PARP was significantly increased by DOXO combined with CPT at subtoxic concentrations of 0.5 µg/ml and 15 μM, resepectively, in SGC7901 cells (p < 0.01, Figure 1(c)).
CPT inhibits the phosphorylation of STAT3 Tyr705 in gastric cancer cells
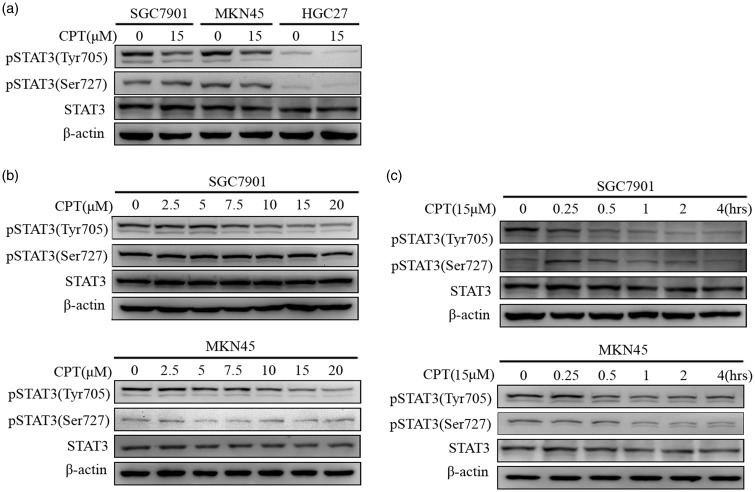
STAT3 contributes to the regulation of apoptosis in cancer cells. For example, CPT preserves the inhibitory effects of STAT3 on carcinomas such as prostate cancer as well as gliomas.46,47 Here, we sought to determine whether CPT suppressed STAT3 phosphorylation in human gastric cancer cells. As shown in Figure 2(a), CPT inhibited the phosphorylation of STAT3 Tyr705 but not STAT3 Ser727 in the three human gastric cancer cell lines without altering the expression levels of total STAT3. Further experiments showed that CPT inhibited phosphorylation of STAT3 Tyr705 in a concentration and time-dependent manner (Figure 2(b) and Figure 2(c)). Furthermore, decreased levels of phosphorylated STAT3 Tyr705 (pTyr705) were detected after 15–30 min incubation with 15 μM CPT.
Figure 2.
CPT inhibits the phosphorylation of STAT3 Tyr705 (pTyr705) in human gastric cancer cells. (a) The human gastric cancer cell lines SGC7901, MKN45, and HGC27 were incubated with 15 μM CPT for 4 h, and the levels of STAT3 pTyr705 and pSer727 were determined using western blotting with β-actin (ACTB) as an internal reference. (b) SGC7901 and MKN45 cells were treated with the indicated concentrations of CPT for 4 h. (c) SGC7901 or MKN45 cells were treated with 15 μM CPT for the indicated times. After treatment, western blotting analysis was used to determine the levels of STAT3 pTyr705 and STAT3 pSer727.
CPT inhibits interleukin 6 (IL6)-induced phosphorylation of STAT3 Tyr705 in gastric cancer cells
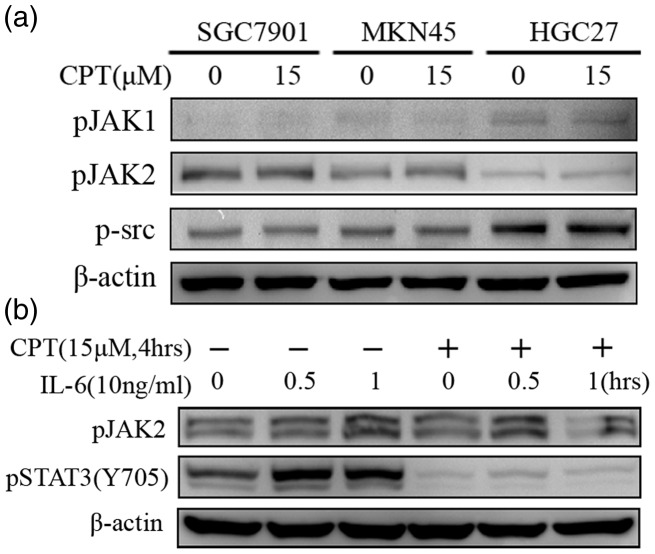
The phosphorylation of STAT3 on Tyr705 is mainly regulated by JAK kinases (JAKs) and SRC that act upstream in the STAT signaling pathway in response to cytokines and growth factors such as (IL-6), epidermal growth factor, oncostatin M, and leukemia inhibitory factor (LIF).36 Therefore, we determined the effects of CPT on the levels of phosphorylation of JAKs and SRC in the gastric cancer cell lines. As shown in Figure 3(a), phosphorylation of JAK1, JAK2, and SRC was not affected by 15 μM CPT.
Figure 3.
CPT inhibits IL6-induced phosphorylation of STAT3 (Y705) in human gastric cancer cells. (a) The human gastric cancer cell lines SGC7901, MKN45, or HGC27 were incubated with 15 μM CPT for 4 h, and the levels of phospho-JAK1, phospho-JAK2, and phospho-SRC were determined using western blotting. (b) HGC27 cells were incubated with 15 µM CPT for 4 h and then stimulated with 10 ng/ml IL-6 for 30 min or 60 min. The levels of STAT3 pTyr705 and phospho-JAK2 were analyzed using western blotting.
Next, we investigated the ability of CPT to inhibit IL-6-induced STAT3 activation in HGC27 cells, because they express low levels of STAT3 pTyr705. The levels of STAT3 pTyr705 in HGC27 cells markedly increased following stimulation with 10 ng/mL IL-6 for 30 min and 60 min, whereas this effect was abolished in the presence of 15 μM CPT (Figure 3(b)).
CPT inhibits STAT3-regulated gene expression in gastric cancer cells
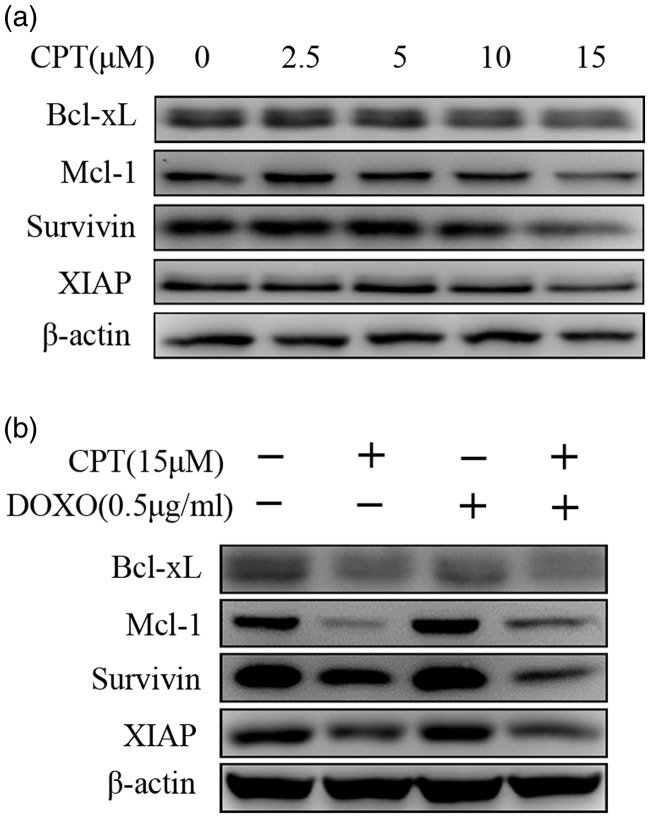
STAT3 regulates the expression of genes involved in its antiapoptotic functions, such as members of the Bcl-2 family and inhibitor of apoptosis proteins (IAPs). To investigate the ability of CPT treatment to downregulate STAT3 target genes, gastric cancer SGC7901 cells were treated with different concentrations of CPT. As shown in Figure 4(a), the levels of Bcl-xL, Mcl-1, survivin, and XIAP were decreased. Furthermore, CPT or CPT combined with DOXO decreased the levels of Bcl-xL, Mcl-1, survivin, and XIAP (Figure 4(b)).
Figure 4.
CPT decreases the expression of proteins encoded by STAT3-regulated genes in SGC7901 cells. (A) SGC7901 cells were treated with indicated concentrations of CPT for 24 h and then subjected to western blotting to detect Bcl-xL, Mcl-1, survivin, and XIAP. (B) SGC7901 cells were treated with 15 µM CPT, 0.5 µg/mL DOXO, or CPT combined with DOXO for 24 h. Cell lysates were subjected to western blotting using antibodies against Bcl-xL, Mcl-1, survivin, XIAP, and ACTB (internal control).
STAT3 siRNA sensitizes gastric cancer cells to DOXO-induced apoptosis
CPT potentiated DOXO-induced apoptosis in human gastric cancer cells (Figure 1) and reduced constitutive and IL-6-stimulated STAT3 activation (Figure 2). Therefore, we asked whether CPT increased the activity of DOXO via suppression of STAT3. To answer this question, we determined the effect of siRNA-mediated STAT3 knockdown on CPT- and DOXO-induced apoptosis in SGC7901 cells transfected with a STAT3-specific siRNA. The levels of p-STAT3 Tyr705, STAT3, Bcl-xL, Mcl-1, survivin, and XIAP were reduced (Figure 5(a)). Moreover, apoptosis was significantly increased in the presence of DOXO+STAT3 siRNA, DOXO + CPT, or DOXO + STAT3 siRNA + CPT, whereas there was no significant effect after exposure to DOXO, STAT3 siRNA, or CPT (Figure 5(b)).
Figure 5.
STAT3 siRNA decreases the expression of STAT3-regulated genes and enhances DOXO-induced apoptosis in SGC7901 cells. (a) SGC7901 cells were transfected with a STAT3-specific siRNA or a nonspecific scrambled siRNA for 24 h. The cells were collected and subjected to western blotting to detect STAT3 pTyr705, STAT3 pSer727, STAT3, Bcl-xL, Mcl-1, survivin, and XIAP. (b) After STAT3 knockdown for 24 h, SGC7901 cells were treated with the indicated concentrations of DOXO for an additional 24 h. Cells were then double-stained with AnnexinV/PI, and the apoptotic rates were determined using flow cytometry. (c) SGC7901 cells transfected for 24 h with the STAT3 siRNA were treated with 15 µM of CPT or 0.5 µg/mL of DOXO for another 24 h. Cells were then double-stained with AnnexinV/PI, and the apoptotic rates were determined using flow cytometry.
Discussion
Accumulating evidence demonstrates that CPT is a potent anticancer drug. For example, the anticancer activities of CPT include inhibition of cell proliferation and angiogenesis as well as induction of apoptosis.20–26 Furthermore, CPT acts cooperatively with numerous chemotherapeutic agents to inhibit the growth of carcinomas,37–38 CPT enhances As2O3-induced apoptosis of the multiple myeloma cell line U266 through activation of the JNK signaling pathway.34 Here, we demonstrate that CPT sensitized human gastric cancer cells to DOXO-induced apoptosis via suppression of the STAT3 signaling pathway.
DOXO is widely used to treat human gastric cancer, although toxicity and drug resistance limit its extensive application and efficacy.7–8 Therefore, methods are urgently required to increase the sensitivity of gastric cancer cells to DOXO. CPT potentiates the cytotoxicity of DOXO in colon and hepatocellular cancer cells that overexpress P-glycoprotein.39,40 However, to our knowledge, no report describes the effects of the combination of CPT and DOXO on gastric cancer. The aim of the current study therefore was to investigate whether CPT increased the anticancer effect of DOXO at low doses (i.e. safe and clinically achievable doses) on gastric cancer cells. Our data demonstrate that cell viability and apoptosis were not significantly affected by 15 µM of CPT or a low dose of DOXO (0.5 µg/ml), while their combination had a strong synergistic effect on reducing cell viability and inducing apoptosis (Figure 1). These results suggest that CPT enhanced DOXO-induced apoptosis in gastric cancer cells, indicating that the combination of DOXO and CPT may serve as potent novel strategy for treating gastric cancer.
STAT3 plays an important role in drug resistance and is closely associated with the progression and prognosis of patients with carcinomas.12 STAT3, which is activated through phosphorylation on Tyr705 by the upstream Janus kinases (JAKs), mediates the transcription and expression of a variety of downstream genes such as those that encode the antiapoptotic proteins XIAP, Bcl-2, Mcl-1 and survivin.41 STAT3 confers resistance to DOXO through its antiapoptotic effects on gastric cancer cells, and abrogation of STAT3 activation reverses the resistance to DOXO of cancer cell lines and tumor cells studied using in vivo xenograft models.12–15 Moreover, CPT directly inhibits the phosphorylation of STAT3 Tyr705.27
Here we show that CPT significantly inhibited constitutive and IL-6-induced phosphorylation of STAT3 Tyr705 in human gastric cancer cells without altering the phosphorylation of STAT3 Ser727 (pSer727), JAK1, and JAK2. This effect was accompanied by a reduction in the levels of Bcl-xL, Mcl-1, survivin, and XIAP, which act as downstream components of STAT3 signaling and are encoded by STAT3-target genes. These results are consistent with previous studies of other tumors.42–44 Therefore, we postulate that the sensitizing effect of CPT on DOXO-induced apoptosis may be caused by inhibition of STAT3 activity by CPT. The down-regulation of Bcl-xL, Mcl-1, survivin, and XIAP likely indicates an association between the inhibition of STAT3 activity and the capacity of CPT to enhance DOXO-induced apoptosis in gastric cancer cells.
As expected, STAT3 knockdown potentiated DOXO-induced apoptosis and decreased the expression levels of proteins encoded by STAT3-regulated genes in gastric cancer cells, in a manner similar to that mediated by CPT treatment. Furthermore, apoptosis was greatly increased in gastric cancer cells treated with DOXO + STAT3 siRNA, DOXO + CPT or DOXO + STAT3 siRNA + CPT, whereas there was no significant effect on cells exposed to DOXO, STAT3-siRNA, or CPT. Other studies show that knockdown of STAT3 using a specific siRNA or an inhibitor does not induce apoptosis, but greatly enhances the sensitivity of cancer cell lines to cisplatin or gefitinib by significantly reducing the expression levels of downstream signaling proteins encoded by STAT3-target genes as well as those of the antiapoptotic proteins Bcl-2, Bcl-xL, and survivin.45–47 These observations indicate that CPT enhances the sensitivity of gastric cancer cells to DOXO through the inhibition of STAT3 activity as well as the inhibition of the expression of the STAT3-target genes Bcl-xL, Mcl-1, survivin, and XIAP.
In summary, the results of our present study demonstrate for the first time that CPT increased the sensitivity of human gastric cancer cells to DOXO by suppressing STAT3 activity via a mechanism that involves the reduction of the levels of the antiapoptotic proteins Bcl-xL, Mcl-1, survivin, and XIAP. Thus, the combination of DOXO and CPT may serve as a novel strategy for treating patients with gastric carcinoma. Further in vivo studies are required to fully elucidate the mechanisms of the anticancer effects of this drug combination.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by grants from the Natural Science Foundation of Zhejiang Province, China (No. LY13H290014), the National Natural Science Foundation of China (No. 81473389, 81403199, 81503581), the Special Foundation of Science Technology Department of Zhejiang Province (No. 2012C13017-1), and the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20123322110001).
References
- 1.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol 2003; 56: 1–9. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 3.Pisani P, Parkin DM, Bray F, et al. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999; 83: 18–29. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 5.Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24: 2903–2909. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald JS, Schein PS, Woolley PV, et al. 5-Fluorouracil, doxorubicin, and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med 1980; 93: 533–536. [DOI] [PubMed] [Google Scholar]
- 7.Pramanik D, Campbell NR, Das S, et al. A composite polymer nanoparticle overcomes multidrug resistance and ameliorates doxorubicin-associated cardiomyopathy. Oncotarget 2012; 3: 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gammella E, Maccarinelli F, Buratti P, et al. The role of iron in anthracycline cardiotoxicity. Front Pharmacol 2014; 5: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong H, Du W, Wang JL, et al. Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer. J Mol Med (Berl) 2012; 90: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 10.Xie K, Huang S. Regulation of cancer metastasis by stress pathways. Clin Exp Metastasis 2003; 20: 31–43. [DOI] [PubMed] [Google Scholar]
- 11.Siewert JR, Böttcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German gastric cancer study. Ann Surg 1998; 228: 449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnston PA, Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv 2011; 11: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giraud AS, Menheniott TR, Judd LM. Targeting STAT3 in gastric cancer. Expert Opin Ther Targets 2012; 16: 889–901. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, Chen M, Shen Y, et al. Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett 2012; 315: 198–205. [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Chen M, Ding X, et al. Proton pump inhibitor selectively suppresses proliferation and restores the chemosensitivity of gastric cancer cells by inhibiting STAT3 signaling pathway. Int Immunopharmacol 2013; 17: 585–592. [DOI] [PubMed] [Google Scholar]
- 16.Yao X, Li G, Xu H, et al. Inhibition of the JAK-STAT3 signaling pathway by ganoderic acid A enhances chemosensitivity of HepG2 cells to cisplatin. Planta Med 2012; 78: 1740–1748. [DOI] [PubMed] [Google Scholar]
- 17.Ryu SY, Oak MH, Kim KM. Inhibition of mast cell degranulation by tanshinones from the roots of Salvia miltiorrhiza. Planta Med 1999; 65: 654–655. [DOI] [PubMed] [Google Scholar]
- 18.Kang BY, Chung SW, Kim SH, et al. Inhibition of interleukin-12 and interferon-gamma production in immune cells by tanshinones from Salvia miltiorrhiza. Immunopharmacology 2000; 49: 355–361. [DOI] [PubMed] [Google Scholar]
- 19.Jin HJ, Li CG. Tanshinone IIA and Cryptotanshinone prevent mitochondrial dysfunction in hypoxia-induced H9c2 cells: association to mitochondrial ROS, intracellular nitric oxide, and calcium levels. Evid Based Complement Alternat Med 2013; 2013: 610–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W, Liu L, Luo Y, et al. Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev Res (Phila) 2012; 5: 778–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park IJ, Kim MJ, Park OJ, et al. Cryptotanshinone induces ER stress-mediated apoptosis in HepG2 and MCF7 cells. Apoptosis 2012; 17: 248–257. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Wang H, Hong L, et al. Cryptotanshinone inhibits breast cancer cell growth by suppressing estrogen receptor signaling. Cancer Biol Ther 2015; 16: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hur JM, Shim JS, Jung HJ, et al. Cryptotanshinone but not tanshinone IIA inhibits angiogenesis in vitro. Exp Mol Med 2005; 37: 133–137. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Wang HJ, Xie W, et al. Cryptotanshinone inhibits lung tumorigenesis and induces apoptosis in cancer cells in vitro and in vivo. Mol Med Rep 2014; 9: 2447–2452. [DOI] [PubMed] [Google Scholar]
- 25.Park IJ, Yang WK, Nam SH, et al. Cryptotanshinone induces G1 cell cycle arrest and autophagic cell death by activating the AMP-activated protein kinase signal pathway in HepG2 hepatoma. Apoptosis 2014; 19: 615–628. [DOI] [PubMed] [Google Scholar]
- 26.Nizamutdinova IT, Lee GW, Son KH, et al. Tanshinone I effectively induces apoptosis in estrogen receptor-positive (MCF-7) and estrogen receptor-negative (MDA-MB-231) breast cancer cells. Int J Oncol 2008; 33: 485–491. [PubMed] [Google Scholar]
- 27.Shin DS, Kim HN, Shin KD, et al. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res 2009; 69: 193–202. [DOI] [PubMed] [Google Scholar]
- 28.Chen W, Luo Y, Liu L, et al. Cryptotanshinone inhibits cancer cell proliferation by suppressing Mammalian target of rapamycin-mediated cyclin D1 expression and Rb phosphorylation. Cancer Prev Res (Phila) 2010; 3: 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Y, Chen W, Zhou H, et al. Cryptotanshinone inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3/ERK and small GTPase pathways. Cancer Prev Res (Phila) 2011; 4: 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Jeong SJ, Kwon TR, et al. Cryptotanshinone enhances TNF-α-induced apoptosis in chronic myeloid leukemia KBM-5 cells. Apoptosis 2011; 16: 696–707. [DOI] [PubMed] [Google Scholar]
- 31.Tse AK, Chow KY, Cao HH, et al. The herbal compound cryptotanshinone restores sensitivity in cancer cells that are resistant to the tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem 2013; 288: 29923–29933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park IJ, Kim MJ, Park OJ, et al. Cryptotanshinone sensitizes DU145 prostate cancer cells to Fas (APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulation. Cancer Lett 2010; 298: 88–98. [DOI] [PubMed] [Google Scholar]
- 33.Ge Y, Yang B, Xu X, et al. Cryptotanshinone acts synergistically with imatinib to induce apoptosis of human chronic myeloid leukemia cells. Leuk Lymphoma 2015; 56: 730–738. [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Xu S, Zhang M, et al. Anticancer activity in human multiple myeloma U266 cells: synergy between cryptotanshinone and arsenic trioxide. Metallomics 2013; 5: 871–878. [DOI] [PubMed] [Google Scholar]
- 35.Ge Y, Cheng R, Zhou Y, et al. Cryptotanshinone induces cell cycle arrest and apoptosis of multidrug resistant human chronic myeloid leukemia cells by inhibiting the activity of eukaryotic initiation factor 4E. Mol Cell Biochem 2012; 368: 17–25. [DOI] [PubMed] [Google Scholar]
- 36.Heinrich PC, Behrmann I, Müller-Newen G, et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 1998; 334(Pt 2): 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang YF, Zhang M, Huang XL, et al. The combination of arsenic and cryptotanshinone induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in breast cancer cells. Metallomics 2015; 7: 165–173. [DOI] [PubMed] [Google Scholar]
- 38.Chang CC, Lai JS, Tsai CS, et al. Proapoptotic and TRAIL-sensitizing constituents isolated from Salvia militiorrhiza (Danshen). J Biosci Bioeng 2013; 116: 516–523. [DOI] [PubMed] [Google Scholar]
- 39.Lee WY, Cheung CC, Liu KW, et al. Cytotoxic effects of tanshinones from Salvia miltiorrhiza on doxorubicin-resistant human liver cancer cells. J Nat Prod 2010; 73: 854–859. [DOI] [PubMed] [Google Scholar]
- 40.Hu T, To KK, Wang L, et al. Reversal of P-glycoprotein (P-gp) mediated multidrug resistance in colon cancer cells by cryptotanshinone and dihydrotanshinone of Salvia miltiorrhiza. Phytomedicine 2014; 21: 1264–1272. [DOI] [PubMed] [Google Scholar]
- 41.Qi QR, Yang ZM. Regulation and function of signal transducer and activator of transcription 3. World J Biol Chem 2014; 5: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li W, Saud SM, Young MR, et al. Cryptotanshinone, a Stat3 inhibitor, suppresses colorectal cancer proliferation and growth in vitro. Mol Cell Biochem 2015; 406: 63–73. [DOI] [PubMed] [Google Scholar]
- 43.Yu HJ, Park C, Kim SJ, et al. Signal transducer and activators of transcription 3 regulates cryptotanshinone-induced apoptosis in human mucoepidermoid carcinoma cells. Pharmacogn Mag 2014; 10(Suppl 3): S622–S629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu L, Li C, Li D, et al. Cryptotanshinone inhibits human glioma cell proliferation by suppressing STAT3 signaling. Mol Cell Biochem 2013; 381: 273–282. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Zhang D, Chen X, et al. Nuclear PKM2 contributes to gefitinib resistance via upregulation of STAT3 activation in colorectal cancer. Sci Rep 2015; 5: 16082–16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji T, Gong D, Han Z, et al. Abrogation of constitutive Stat3 activity circumvents cisplatin resistant ovarian cancer. Cancer Lett 2013; 341: 231–239. [DOI] [PubMed] [Google Scholar]
- 47.LaStayo PC, Marcus RL, Dibble LE, et al. Eccentric exercise versus usual-care with older cancer survivors: the impact on muscle and mobility–an exploratory pilot study. BMC Geriatr 2011; 11: 5–5. [DOI] [PMC free article] [PubMed] [Google Scholar]