Abstract
Objective
To analyse the role of serum cartilage oligomeric matrix protein (COMP) levels in the differential diagnosis of rheumatoid arthritis (RA).
Methods
This case–control study analysed the clinical and laboratory characteristics of patients with RA and healthy control subjects. The diagnostic ability of COMP for RA was evaluated by comparing it with anti-cyclic citrullinated peptide antibody levels. The sensitivity, specificity, positive and negative predictive values were calculated.
Results
The study enrolled 82 patients with RA and 34 healthy control subjects. The serum COMP level was significantly higher in patients with RA compared with control subjects (mean ± SD 29.51 ± 9.21 ng/ml versus 17.85 ± 5.55 ng/ml, respectively). The serum COMP level was significantly higher in patients with active RA compared with patients with RA in remission (mean ± SD 33.08 ± 8.80 ng/ml versus 24.94 ± 7.65 ng/ml, respectively). The cut-off value for COMP to discriminate patients with RA from healthy individuals was 21.51 ng/ml (sensitivity 0.817, specificity 0.882, positive predictive value 0.944, negative predictive value 0.667, and accuracy 0.836).
Conclusion
The serum COMP level has the potential to be used as a biological marker for differentiating between patients with RA and healthy individuals.
Keywords: Cartilage oligomeric matrix protein, rheumatoid arthritis, anti-cyclic citrullinated peptide antibody
Introduction
Rheumatoid arthritis (RA) is a complex, chronic autoimmune inflammatory joint disease that is characterized by progressive erosive symmetrical joint destruction and systemic extra-articular manifestations, affecting approximately 0.5%–1.0% of the adult population worldwide.1 Research has shown that the most significant characteristic of RA is proliferative and inflammatory synovitis of the peripheral joints, which is accompanied by progressive and irreversible damage to the articular cartilage and bone that may cause joint deformity and disability.2,3 It is very important that diagnosis and treatment are initiated in the early stages of RA, because this has been shown to be very valuable in slowing down progression of the disease.4,5 Currently, the diagnosis and staging of patients with RA is mainly based on the clinical symptoms, imaging results and some traditional laboratory tests.6,7 These methods are often used to diagnose RA during the middle-to-late disease period when treatment is not able to effectively control the progressive articular and bone damage caused by the disease.8 Research has confirmed that some biological markers reflecting cartilage degradation provide the possibility of early diagnosis.7 Biomarkers may serve a wide range of purposes in clinical practice, but biomarker research must be the subject of a quantitative surrogate validation schema to ensure clinical and statistical validity.9,10
Cartilage oligomeric matrix protein (COMP), a cartilage-derived marker of cartilage breakdown, is a prognostic factor in early RA.7,11,12 It can be detected in biological fluids, including synovial fluid and serum in patients with RA and other diseases, such as osteoarthritis and articular trauma.7 COMP is an extracellular matrix protein mainly localized to tendon, cartilage, and pericartilage tissues.13 During RA progression, the pathophysiological processes involve the digestion and dissolution of the intercellular components of the connective tissue by protease-derived hydrolysis.14 Inflammatory synovium has been considered as a potential tissue source of COMP since the molecule has been detected in the synovium in both RA and osteoarthritis.15–18 A study concluded that serum COMP was a novel indicator for the diagnosis of early RA and a promising tool to identify patients with significant joint damage.17 Some studies have demonstrated that COMP has the potential to be a diagnostic and prognostic indicator, a marker of the disease severity and a marker of the effect of treatment.12,19
In contrast, there are opposing views about both (i) the potential use of COMP as a biological marker for RA diagnosis and prognosis, and (ii) the sensitivity and specificity of COMP in early RA diagnosis,20–22 which highlight the need for a greater understanding of the biological processes involved in RA disease progression. It also remains unknown as to whether there is an optimal cut-off value for COMP that is able to discriminate patients with RA from healthy subjects, which would be a key feature for standardizing the use of COMP for the diagnosis of RA. This present case–control study investigated the role of COMP in the diagnosis of patients with RA by comparing it with anti-cyclic citrullinated peptide antibody (anti-CCP) level, which is another laboratory marker that has been shown to be valuable in the early diagnosis and prognosis of RA.23,24
Patients and methods
Study participants
This case–control study enrolled consecutive patients with RA, who satisfied the revised criteria for RA recommended by the 2010 American College of Rheumatology,25 between January 2011 and February 2013 in the Department of Rheumatology and Immunology, Weifang People’s Hospital, Weifang City, Shandong Province, China. The inclusion criteria were as follows: (i) confirmed diagnosis of RA; (ii) RA routine treatment naïve; (iii) ≥18 years; (iv) willingness to participate in the study; (v) resident in Weifang City for >6 months; and (vi) lack of osteoarthritis or any other inflammatory articular diseases. The exclusion criteria were as follows: (i) patients with osteoarthritis, rheumatic arthritis, or gouty arthritis; (ii) those who had received RA routine treatment; (iii) <18 years; (iv) resident in Weifang City ≤6 months; (v) did not agree to participate in the study; (vi) suffered from a severe form of the disease or other severe diseases and could not participate in the study.
The healthy control subjects were recruited from the Health Evaluation Clinic of Weifang People’s Hospital during the same study period. They were healthy subjects with no history of rheumatic disease or autoimmune disease, no other history of infectious or chronic inflammatory autoimmune diseases and they were unrelated to the patients. They were matched to the patients with RA based on sex and age ( ±2 years).
The study protocol was approved by the Ethical Committee of Weifang People’s Hospital (no. WRYL11016). Participants were interviewed and informed of the nature of the study. Each study participant, or their legal representative, provided written informed consent.
Physical examinations and laboratory tests
All study participants underwent a routine medical history and physical examination, which were undertaken by three Associate Chief Physicians (F.L., X.W. & X.Z.) under the supervision of an independent rheumatologist in the Department of Rheumatology and Immunology, Weifang People’s Hospital. The patients’ age, disease duration, the duration of morning stiffness, joint tenderness index, joint swelling index, joint activity pain index, and joint resting pain index were recorded.26 X-rays of the hands were undertaken using an Axiom Aristos VX Plus digital radiography system (Siemens, Erlangen, Germany) in order to identify the extent of joint bone damage so that the stage of the disease could be recorded. The stages of joint bone damage were defined as follows (where * identifies items that are necessary for classification):27,28 stage I (early stage) includes (i) no abnormal change on X-ray examination and (ii) visible osteoporosis under the joint surface; stage II (interim stage) includes *(i) regional osteoporosis, with mild cartilage damage, with or without mild subchondral bone destruction, *(ii) visible restricted joint activities with no joint deformities, (iii) adjacent muscle atrophy, and (iv) abarticular soft tissue lesions, e.g. nodules and tenosynovitis; stage III (severe stage) includes *(i) osteoporosis, bone and cartilage destruction, *(ii) joint deformities, such as subluxation, feet lateral deflection, with no joint stiffness, (iii) a wide range of muscle atrophy, and (iv) abarticular soft tissue lesions with nodules and tenosynovitis; and stage IV (late stage) includes *(i) joint stiffness and (ii) each item included in stage III.
A 5-ml sample of venous blood was collected into IMPROVACUTER® EDTA K2 tubes (IMPROVE MEDICAL Technology, Guangzhou, China) before the patients started treatment with prednisolone and/or disease modifying anti-rheumatic drugs. Blood samples were centrifuged at 2000 g at room temperature for 20 min in a BY-320 C centrifuge (Baiyang Medical Instrument Company, Beijing, China) and stored at −80℃ until analysis. The blood samples were analysed by routine methods for erythrocyte sedimentation rate (ESR) (Westergren's blood sedimentation tube; Hull Medical Science and Technology, Hefei, China), platelet count (PLT) (SYSMEX XE-2100™ Automated Haematology System; SYSMEX, Kobe, Japan) and C-reactive protein (CRP) (IMMAGE® 800 Turbidimetric Inhibition Immunoassay System; Beckman Coulter, Brea, CA, USA). Rheumatoid factor (RF) was measured using the latex agglutination test (Rheumatoid Factor Reagent Kit; Rongchuan Biotechnology Company, Shanghai, China) and a positive titre was >1/20. Anti-CCP levels were analysed using an ImmunoscanCCPlus® enzyme-linked immunosorbent assay (ELISA) (Euro Diagnostica, Malmö, Sweden) according to the manufacturer’s instructions and the minimum detectable concentration was 0.1 U/l. Serum COMP levels were measured using the Human COMP ELISA assay (Kamiya Biomedical Company, Seattle, WA, USA) according to the manufacturer’s instructions. The minimum detectable concentration was 0.4 ng/ml, and the intra-assay and inter-assay coefficients of variation were both <5%.
Patient subgroups
The patients with RA were divided into two groups according to the information obtained from the physical examination, laboratory tests, and X-ray imaging.6,25,27 Patients were considered to have active disease if four of five of the following items were satisfied: (i) there was moderate joint pain at rest; (ii) the duration of morning stiffness was ≥1 h; (iii) there was swelling of > three joints; (iv) there was pressing pain in > eight joints; (v) ESR > 30 mm/h or CRP > 8 mg/l. Patients were considered to be in remission if ≥ five of the following items were satisfied and had been in existence for ≥2 months: (i) the duration of morning stiffness was ≤15 min; (ii) there is no lack of strength; (iii) there was no joint pain at rest; (iv) there was no joint pain or pressing pain when undertaking activity; (v) there was no joint or sheath soft tissue swelling; (vi) ESR ≤ 30 mm/h (for females) or ≤20 mm/h (for males).
The patients with RA were divided into two groups according to the information that was obtained from the X-ray imaging of the patient’s hands: patients with joint bony damage as demonstrated by abnormal changes observed on the X-ray examination (stages II–IV); and patients without joint bony damage as confirmed by the lack of abnormal changes on the X-ray examination (stage I).27
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 19.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Continuous data are presented as the mean ± SD. Differences in continuous variables between groups were determined using Student’s t-test or the Mann–Whitney U-test. Categorical data are presented as n of patients (%). Differences between categorical variables were analysed using Pearson’s χ2-test or Fisher’s exact test. The sensitivity, specificity, and positive and negative predictive values (PPV and NPV, respectively) of COMP and anti-CCP for the diagnosis of RA were assessed. Optimum cut-off values are calculated to optimize sensitivity and specificity (i.e. the Youden index). Receiver-operating characteristic (ROC) curves were plotted and the areas under the ROC curves were calculated to assess the performance of each marker to distinguish RA. A P-value < 0.05 was considered statistically significant (two-tailed).
Results
This case–control study enrolled 82 patients with RA and 34 healthy control subjects. There was no significant difference in the mean ± SD age of the group of patients with RA compared with the control group (49.93 ± 11.15 versus 47.60 ± 15.49 years, respectively; Student’s t-test). Among the 82 patients with RA, the mean ± SD disease duration was 3.79 ± 5.45 years, the mean ±SD duration of morning stiffness was 1.47 ± 1.67 h, the mean ± SD joint tenderness index score was 11.91 ± 16.82, the mean ± SD joint swelling index score was 10.37 ± 14.16, the mean ± SD joint activity pain index score was 17.93 ± 20.83, and the mean ± SD joint resting pain index score was 3.39 ± 7.54.
The baseline laboratory characteristics of the study population are presented in Table 1. The serum COMP and anti-CCP antibody levels were significantly higher in patients with RA compared with the control group (P < 0.0001 for both comparisons). There were no significant difference in the ESR, CRP, PLT or RF levels between patients with RA and healthy control subjects.
Table 1.
Baseline laboratory characteristics for patients with rheumatoid arthritis (RA) and healthy control subjects who participated in a study to determine the role of serum cartilage oligomeric matrix protein (COMP) levels in the diagnosis of RA.
| Characteristic | Patients with RA n = 82 | Healthy control subjects n = 34 | Statistical significancea |
|---|---|---|---|
| COMP, ng/ml | 29.51 ± 9.21 | 17.85 ± 5.55 | P < 0.0001 |
| ESR, mm/1 h | 10.94 ± 6.85 | 8.53 ± 6.53 | NS |
| CRP, mg/dl | 4.90 ± 3.30 | 3.87 ± 2.76 | NS |
| PLT, × 109/l | 187.60 ± 98.76 | 164.10 ± 82.70 | NS |
| RF, IU/ml | 125.10 ± 65.84 | 101.50 ± 60.36 | NS |
| Anti-CCP, RU/ml | 65.57 ± 58.62 | 21.48 ± 24.45 | P < 0.0001 |
Data presented as mean ± SD.
Differences in continuous variables between groups were determined using Student’s t-test or the Mann–Whitney U-test.
ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; PLT, platelet count; RF, rheumatoid factor; CCP, cyclic citrullinated peptide; NS, no significant between-group difference (P ≥ 0.05).
The serum COMP levels were compared among different subgroups of patients with RA and healthy control subjects (Table 2). The serum COMP levels were significantly higher in the patients with RA compared with the healthy control subjects (P < 0.0001).The serum COMP levels were significantly higher in the patients with RA with active disease compared with patients with RA in remission (P < 0.0001). The serum COMP levels were significantly higher in the patients with RA in remission compared with healthy control subjects (P < 0.0001).The serum COMP levels were significantly higher in the patients with RA with joint bony damage compared with patients with RA without joint bony damage (P = 0.0156).There was no significant difference in the serum COMP level between male and female patients with RA.
Table 2.
Comparison of serum cartilage oligomeric matrix protein (COMP) levels among different subgroups of patients with rheumatoid arthritis (RA) and healthy control subjects.
| Subgroups | n | COMP, ng/ml | Statistical significancea |
|---|---|---|---|
| Patients with RA | 82 | 29.51 ± 9.21 | P < 0.0001 |
| Healthy control subjects | 34 | 17.85 ± 5.55 | |
| Male patients with RA | 21 | 27.01 ± 10.25 | NS |
| Female patients with RA | 61 | 30.37 ± 8.75 | |
| Patients with active RA | 46 | 33.08 ± 8.80 | P < 0.0001 |
| Patients with RA in remission | 36 | 24.94 ± 7.65 | |
| Patients with RA in remission | 36 | 24.94 ± 7.65 | P < 0.0001 |
| Healthy control subjects | 34 | 17.85 ± 5.55 | |
| Patients with RA with joint bony damage | 44 | 31.77 ± 8.21 | P = 0.0156 |
| Patients with RA without joint bony damage | 38 | 26.88 ± 9.71 |
Data presented as mean ± SD.
Differences in continuous variables between groups were determined using Student’s t-test or the Mann–Whitney U-test.
NS, no significant between-group difference (P ≥ 0.05).
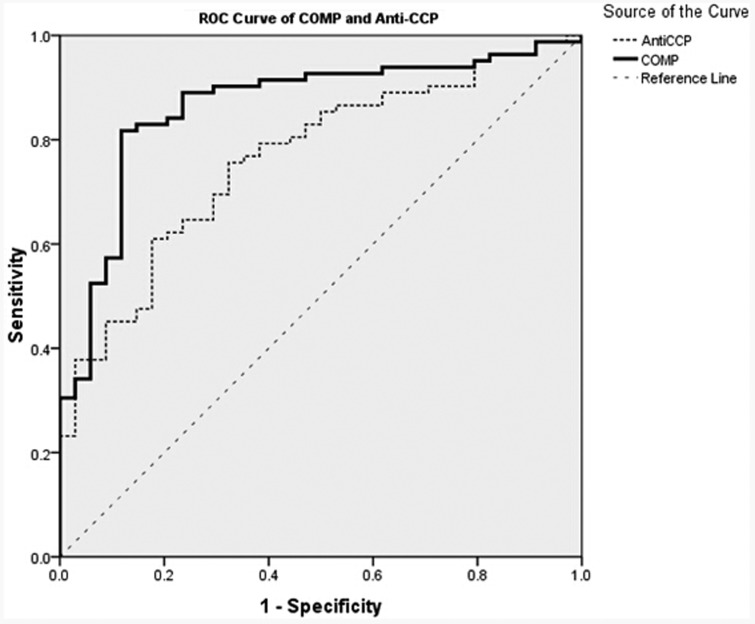
The ROC of COMP and anti-CCP for discriminating RA patients from healthy control subjects were analysed. The areas under the curves (AUC) for COMP (AUC 0.864; 95% confidence interval [CI] 0.790, 0.937) was significantly greater than that of anti-CCP (AUC 0.764; 95% CI 0.674, 0.854; P = 0.0015) (Figure 1). Optimal cut-off values for COMP and anti-CCP were 21.51 ng/ml and 34.76 RU/ml, respectively. At these cut-off values, diagnostic validity such as sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated (Table 3).
Figure 1.
Receiver operating characteristic curve analysis for serum cartilage oligomeric matrix protein (COMP) and anti-cyclic citrullinated peptide antibody (anti-CCP) calculated using data from patients with rheumatoid arthritis (n = 82) and healthy control subjects (n = 34).
Table 3.
Diagnostic validity of serum cartilage oligomeric matrix protein (COMP) and anti-cyclic citrullinated peptide antibody (anti-CCP) in differentiating patients with rheumatoid arthritis (n = 82) from healthy control subjects (n = 34).
| Marker | Cut-off value | AUC (95% CI) | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|
| COMP | 21.51 ng/ml | 0.864 (0.790, 0.937) | 0.817 | 0.882 | 0.944 | 0.667 | 0.836 |
| Anti-CCP | 34.76 RU/ml | 0.764 (0.674, 0.854) | 0.610 | 0.824 | 0.893 | 0.467 | 0.672 |
AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Discussion
The main findings of this present study include the following: (i) serum COMP levels were significantly higher in patients with RA compared with healthy control subjects; (ii) serum COMP levels were significantly higher in patients with active RA compared with patients with RA in remission; (iii) serum COMP levels in patients with RA who also had joint bony damage were significantly higher compared with patients with RA who did not have joint bony damage; and (iv) a serum COMP level of 21.51 ng/ml was the optimal cut-off value for discriminating between patients with RA and healthy control subjects. Based on the above findings, this present study demonstrated that COMP could be a useful laboratory marker for discriminating between patients with RA and healthy individuals, and it could also be used to judge the severity of the disease; findings that were in accordance with other research.8 A previous study concluded that serum COMP was significantly higher in patients with RA compared with healthy subjects; and it was also significantly higher in patients with joint destruction compared with those patients in the early stages of the disease, but its levels were affected by age, disease duration, and body mass index.29 In contrast, another study reported a sensitivity of 15%–48% and a specificity of 66%–69% for COMP as a marker for RA.20 It has also been suggested that the usefulness of testing for COMP as a marker of joint damage should be confirmed by additional and preferably longitudinal studies.21 In addition, this present study is the first to demonstrate the optimal cut-off value of COMP for discriminating between patients with RA and healthy individuals.
This present study found that the serum COMP level was not only a useful laboratory marker for discriminating between patients with RA and healthy control subjects, but it also discriminated between the different stages of joint bone damage. Other methods exist for the diagnosis of RA and for the determination of disease severity, such as RF, CRP, ESR, anti-CCP and bone imaging.30,31 In this present study, the RF, CRP, ESR and anti-CCP levels were also measured. Although the anti-CCP levels were significantly higher in the patients with RA compared with the healthy control subjects, its predictive accuracy was not as high as that determined for COMP (0.672 versus 0.836, respectively). There were no significant differences in RF, CRP, and ESR between the patients with RA and the healthy control subjects. Anti-CCP levels demonstrated a high specificity (0.824) and low sensitivity (0.610) for discriminating between patients with RA and healthy control subjects. Another study reported that CRP and ESR may not reflect the early stage of bony damage in RA and their sensitivity and specialty were low when used for RA in clinical practice.32 The imaging features of bones often change during the bone destructive stages of RA and bony damage often causes deformation.7 Thus, an early diagnosis based on the results of a physical examination, radiographs and ESR may not be useful for establishing a therapeutic strategy. Therefore, a method with high sensitivity and specificity is needed for the early stage evaluation of bone joint dysfunction.
In this present study, the serum COMP level demonstrated an acceptable diagnostic performance for discriminating between patients with RA and healthy control subjects. A COMP level of 21.51 ng/ml was the optimal cut-off value for discriminating RA patients from healthy individuals. The AUC of COMP to predict RA was high (0.864), and when using the COMP cut-off of 21.51 ng/ml to rule out and rule in the presence of RA, the percentage of correctly classified patients was 81.7% (i.e. sensitivity) and the percentage correctly classified as not having RA was 88.2% (i.e. specificity). However, it should be noted that COMP levels can be influenced by factors such as muscle mass, sex, age and diet.7
This present study had several limitations. First, the sample was selected from a city-level hospital, which may cause some limitations in the extrapolation of results; thus, when extrapolating the results of this study, the representativeness of the sample should be considered. Secondly, the patients in the study had RA with a relatively long disease duration, so the individual’s COMP values may have been affected by multiple factors, which might have then influenced the optimal cut-off value.
In conclusion, this present study demonstrated that serum COMP levels have the potential to be used as a biological marker of cartilage metabolism in RA. To our knowledge, this is the first study to demonstrate the diagnostic ability of COMP for differentiating between patients with RA and healthy individuals. These findings also indicated that a cut-off value of 21.51 ng/ml for serum COMP could be useful for the discriminating between patients with RA and healthy individuals. The monitoring of COMP levels in serum could also be a helpful method for assessing the presence and progression of RA.
Acknowledgements
We would like to thank the staff at Weifang People’s Hospital as they provided valuable assistance with the data collection.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the Foundation for Outstanding Young Scientist in Shandong Province: ‘Analysis on the molecular mechanism of EDM1 and PSACH in different clinical phenotypes caused by COMP gene mutations’ (no. BS2011SW050).
References
- 1.Sokka T, Kautiainen H, Toloza S, et al. QUEST-RA: quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann Rheum Dis 2007; 66: 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rindfleisch JA, Muller D. Diagnosis and management of rheumatoid arthritis. Am Fam Physician 2005; 72: 1037–1047. [PubMed] [Google Scholar]
- 3.Scott DL, Smith C, Kingsley G. Joint damage and disability in rheumatoid arthritis: an updated systematic review. Clin Exp Rheumatol 2003; 21(5 Suppl 31): S20–S27. [PubMed] [Google Scholar]
- 4.Emery P, Solem C, Majer I, et al. A European chart review study on early rheumatoid arthritis treatment patterns, clinical outcomes, and healthcare utilization. Rheumatol Int 2015; 35: 1837–1849. [DOI] [PubMed] [Google Scholar]
- 5.Contreras-Yáñez I, Pascual-Ramos V. Window of opportunity to achieve major outcomes in early rheumatoid arthritis patients: how persistence with therapy matters. Arthritis Res Ther 2015; 17: 177–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 3: 315–324. [DOI] [PubMed] [Google Scholar]
- 7.Tseng S, Reddi AH, Di Cesare PE. Cartilage oligomeric matrix protein (COMP): a biomarker of arthritis. Biomark Insights 2009; 4: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mølbaek K, Hørslev-Petersen K, Primdahl J. Diagnostic delay in rheumatoid arthritis: a qualitative study of symptom interpretation before the first visit to the doctor. Musculoskeletal Care 2016; 14: 26–36. [DOI] [PubMed] [Google Scholar]
- 9.Lassere MN, Johnson KR, Boers M, et al. Definitions and validation criteria for biomarkers and surrogate endpoints: development and testing of a quantitative hierarchical levels of evidence schema. J Rheumatol 2007; 34: 607–615. [PubMed] [Google Scholar]
- 10.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89–95. [DOI] [PubMed] [Google Scholar]
- 11.Zhu TH, Cai CY, Zhang L. Research progress of biomarker COMP in osteoarthritis. Zhongguo Gu Shang 2010; 23: 959–961. in Chinese, English Abstract). [PubMed] [Google Scholar]
- 12.Morozzi G, Fabbroni M, Bellisai F, et al. Cartilage oligomeric matrix protein level in rheumatic diseases: potential use as a marker for measuring articular cartilage damage and/or the therapeutic efficacy of treatments. Ann N Y Acad Sci 2007; 1108: 398–407. [DOI] [PubMed] [Google Scholar]
- 13.Di Cesare PE, Carlson CS, Stollerman ES, et al. Expression of cartilage oligomeric matrix protein by human synovium. FEBS Lett 1997; 412: 249–252. [DOI] [PubMed] [Google Scholar]
- 14.Wasserman AM. Diagnosis and management of rheumatoid arthritis. Am Fam Physician 2011; 11: 1245–1252. [PubMed] [Google Scholar]
- 15.Saxne T, Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol 1992; 31: 583–591. [DOI] [PubMed] [Google Scholar]
- 16.Neidhart M, Hauser N, Paulsson M, et al. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol 1997; 36: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 17.Andersson ML, Svensson B, Petersson IF, et al. Early increase in serum-COMP is associated with joint damage progression over the first five years in patients with rheumatoid arthritis. BMC Musculoskelet Disord 2013; 14: 229–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das BR, Roy A, Khan FR. Cartilage oligomeric matrix protein in monitoring and prognostication of osteoarthritis and its utility in drug development. Perspect Clin Res 2015; 6: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong Z, Munneke M, Vilim V, et al. Value of serum cartilage oligomeric matrix protein as a prognostic marker of large-joint damage in rheumatoid arthritis–data from the RAPIT study. Rheumatol (Oxford) 2008; 47: 868–871. [DOI] [PubMed] [Google Scholar]
- 20.Nikolaisen C, Rekvig OP, Nossent HC. Diagnostic impact of contemporary biomarker assays for rheumatoid arthritis. Scand J Rheumatol 2007; 36: 97–100. [DOI] [PubMed] [Google Scholar]
- 21.Bender AL, da Silveira IG, von Mühlen CA, et al. High specificity but low sensitivity of the cartilage oligomeric matrix protein (COMP) test in rheumatoid arthritis and osteoarthritis. Clin Chem Lab Med 2010; 48: 569–570. [DOI] [PubMed] [Google Scholar]
- 22.Wisłowska M, Jabłońska B. Serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and knee osteoarthritis. Clin Rheumatol 2005; 24: 278–284. [DOI] [PubMed] [Google Scholar]
- 23.Kroot EJ, de Jong BA, van Leeuwen MA, et al. The prognostic value of anti-cyclic citrullinated peptide antibody in patients with recent-onset rheumatoid arthritis. Arthritis Rheum 2000; 8: 1831–1835. [DOI] [PubMed] [Google Scholar]
- 24.Lindqvist E, Eberhardt K, Bendtzen K, et al. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis 2005; 64: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62: 2569–2581. [DOI] [PubMed] [Google Scholar]
- 26.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005; 23(5 Suppl 39): S100–S108. [PubMed] [Google Scholar]
- 27.Chinese Rheumatology Branch of Chinese Medical Association. Guide for diagnosis and treatment of rheumatoid arthritis. Chin J Rheumatol 2010; 4: 265–270. in Chinese). [Google Scholar]
- 28.Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc 1949; 140: 659–662. [DOI] [PubMed] [Google Scholar]
- 29.El Defrawy AO, Gheita TA, Raslan HM, et al. Serum and synovial cartilage oligomeric matrix protein levels in early and established rheumatoid arthritis. Z Rheumatol 2015. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 30.Wollheim FA. Predictors of joint damage in rheumatoid arthritis. APMIS 1996; 104: 81–93. [DOI] [PubMed] [Google Scholar]
- 31.Lee YH, Bae SC, Song GG. Diagnostic accuracy of anti-MCV and anti-CCP antibodies in rheumatoid arthritis: a meta-analysis. Z Rheumatol 2015; 74: 911–918. [DOI] [PubMed] [Google Scholar]
- 32.Shen R, Ren X, Jing R, et al. Rheumatoid factor, anti-cyclic citrullinated peptide antibody, C-reactive protein, and erythrocyte sedimentation rate for the clinical diagnosis of rheumatoid arthritis. Lab Med 2015; 46: 226–229. [DOI] [PubMed] [Google Scholar]