Abstract
Objective
To investigate the potential association between serum folate levels and colorectal adenoma (CRA) occurrence and recurrence.
Methods
This prospective study measured baseline serum folate levels in outpatients who were screened for CRA using colonoscopy. Participants were then randomly selected to produce one group with CRA and one without CRA. These two subgroups underwent further follow-up observations of colonoscopy to determine the occurrence of new and recurrent CRA.
Results
A total of 1310 participants were screened at baseline: 888 were healthy subjects without CRA; and 422 had CRA. Two subgroups were randomly selected (n = 200 per group) for follow-up. In the overall population, baseline serum folate levels were significantly lower in patients with CRA or advanced CRA (A-CRA) compared with healthy participants without CRA. Similar findings were shown for the follow-up study in terms of the association between CRA and A-CRA occurrence and recurrence and baseline serum folate levels. After controlling for confounders, increased serum folate was associated with a reduced risk of occurrence of CRA (odds ratio [OR] 0.993, 95% confidence interval [CI] 0.924, 1.066) and recurrence of CRA (OR 0.749, 95% CI 0.322, 1.742).
Conclusions
Higher serum folate levels may be protective against CRA and/or A-CRA.
Keywords: Folate levels, colorectal adenoma, advanced colorectal adenoma, confounders
Introduction
Colorectal cancer (CRC) is the third most frequently diagnosed malignancy and has the fifth highest death rate in China.1 Over 75% of CRC and 95% of adenocarcinomas may be derived from adenomatous polyps.2 CRC is thought to be the result of a multistep evolutionary process that involves a precursor lesion, the adenoma.3 Folate insufficiency has been suggested as a possible mechanism of CRA occurrence and CRC progression because optimal levels of folate and the other B vitamins are required for one-carbon metabolism.4 Randomized clinical trials and meta-analyses worldwide have provided supportive evidence of the protective effects of folate,5,6 and the critical serum folic acid level has been determined.7 Our research group demonstrated an antineoplastic effect of folate in an animal model8 and then performed a randomized clinical trial in healthy Chinese subjects aged over 50 years with 1 mg/day supplementation;9 both studies provided evidence supporting the use of folate. Nevertheless, other studies reported folic acid supplementation may not inhibit CRA occurrence10 or recurrence;11,12 therefore, the effect of folate on CRA remains uncertain.
Conflicting results regarding the efficacy of folate have mainly come from clinical trials that measured the effect of folate supplements rather than analysing serum levels of folate.10 Meanwhile, few of the studies considered advanced colorectal adenoma (A-CRA),13,14 which is most likely to progress to CRC. This present study investigated the potential association between serum folate levels and CRA and A-CRA occurrence and recurrence in a Chinese population.
Patients and methods
Study participants and study design
This study recruited consecutive patients who went to the outpatient clinic of the Division of Gastroenterology and Hepatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China with a digestive system complaint. These patients were recommended to undergo a colonoscopy between May 2006 and October 2010. All of them were without a history of regular folate intake, former resection or a diagnosis of CRA, polyposis syndrome, inflammatory bowel disease and other risk factors for CRA. The initial data from patients found to have an adenoma at baseline were matched for age, sex, residential location (urban or rural), educational history, and economic history to those without a baseline adenoma in order to reduce the effect of bias as much as possible on the demographic and clinical characteristics. All study participants underwent a complete colonoscopy as described below, during which all identified adenomatous polyps were removed and biopsied. Blood samples for serum folate were collected before the colonoscopy was undertaken. Colonoscopy and pathology results enabled a diagnosis of CRA or A-CRA.
A control study was carried out at baseline to observe the relationship between serum folate levels and the presence of CRA or A-CRA. Then, cohort study was carried out to determine any change of CRA and A-CRA occurrence and recurrence after follow-up in participants with a normal baseline colonoscopy and in patients with diagnosed CRA who were randomly selected using a computer-generated randomization schedule based on prospective observations. The selected study participants were then asked to complete a questionnaire concerning related confounders. Conditional logistic regression was used to eliminate confounders and to investigate factors that were independently associated with CRA. Follow-up started from the first colonoscopy or polypectomy.
The study protocol was approved by the Institutional Review Board of Shanghai Jiao Tong University School of Medicine, Renji Hospital, Shanghai, China. All 1310 study participants were provided written informed consent.
Follow-up procedures
All randomly selected participants were asked to complete the standard questionnaire by telephone, which collected the following data: body mass index (BMI); family history of colorectal diseases, including the previous three generations; drug use history, including nonsteroidal anti-inflammatory drugs (NSAIDs), proton pump inhibitors (PPIs), prokinetics, antibiotics, and microbial drugs; gastroenterology health history, including all diseases that may be related to CRA; history of systematic chronic disease, including cardiovascular diseases and endocrine diseases; and nutritional intake, such as vegetable intake, barbecue/grilled food intake, and pickled food intake. Follow-up colonoscopies and pathology reports were performed 12–18 months after the first colonoscopy in the selected subjects to determine the new occurrence or recurrence of CRA/A-CRA.
Diagnosis of CRA and A-CRA
All colonoscopies or polypectomies were performed by skilled endoscopists who were unaware of the clinical details, using a CF-H260 Series colonoscope (Olympus, Tokyo, Japan) with a rigorous withdrawal time of at least 7 min to allow for a full examination of the colorectal mucosa.15 Physicians provided the final CRA or A-CRA confirmation after reviewing the medical records, including the colonoscopy and pathology reports. Additional details of polyp characteristics, including locations, number, size, histological type and grade of dysplasia, were also extracted from the medical records. A-CRA was defined as a large lesion (≥1 cm) or one with advanced histology (tubulovillous or villous adenomas, high-grade dysplasia).16
Folic acid evaluation
Serum folate was evaluated at the beginning of the study in all participants before the colonoscopy was undertaken using a 5-ml peripheral venous blood sample collected after an overnight fast. Serum samples were stored at −20℃ immediately after they were collected. An automated chemiluminescence system (Access® Folate; Beckman Coulter, Brea, CA, USA) was used to determine baseline folic acid concentrations in serum. The minimum detectable concentration was 1.0 ng/ml. Intra- and inter assay coefficients of variation were both < 15%.
Statistical analyses
Baseline characteristics and serum folate levels were compared using bilateral Student’s t-test of mean ± SD for continuous variables, and by Mantel–Haenszel χ2-test for categorical variables of confounders. In Mantel–Haenszel χ2-test statistics, each group included at least 15 patients to minimize bias. All the other variables in the questionnaire were investigated as potential confounders if they were differently distributed between different groups (Mantel–Haenszel χ2-test, P < 0.1), by removing them one at a time from multivariate models containing the main exposure variables of interest. Folate and all other continuous variables that were categorized for the odds ratio (OR) calculations were calculated as split variable by cut-off values in logistic regression. The folate cut-off value was determined by receiver operating characteristic (ROC) curve analysis. Other variables were simply determined as low and high based on established values. The ORs and 95% confidence intervals (CIs) were then estimated for the remaining confounders using a conditional logistic regression model using the GENMOD procedure (SAS version 8.0; SAS Institute, Cary, NC, USA) to assess the association between serum folate levels and CRA and A-CRA without bias. A P-value ≤0.05 was considered statistically significant.
Results
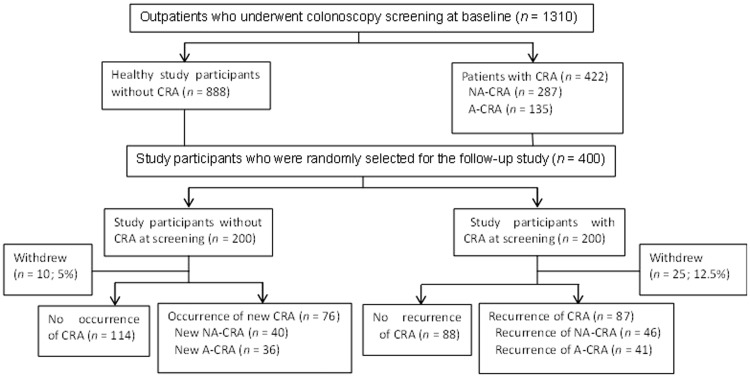
The overall study population consisted of 1310 eligible outpatients (males 606; females 704) aged from 13 to 88 years. A flow chart describing the study process is presented in Figure 1. Because of the follow-up time limitation, a total of 200 study participants (males 99; females 101) with a normal baseline colonoscopy (i.e. without CRA) and 200 study participants (males 100; females 100) with diagnosed CRA were randomly selected for follow-up.
Figure 1.
Flow chart illustrating the study process and how the various subgroups of study participants were analysed during the initial screening part of the study and during the follow-up period. CRA, colorectal adenoma; NA-CRA, non-advanced colorectal adenoma; A-CRA, advanced colorectal adenoma.
In the overall study population (n = 1310), patients with CRA or A-CRA were significantly older than healthy study participants without CRA (P = 0.005 and P = 0.020, respectively) (Table 1). The mean folate levels of patients with CRA or A-CRA were significantly lower compared with that of the healthy study participants without CRA (P = 0.029 and P = 0.002, respectively). When the two sexes were analysed separately, the folate levels remained lower in the CRA and A-CRA groups compared with the healthy study participants without CRA, but the differences did not reach statistical significance.
Table 1.
Association between the presence of colorectal adenomas (CRA) or advanced colorectal adenomas (A-CRA) and serum folate levels in the overall study population (n = 1310) that was screened at the start of the study for the presence of CRA.
| Group | n | Age, years | Statistical significancea | Serum folate, ng/ml | Statistical significancea | |
|---|---|---|---|---|---|---|
| Total | Without CRA | 888 | 56.78 ± 12.02 | 7.76 ± 4.25 | ||
| CRA | 422 | 58.78 ± 12.08 | P = 0.005 | 7.26 ± 3.67 | P = 0.029 | |
| A-CRA | 135 | 58.74 ± 12.62 | P = 0.020 | 6.95 ± 3.72 | P = 0.002 | |
| Males | Without CRA | 381 | 54.63 ± 13.55 | 7.11 ± 3.99 | ||
| CRA | 225 | 56.01 ± 12.86 | NS | 6.78 ± 3.95 | NS | |
| A-CRA | 83 | 58.23 ± 13.35 | NS | 6.43 ± 3.15 | NS | |
| Females | Without CRA | 507 | 57.37 ± 10.70 | 8.24 ± 4.57 | ||
| CRA | 197 | 60.01 ± 11.02 | 0.004 | 7.80 ± 3.70 | NS | |
| A-CRA | 52 | 59.56 ± 11.45 | NS | 7.78 ± 4.40 | NS | |
Data presented as mean ± SD.
Compared with healthy participants without CRA at baseline (Without CRA group); Student’s t-test.
CRA, colorectal adenoma; A-CRA, advanced colorectal adenoma; NS, no significant difference (P > 0.05).
A total of 200 study participants were randomly selected from each group (with and without CRA) for the follow-up procedure. The median follow-up time for the participants without CRA was 2.1 years compared with 1.8 years for patients with CRA. During the follow-up period, 10 participants without CRA withdrew from the study: five lost contact with the research team, three refused to complete the questionnaire, and two refused to be re-examined via a colonoscopy. Twenty-five patients with CRA withdrew during follow-up: four died from CRC, seven lost contact with the research team, six refused to complete the questionnaire, four developed other diseases that may impact CRA recurrence, and four refused to be re-examined via a colonoscopy.
After follow-up, healthy participants with a new occurrence of CRA (n = 76) or A-CRA (n = 36) were significantly older compared with healthy participants who remained free of CRA (n = 114) (P = 0.001 and P = 0.001, respectively) (Table 2). The mean folate levels of healthy participants with a new occurrence of CRA or A-CRA were significantly lower compared with that of the healthy study participants who remained free of CRA (P = 0.046 and P = 0.023, respectively).
Table 2.
Association between the new occurrence of colorectal adenomas (CRA) or advanced colorectal adenomas (A-CRA) and serum folate levels in the healthy study participants (n = 190) who were screened at the start of the study and found not to have CRA.
| Group | n | Age, years | Statistical significancea | Serum folate, ng/ml | Statistical significancea | |
|---|---|---|---|---|---|---|
| Total | Without CRA | 114 | 58.25 ± 11.76 | 7.89 ± 3.77 | ||
| CRA | 76 | 64.10 ± 8.99 | P = 0.001 | 6.75 ± 3.86 | P = 0.046 | |
| A-CRA | 36 | 64.28 ± 8.21 | P = 0.001 | 6.14 ± 3.77 | P = 0.023 | |
| Males | Without CRA | 53 | 56.45 ± 14.76 | 7.17 ± 3.16 | ||
| CRA | 36 | 64.64 ± 9.61 | P = 0.002 | 7.52 ± 4.10 | NS | |
| A-CRA | 14 | 67.00 ± 8.40 | P = 0.013 | 7.36 ± 4.49 | NS | |
| Females | Without CRA | 61 | 59.80 ± 8.13 | 8.51 ± 4.16 | ||
| CRA | 40 | 63.62 ± 8.48 | P = 0.025 | 6.07 ± 3.54 | P = 0.003 | |
| A-CRA | 22 | 62.54 ± 7.78 | NS | 5.37 ± 3.11 | P = 0.001 | |
Data presented as mean ± SD.
Compared with healthy participants without CRA at baseline who did not develop CRA during follow-up (Without CRA group); Student’s t-test.
CRA, colorectal adenoma; A-CRA, advanced colorectal adenoma; NS, no significant difference (P > 0.05).
After follow-up, patients with CRA (n = 87) at baseline who then experienced a CRA recurrence and those patients with CRA (n = 41) who experienced an A-CRA recurrence were older compared with patients with baseline CRA (n = 88) but without CRA recurrence (Table 3), but the differences did not reach statistical significance. The mean serum folate levels in patients with CRA (n = 87) at baseline who then experienced a CRA recurrence and in those patients with CRA (n = 41) who experienced an A-CRA recurrence were significantly lower compared with that of the patients with baseline CRA (n = 88) without adenoma recurrence (P = 0.002 and P = 0.002, respectively).
Table 3.
Association between the recurrence of colorectal adenomas (CRA) or advanced colorectal adenomas (A-CRA) and serum folate levels in the patients (n = 175) who were screened at the start of the study and found to have CRA.
| Group | n | Age, years | Statistical significancea | Serum folate, ng/ml | Statistical significancea | |
|---|---|---|---|---|---|---|
| Total | Without CRA recurrence | 88 | 60.81 ± 10.77 | 8.04 ± 4.27 | ||
| CRA recurrence | 87 | 62.21 ± 10.36 | NS | 6.29 ± 3.04 | P = 0.002 | |
| A-CRA recurrence | 41 | 64.00 ± 8.70 | NS | 6.01 ± 3.02 | P = 0.002 | |
| Males | Without CRA recurrence | 45 | 60.69 ± 10.65 | 7.55 ± 4.31 | ||
| CRA recurrence | 55 | 61.06 ± 11.59 | NS | 5.82 ± 2.81 | P = 0.017 | |
| A-CRA recurrence | 28 | 62.82 ± 9.08 | NS | 5.46 ± 2.27 | P = 0.021 | |
| Females | Without CRA recurrence | 43 | 60.93 ± 11.02 | 8.55 ± 4.22 | ||
| CRA recurrence | 32 | 64.19 ± 7.57 | NS | 7.09 ± 3.29 | NS | |
| A-CRA recurrence | 13 | 66.54 ± 7.53 | NS | 7.17 ± 4.08 | NS | |
Data presented as mean ± SD.
Compared with patients with CRA at baseline who did not develop CRA during follow-up (Without CRA recurrence group); Student’s t-test.
CRA, colorectal adenoma; A-CRA, advanced colorectal adenoma; NS, no significant difference (P > 0.05).
Colorectal adenoma is not only an age-related disease, but it is also influenced by many confounders. This present study performed a stratified analysis of confounders. The logistic regression analysis of categorical information was made using the folate cut-off value of 4.55 ng/ml, a BMI of 23.8 kg/m2, and age 50. The results of the logistic regression analysis (Table 4) showed that increased serum folate was associated with a reduced risk of CRA occurrence (OR 0.993; 95% CI 0.924, 1.066; P = 0.0371) and recurrence (OR 0.749; 95% CI 0.322, 1.742; P = 0.0028) during the follow-up period. A history of gastrointestinal drug use was associated with a lower risk of CRA occurrence (OR 0.242; 95% CI 0.134, 0.437; P < 0.0001) and recurrence (OR 0.453; 95% CI 0.211, 0.971; P = 0.0418) during the follow-up period. When BMI and age increase, or when there is a family history of colorectal diseases, the risk of CRA occurrence increased significantly (P < 0.05 for all comparisons). There was no significant association between alcohol and cigarette consumption and risk of CRA occurrence or recurrence.
Table 4.
Logistic regression analysis of the association between various clinicopathological characteristics and the risk of developing primary and recurrent colorectal adenomas (CRA).
| Estimate (standard error) | OR | 95% CI | Statistical significancea | |
|---|---|---|---|---|
| Primary CRA occurrence analyses | ||||
| Folate level | −0.0075 (0.0364) | 0.993 | 0.924, 1.066 | P = 0.0371 |
| Age | 0.3597 (0.4451) | 1.433 | 0.599, 3.428 | P = 0.0419 |
| BMI | 0.8314 (0.3006) | 2.297 | 1.274, 4.140 | P = 0.0057 |
| Family history of colorectal diseases | 0.9227 (0.4053) | 2.516 | 1.137, 5.5689 | P = 0.0228 |
| History of gastroenterology drug use | −1.4187 (0.3018) | 0.242 | 0.134, 0.437 | P < 0.0001 |
| CRA recurrence analyses | ||||
| Folate level | −0.2884 (0.4304) | 0.749 | 0.322, 1.742 | P = 0.0028 |
| History of gastroenterology drug use | −0.7917 (0.3889) | 0.453 | 0.211, 0.971 | P = 0.0418 |
The logistic regression analysis was divided into two parts: the upper half of the table presents the results of the analyses of the overall study population (n = 1310) as screened at baseline; and the lower half of the table presents the results of the analysis of the subgroup with CRA (n = 175) at baseline with or without recurrent CRA during the follow-up study.
OR, odds ratio; CI, confidence interval.
Discussion
There has been little research on the primary prevention of CRA, especially in China. The key characteristic of this present study was the analysis of baseline serum folate levels and the determination of their relationship with the new occurrence and recurrence of CRA and A-CRA. The results of the initial analysis of the overall study population (n = 1310) were similar to those demonstrated by the follow-up study, which showed that those who developed new or recurrent CRA or A-CRA during follow-up had lower baseline serum folate levels.
Serum folate is predominately N5-methyltetrahydrofolicacid(5-MTHF) monoglutamate,17 supplemental folate is pterolglutamic acid that is fully oxidized and bioavailable, and dietary folate is a mixture of polyglutamated folates and 5-MTHF in combination with certain food constituents that can influence folate bioavailability.18 Folate functions in one-carbon metabolism and in methylation reactions and nucleotide synthesis.19,20 An association between folate and p53 expression has been observed,21 which linked it to the metabolism of CRC. When folate concentrations in intestinal epithelial cells reduce significantly, the level of DNA methylation reduces and hypermethylation levels increase.22 Oncogenes are reactivated or abnormally expressed because of inadequate methylation, meanwhile tumour suppressor genes are inhibited because of excessive hypermethylation.23 This synergy of various factors results in tumorigenesis. The mechanisms that might explain how folic acid impacts on colorectal cancer in addition to DNA methylation and DNA repair have been hypothesized. For example, one suggestion is that folic acid could decrease epidermal growth factor receptor (EGFR) expression and tyrosine kinase activity to regulate cancer occurrence and development; which is supported by the finding that folic acid supplementation strongly inhibited the expression and activity of EGFR in colorectal cancer cell lines.24
Strategies to prevent CRC development and progression have received significant attention. For example, previous observational epidemiological studies, though not entirely consistent, tend to support the hypothesis that adequate intake of folate may reduce the risk of colorectal neoplasia.25–27 However, a randomized trial has shown negative results.28 The key point is that none of those studies attempted to evaluate baseline serum folate levels. Data on CRA and A-CRA recurrence from randomized trials of polypectomy patients are very limited. A case–control study29 and some meta-analyses15,30 found no statistically significant decrease or increase in recurrence of new adenomas in patients with a polypectomy history. Investigations about the relationship between baseline serum folate levels and A-CRA are rare.13 Therefore, this present study investigated the occurrence and recurrence of A-CRA, which is more significant than CRA in CRC progression.13 The findings of the present study are consistent with other randomized trials,31,32 which suggested that folic acid might be an effective chemopreventive agent for colorectal neoplasia, including CRA, A-CRA and CRC. The present findings are also consistent with the view that more CRA and A-CRA are detected in people over the age of 50 years because invasive disease of the colorectal mucosa increases with age.33 There were also negative results in many other studies, but different methods were used to extract and determine folate concentrations, which makes the results unreliable.34,35 Most articles agree that folate levels play a dual role in the progression of CRA:36,37 folate deficiency or folate levels above a specific high value might both be associated with an increased risk of CRA, which would lead to different results being generated in different studies. Negative results might be subject to statistical as well as ethnological bias,38 as demonstrated by the fact that in the US, Canada, some countries in Central and South America and in Europe people are getting much higher doses of folic acid because it has been added as a supplement to flour products. Some data in the literature are from people with this type of high exposure to folic acid,10 which might actually increase the risk of CRA rather than simply nullifying any protective effect. This present study restricted the participants’ diets as much as possible, which was helped by the fact that no folate-fortified foods are currently available for purchase in China.9 Variable dietary intake of folic acid might potentially explain why the results from both observational studies and clinical trials in Caucasian populations differ from those undertaken in China.
Alcohol is a known folate inhibitor that affects dietary methyl supply and therefore is associated with CRA and CRC.39–41 In the present study, some factors were independent risk factors of CRA occurrence (age, BMI and family history of colorectal diseases), and two were independent protective factors (folic acid and a history of gastroenterology drug use). Visceral fat obesity has been confirmed as an independent risk factor for CRA,42 which has been suggested to bring about its affects by increasing insulin-like growth factor 1, leading to the stimulation of colorectal epithelium proliferation.43 Meanwhile, approximately 30% of CRC is related to genetic factors.44 NSAIDs inhibit CRA progression through inflammatory factors.45,46 Compared with research describing western populations that has shown significant associations between CRA occurrence and alcohol and cigarette consumption,39–41,47 the current nonsignificant findings in a Chinese population might be the result of differences in alcohol and cigarette consumption compared with other populations. In the present study, the protective factors for CRA recurrence during follow-up were the same as for CRA occurrence (i.e. folate levels and a history of gastroenterology drug use), but the risk factors were not significant (i.e. age, BMI, and a family history of colorectal diseases). These current results suggest that once patients have developed CRA, only serum folate levels and gastroenterology drug use will impact on their clinical prognosis, but the lack of significance of other factors may be due to the lack of power resulting from the small number of incident cases in each analysis. The difference might be hypothesized to be due to the colorectal mucosa with CRA acquiring a level of clinical susceptibility to further CRA formation that is no longer affected by age and BMI. It has been suggested that CRA occurrence is associated with a ‘folate-related pathway’, which explains how CRA is mainly decreased through folate intake. However, a recent large randomized trial in females showed that folate intake combined with vitamin B6 and vitamin B12 did not affect CRA development, but the trial also indicated that the negative results might have been the result of national guidelines that recommended that females of a specified age should receive regular supplemental folate, so few study participants would have had folate deficiency.10 None of the participants in the present study took regular folate, vitamin B6 or vitamin B12. Although women in China, especially in Shanghai, have recently started to take folic acid before and at the beginning of pregnancy, over 95% of the female participants in the present study were over 50 years old; and 30 years ago, folic acid supplements were not common. Therefore, in our opinion, the results of the current study are reliable.
In addition to the analysis of the relationship between baseline folate levels and the occurrence and recurrence of CRA, this present study also undertook a subgroup analysis that included both the occurrence and recurrence of A-CRA in relation to the baseline folate levels. The large number of study participants should have minimized the bias caused by confounders. Uniform, blinded follow-up also prevented differential ascertainment of the data. This present study observed a descending trend of serum folate and did not identify a dual role for folate similar to that observed in other studies,36,37 but this might be because Asian people generally ingest less folate than other races.48 Some of the results in the present study were not significantly different, but the trends in folate levels were still matched and larger sample capacity trials should be used to confirm these preliminary findings. A recent report suggested that folic acid fortification of the US food supply did not alter the association between folate and CRC risk in postmenopausal women, neither when measured by baseline serum folate nor by red blood cell folate.49 In this present study, folate level as well as age in the female subgroup were not significantly different for the recurrence of CRA or A-CRA after follow-up in patients that had CRA at baseline, and one possible reason is that most of the female participants were postmenopausal. There is some evidence that shows that higher folic acid intakes enhance the recurrence of A-CRA,11 so the effect of folate on CRA recurrence in women requires further investigation.
This present study had a number of limitations. First, participants came to the clinic spontaneously and they were not randomly selected from the community, so the presence of clinical symptoms should be assumed and may have caused bias. Secondly, serum folate levels were not continuously recorded while the CRA was occurring, which if they had been it would have made the results more reliable. Finally, the follow-up period was not long enough, so future studies are recommended to re-examine patients after at least 2 years. Although all colonoscopies made sure that most of the intestinal mucosa was examined, there was still a possibility that some of the CRAs were missed during the original baseline colonoscopy or not completely excised. A longer follow-up period would provide more of a chance to accurately measure the rate of development of new CRAs and recurrent CRAs. Future research should pay close attention to these limitations in order to obtain more reliable results.
In conclusion, data from this present study suggest that higher serum folate is protective against CRA and A-CRA in Chinese patients. The findings also showed that post-polypectomy patients with lower baseline serum folate levels have a higher risk of recurrence of CRA and A-CRA. These data demonstrate the potential chemopreventive effects of folic acid. The follow-up period in this present study was relatively short, so adequate population surveillance should continue to monitor the long-term effects of folate. Further larger, well-designed randomized trials with longer follow-up periods are needed to confirm these preliminary findings.
Acknowledgements
We sincerely thank the doctors from the Division of Gastroenterology and Hepatology of Renji Hospital for their help recruiting patients and for undertaking the colonoscopy and biopsy examinations.
Authors’ contributions
Hui Ding undertook most of the study design and follow-up, and performed the statistical analyses and drafted the manuscript. Qin-Yan Gao helped design the study and modified the final manuscript. Hui-Min Chen carried out the recruitment of the study participants and the baseline folate level evaluation. All authors read and approved the final manuscript.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2.Collins BD. Risk of proximal colonic neoplasms in asymptomatic adults older than 50 years found to have distal hyperplastic polyps on routine colorectal cancer screening. Perm J 2010; 14: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez ME, Giovannucci E, Jiang R, et al. Folate fortification, plasma folate, homocysteine and colorectal adenoma recurrence. Int J Cancer 2006; 119: 1440–1446. [DOI] [PubMed] [Google Scholar]
- 4.Powers HJ. Interaction among folate, riboflavin, genotype, and cancer, with reference to colorectal and cervical cancer. J Nutr 2005; 135(12 Suppl): 2960S–2966S. [DOI] [PubMed] [Google Scholar]
- 5.Figueiredo JC, Mott LA, Giovannucci E, et al. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int J Cancer 2011; 129: 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson TM, Weinstein SJ, Pfeiffer RM, et al. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am J Clin Nutr 2011; 94: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimori S, Gudis K, Takahashi Y, et al. Determination of the minimal essential serum folate concentration for reduced risk of colorectal adenoma. Clin Nutr 2011; 30: 653–658. [DOI] [PubMed] [Google Scholar]
- 8.Lin YW, Wang JL, Chen HM, et al. Folic acid supplementary reduce the incidence of adenocarcinoma in a mouse model of colorectal cancer: microarray gene expression profile. J Exp Clin Cancer Res 2011; 30: 116–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao QY, Chen HM, Chen YX, et al. Folic acid prevents the initial occurrence of sporadic colorectal adenoma in Chinese older than 50 years of age: a randomized clinical trial. Cancer Prev Res (Phila) 2013; 6: 744–752. [DOI] [PubMed] [Google Scholar]
- 10.Song Y, Manson JE, Lee IM, et al. Effect of combined folic acid, vitamin B(6), and vitamin B(12) on colorectal adenoma. J Natl Cancer Inst 2012; 104: 1562–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007; 297: 2351–2359. [DOI] [PubMed] [Google Scholar]
- 12.Carroll C, Cooper K, Papaioannou D, et al. Meta-analysis: folic acid in the chemoprevention of colorectal adenomas and colorectal cancer. Aliment Pharmacol Ther 2010; 31: 708–718. [DOI] [PubMed] [Google Scholar]
- 13.Stegeman I, de Wijkerslooth TR, Stoop EM, et al. Colorectal cancer risk factors in the detection of advanced adenoma and colorectal cancer. Cancer Epidemiol 2013; 37: 278–283. [DOI] [PubMed] [Google Scholar]
- 14.Ruco A, Stock D, Hilsden RJ, et al. Evaluation of a clinical risk index for advanced colorectal neoplasia among a North American population of screening age. BMC Gastroenterol 2015; 15: 162–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schachschal G, Mayr M, Treszl A, et al. Endoscopic versus histological characterisation of polyps during screening colonoscopy. Gut 2014; 63: 458–465. [DOI] [PubMed] [Google Scholar]
- 16.Wu K, Platz EA, Willett WC, et al. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am J Clin Nutr 2009; 90: 1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagley PJ, Selhub J. A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci U S A 1998; 95: 13217–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seyoum E, Selhub J. Properties of food folates determined by stability and susceptibility to intestinal pteroylpolyglutamate hydrolase action. J Nutr 1998; 128: 1956–1960. [DOI] [PubMed] [Google Scholar]
- 19.Kim YI. Role of folate in colon cancer development and progression. J Nutr 2003; 133(11 Suppl 1): 3731S–3739S. [DOI] [PubMed] [Google Scholar]
- 20.Choi SW, Mason JB. Folate status: effects on pathways of colorectal carcinogenesis. J Nutr 2002; 132(8 Suppl): 2413S–2418S. [DOI] [PubMed] [Google Scholar]
- 21.Schernhammer ES, Ogino S, Fuchs CS. Folate and vitamin B6 intake and risk of colon cancer in relation to p53 expression. Gastroenterology 2008; 135: 770–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsend JH, Davis SR, Mackey AD, et al. Folate deprivation reduces homocysteine remethylation in a human intestinal epithelial cell culture model: role of serine in one-carbon donation. Am J Physiol Gastrointest Liver Physiol 2004; 286: G588–G595. [DOI] [PubMed] [Google Scholar]
- 23.Wang LS, Kuo CT, Cho SJ, et al. Black raspberry-derived anthocyanins demethylate tumor suppressor genes through the inhibition of DNMT1 and DNMT3B in colon cancer cells. Nutr Cancer 2013; 65: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaszewski R, Millar B, Hatfield JS, et al. Folic acid reduces nuclear translocation of beta-catenin in rectal mucosal crypts of patients with colorectal adenomas. Cancer Lett 2004; 206: 27–33. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr 2002; 132(8 Suppl): 2350S–2355S. [DOI] [PubMed] [Google Scholar]
- 26.Lee JE, Willett WC, Fuchs CS, et al. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr 2011; 93: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YI. Folate and colorectal cancer: an evidence-based critical review. Mol Nutr Food Res 2007; 51: 267–292. [DOI] [PubMed] [Google Scholar]
- 28.Zhang SM, Cook NR, Albert CM, et al. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA 2008; 300: 2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clapin HF, Fritschi L, Iacopetta B, et al. Dietary and supplemental folate and the risk of left-and right-sided colorectal cancer. Nutr Cancer 2012; 64: 937–945. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim EM, Zekri JM. Folic acid supplementation for the prevention of recurrence of colorectal adenomas: metaanalysis of interventional trials. Med Oncol 2010; 27: 915–918. [DOI] [PubMed] [Google Scholar]
- 31.Jaszewski R, Misra S, Tobi M, et al. Folic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trial. World J Gastroenterol 2008; 14: 4492–4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueiredo JC, Levine AJ, Grau MV, et al. Colorectal adenomas in a randomized folate trial: the role of baseline dietary and circulating folate levels. Cancer Epidemiol Biomarkers Prev 2008; 17: 2625–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond SJ, Enestvedt BK, Jiang Z, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest Endosc 2011; 74: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yetley EA, Pfeiffer CM, Phinney KW, et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr 2011; 94: 313S–321S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drammeh BS, Schleicher RL, Pfeiffer CM, et al. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clin Chem 2008; 54: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 36.Kim YI. Folic acid supplementation and cancer risk: point. Cancer Epidemiol Biomarkers Prev 2008; 17: 2220–2225. [DOI] [PubMed] [Google Scholar]
- 37.Sauer J, Mason JB, Choi SW. Too much folate: a risk factor for cancer and cardiovascular disease? Curr Opin Clin Nutr Metab Care 2009; 12: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natarajan L, Flatt SW, Sun X, et al. Validity and systematic error in measuring carotenoid consumption with dietary self-report instruments. Am J Epidemiol 2006; 163: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimori S, Kishida T, Yonezawa M, et al. Mean corpuscular volume may be a useful index of risk for colorectal adenoma in middle-aged Japanese men. Am J Gastroenterol 2000; 95: 793–797. [DOI] [PubMed] [Google Scholar]
- 40.Halsted CH, Villanueva JA, Devlin AM, et al. Metabolic interactions of alcohol and folate. J Nutr 2002; 132(8 Suppl): 2367S–2372S. [DOI] [PubMed] [Google Scholar]
- 41.Mason JB, Choi SW. Effects of alcohol on folate metabolism: implications for carcinogenesis. Alcohol 2005; 35: 235–241. [DOI] [PubMed] [Google Scholar]
- 42.Kang HW, Kim D, Kim HJ, et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am J Gastroenterol 2010; 105: 178–187. [DOI] [PubMed] [Google Scholar]
- 43.Bruce WR, Wolever TM, Giacca A. Mechanisms linking diet and colorectal cancer: the possible role of insulin resistance. Nutr Cancer 2000; 37: 19–26. [DOI] [PubMed] [Google Scholar]
- 44.McGrath DR, Spigelman AD. Hereditary colorectal cancer: keeping it in the family – the bowel cancer story. Intern Med J 2002; 32: 325–330. [DOI] [PubMed] [Google Scholar]
- 45.Al-Kharusi MR, Smartt HJ, Greenhough A, et al. LGR5 promotes survival in human colorectal adenoma cells and is upregulated by PGE2: implications for targeting adenoma stem cells with NSAIDs. Carcinogenesis 2013; 34: 1150–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macarthur M, Hold GL, El-Omar EM. Inflammation and cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol 2004; 286: G515–G520. [DOI] [PubMed] [Google Scholar]
- 47.Botteri E, Iodice S, Raimondi S, et al. Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology 2008; 134: 388–395. [DOI] [PubMed] [Google Scholar]
- 48.Ferrari A, de Carvalho AM, Steluti J, et al. Folate and nutrients involved in the 1-carbon cycle in the pretreatment of patients for colorectal cancer. Nutrients 2015; 7: 4318–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neuhouser ML, Cheng TY, Beresford SA, et al. Red blood cell folate and plasma folate are not associated with risk of incident colorectal cancer in the Women’s Health Initiative observational study. Int J Cancer 2015; 137: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]