Abstract
Objective
Continuous invasive arterial blood pressure (IBP) monitoring remains the gold standard for BP measurement, but traditional oscillometric non-invasive intermittent pressure (NIBP) measurement is used in most low-to-moderate risk procedures. This study compared non-invasive continuous arterial BP measurement using a Nexfin® monitor with NIBP and IBP monitors.
Methods
This was a single-centre, prospective, pilot study in patients scheduled for elective orthopaedic surgery. Systolic BP, diastolic BP and mean arterial blood pressure (MAP) were measured by Nexfin®, IBP and NIBP at five intraoperative time-points. Pearson correlation coefficients, Bland–Altman plots and trending ability of Nexfin® measurements were used as criteria for success in the investigation of measurement reliability.
Results
A total of 20 patients were enrolled in the study. For MAP, there was a sufficient correlation between IBP/Nexfin® (Pearson = 0.75), which was better than the correlation between IBP/NIBP (Pearson = 0.70). Bland–Altman analysis of the data showed that compared with IBP, there was a higher percentage error for MAPNIBP (30%) compared with MAPNexfin® (27%). Nexfin® and NIBP underestimated systolic BP; NIBP also underestimated diastolic BP and MAP. Trending ability for MAPNexfin® and MAPNIBP were comparable to IBP.
Conclusion
Non-invasive BP measurement with Nexfin® was comparable with IBP and tended to be more precise than NIBP.
Keywords: Blood pressure, monitoring, non-invasive arterial pressure, invasive arterial pressure, Nexfin® Trial registration: ClinGov. NCT01263990
Introduction
Continuous invasive arterial pressure measurement using an indwelling catheter is the gold standard method for continuous blood pressure (BP) monitoring.1 However, invasive monitoring devices are associated with certain risks such as infection, bleeding and ischaemia.2 Newer non-invasive approaches of continuous arterial pressure monitoring represent promising alternatives. Based on the volume-clamp method,3 the Nexfin® monitor (Edwards LifeSciences Corporation, Irvine, CA, USA), a device that measures cardiac output continuously by an inflatable finger cuff, provides the clinician with a continuous reconstructed arterial pressure waveform after physiological calibration.4–8 In cardiothoracic surgery, BP measured noninvasively with a Nexfin® finger cuff that uses photoplethysmographic technology, was highly comparable with BP measured invasively both intraoperatively and postoperatively9,10 However, in critically ill patients, Nexfin® derived measurements did not correlate with traditional invasive measurements.11
Reconstructive orthopaedic surgery is expected to increase as the proportion of the population over 64 years increases.12 Indeed, patients undergoing reconstructive orthopaedic surgery are often elderly with a high incidence of comorbidity (e.g. obesity, coronary artery disease [CAD]). Several studies have shown that acute haemodynamic impairment, including arterial hypotension, leads to increased complication rates in both surgical and elderly patients.13–15 The use of a non-invasive device for continuous BP monitoring in moderate risk orthopaedic surgery might be beneficial if it is shown to provide reliable measurements.16
The purpose of this pilot study was to investigate whether non-invasive continuous arterial pressure measurements using the Nexfin® monitor were comparable with those obtained by traditional oscillometric non-invasive intermittent BP (NIBP) monitoring and continuous invasive BP (IBP) measurements in patients undergoing moderate risk orthopaedic surgery.
Patients and methods
Patients
Patients were eligible for inclusion in this prospective single-centre study if they were ≥18 years of age, required total hip or knee replacement under general anaesthesia, had a requirement for invasive arterial pressure monitoring due to pre-existing disease or general condition (e.g. obesity with a body mass index [BMI] >30 kg/m2), CAD, peripheral artery disease and had limited exercise capacity (i.e. were moderate risk).17 Patients were excluded from the study if they presented with atrial fibrillation, peripheral vascular disease (i.e. Fontaine stadium >IIa),18 scleroderma, an arteriovenous shunt, valvular heart disease, an acute infection and/or systemic inflammatory response syndrome/sepsis, required intraoperative transfusion of >4 units of packed red blood cells, or were pregnant and/or breastfeeding.
Following Institutional Review Board approval by the Charité Ethics Committee and patientś written informed consent, the study took place at Campus Charité Mitte and Campus Virchow-Klinikum, Charité - Universitätsmedizin Berlin, Charitéplatz, Berlin, Germany between April 2011 and April 2012 (study protocol number: EA1/199/10) (trial registration: ClinGov. NCT01263990).
Perioperative management
For those patients who required premedication, 0.05 – 0.1 mg kg−1 midazolam was given orally approximately 1 h before surgery. After the insertion of a peripheral venous line and assessment of baseline vital signs (i.e. heart rate and pulse oximetry), oscillometric NIBP was recorded on the ipsilateral arm in relation to the operative site. An arterial line was placed into the radial artery contralateral to the NIBP using local anaesthesia and was subsequently connected to a pressure transducer. An appropriate finger cuff connected to the Nexfin® device was then placed on the medial phalanx of the second or third finger on the same side of the body that was used for the IBP assessment.
General anaesthesia was conducted according to our institutional standard operating procedures. In brief, anaesthesia was induced with 1.5–3 mg kg−1 propofol and 1–3 µg.kg−1 fentanyl followed by 0.1–0.15 mg kg−1 cisatracurium to facilitate orotracheal intubation. After tracheal intubation, the patients were pressure controlled ventilated with a tidal volume of ≥8–10 ml kg19,20 and a respiratory rate adjusted to maintain normocapnia (i.e. end tidal CO2 30–35 mmHg, inspired oxygen fraction 0.35). Anaesthesia was maintained with either sevoflurane (1 MAC Vol% end tidal) or 6 mg kg−1 h−1 propofol, both without nitrous oxide. Repeated doses of cisatracurium were administered according to clinical demand. For hip surgery, patients were positioned supine with the ipsilateral arm elevated in a 90° angle to allow optimized access to the hip. For knee surgery, patients were positioned in a supine position with both arms outstretched. Sevoflurane or propofol were discontinued before completion of skin closure. Patients were extubated when they responded to verbal commands and respiratory efforts were within the normal clinical range. Patients were transferred to the recovery room and received oxygen via a facemask at rate of 2–4 l.min−1 if needed. Patients remained in the recovery room until they fulfilled the standard criteria for discontinuation of monitoring.
Haemodynamic measurements
Following zero calibration of the IBP transducer (TruWave™ Pressure Monitoring Set; Edwards Lifesciences, Irvine, CA, USA) and physiological calibration of the Nexfin® monitor, six BP measurements using the Riva-Rocci technique,8 were taken (i.e. immediately before induction [T0] and at 3, 15, 20 and 30 min after induction of general anaesthesia as well as immediately before the end of surgery [TEnd]) using all three methods of monitoring. At each assessment, systolic BP, diastolic BP, and mean arterial pressure (MAP) were recorded by four of the authors (F.B., M.H., J.S. and S.T.). Nexfin® values were taken immediately before insufflation of the NIBP cuff to avoid triggering the need for Physiocal®, which analyses the curvature and sharpness of the plethysmogram during short periods of steady cuff pressure levels.5 Multiple measurements were taken with Nexfin® and IBP at each of the six time-points because they are continuous measuring devices and the mean of the values was subsequently calculated.
Statistical analyses
This study was approved by the institutional review board as a pilot study because of its intention to provide background information for the preparation of a larger investigation. In this respect, no formal power analysis was performed and the design and patient group were chosen to inform the study design and protocol of the larger study.21 The study was performed in moderate risk orthopaedic surgery patients undergoing knee and hip replacement because the Nexfin® device could be used to provide continuous BP measurement without the risk of invasive arterial cannulation-related side-effects.16 Measurements recorded from the IBP device were used as the ‘gold standard’ reference measurements. For data collection and analyses, Microsoft Excel 2010 for Windows® (Microsoft, Redmond, WA, USA), MedCalc (MedCalc Software, Ostend, Belgium) and R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria) were used.
Pearson correlation coefficients and Bland–Altman plots were used to evaluate the correlation between the measurements acquired by the three devices. The Bland–Altman method uses the plot of the difference in BP for each patient (i.e. IBP – Nexfin® or IBP – NIBP) against the mean of the two measurements.22,23 The Bland–Altman method calculates the mean difference between two methods of measurement (‘bias’) and 95% limits of agreement (LOA) as the mean difference (2 SD). The smaller the range is between these two limits, the better is the agreement. A mean percentage error (PE) <30% also indicated sufficient agreement between the devices.22,23 In addition, polar plots were used to compare trending ability between the three methods with the accepted range of ‘angle/angular bias ±30°’ reflecting the 95% confidence interval.24,25 Regression analyses were also performed to adjust for confounders in morphometric data. All tests were part of an exploratory data analysis and so no adjustments for multiple testing were made. In all analyses, a P-value <0.05 was taken to indicate statistical significance.
Results
Data were available from 20 patients who underwent moderate risk reconstructive orthopaedic surgery. The baseline demographic and surgery-related data of the study participants are shown in Table 1. The Nexfin® finger cuff could be easily placed on all patients and no adverse outcomes were observed.
Table 1.
Demographic baseline characteristics for patients undergoing hip or knee surgery and monitored with a Nexfin® or conventional arterial blood pressure monitors.
| Characteristic | Patient group n = 20 |
|---|---|
| Age, years | 68 ± 10 |
| Height, cm | 171 ± 7 |
| Weight, kg | 78 ± 21 |
| Body mass index, kg/m2 | 29.9 ± 6.7 |
| Surgical procedure | |
| Hip replacement | 18 |
| Knee replacement | 2 |
Data presented as mean ± SD or n of patients.
Nexfin® versus IBP measurements
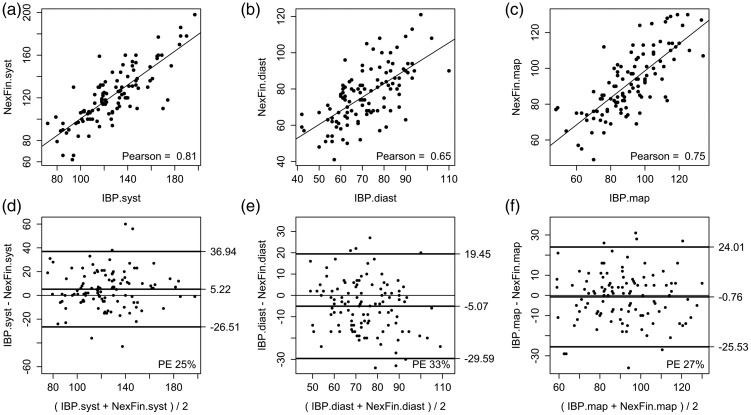
A comparison of the pooled data from all systolic BP, diastolic BP and MAP measurements made with the IBP device and with the Nexfin® device are shown in Table 2. Figure 1 shows the Pearson correlation plots (A to C) and the Bland–Altman plots (D to F) for the comparison between the mean Nexfin® and IBP measurements for systolic BP, diastolic BP and MAP.
Table 2.
Comparison of blood pressure (BP) measurements (Riva-Rocci technique8) taken by the Nexfin® device and invasive BP (IBP) device (n = 20).
| Data from Bland–Altman plots |
||||
|---|---|---|---|---|
| Mean ± SD difference (bias) (mmHg) | Limits of agreement (mmHg) | Percentage error (%) | Pearson correlation coefficient analysis | |
| Systolic BP | 5 ± 16 | −26.5, 36.9 | 25 | 0.81 |
| Diastolic BP | −5 ± 12 | −29.6, 19.5 | 33 | 0.65 |
| Mean arterial pressure | −1 ± 13 | −25.5, 24.0 | 27 | 0.75 |
Figure 1.
Pearson correlation plots between Nexfin® (NexF) and invasively measured blood pressure (IBP) for mean (a) systolic (syst), (b) diastolic (diast), and (c) mean arterial blood pressure (map) values. Bland–Altman plots between Nexfin® and IBP for mean (d) systolic (syst), (e) diastolic (diast), and (f) mean arterial blood pressure (map) measurements.
Measurements from the Nexfin® device correlated well with the IBP measurements as indicated by the Pearson correlation coefficients (Table 2). For MAP, there was a sufficient correlation between IBP/Nexfin® (Pearson = 0.75). Nexfin® underestimated systolic BP as indicated by positive mean difference (bias) whereas diastolic BP and MAP were slightly overestimated as shown by negative mean differences (Table 2). The PE of the systolic BPNexfin® and MAPNexfin® were within the clinically accepted range (i.e. <30%). However, the PE for diastolic BPNexfin® (33%) was greater than the accepted range.
Regression analysis showed that age had an impact on BMI, height and weight (P < 0.05) and so these parameters were adjusted accordingly. Further analyses showed that BMI and weight/height had no impact on the mean difference (bias) of MAPNexfin® with respect to MAPIBP (BMI univariate, P = 0.48; BMI adjusted for age, P = 0.51; weight and height adjusted for age, P = 0.29 and P = 0.27, respectively).
NIBP versus IBP measurements
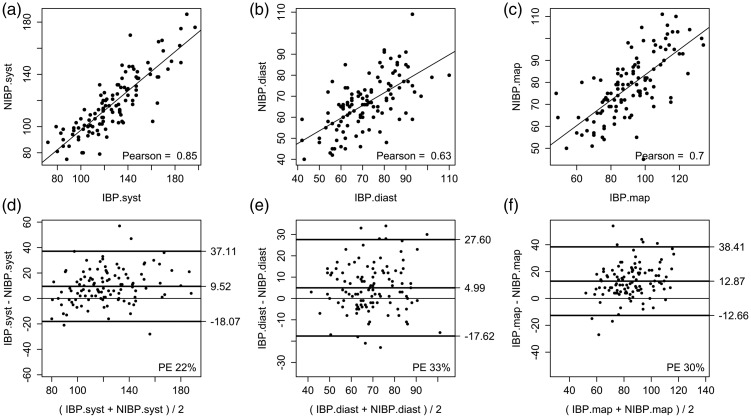
A comparison of the pooled data from all systolic BP, diastolic BP and MAP measurements made by the IBP device and the NIBP device are shown in Table 3. Figure 2 shows the Pearson correlation plots (A to C) and the Bland–Altman plots (D to F) for the comparison between NIBP and IBP measurements for systolic BP, diastolic BP and MAP.
Table 3.
Comparison of blood pressure (BP) measurements (Riva-Rocci technique8) taken by traditional oscillometric, non-invasive pressure (NIBP) and invasive BP (IBP) devices (n = 20).
| Data from Bland–Altman plots |
||||
|---|---|---|---|---|
| Mean ± SD difference (bias) (mmHg) | Limits of agreement (mmHg) | Percentage error (%) | Pearson correlation coefficient analysis | |
| Systolic BP | 10 ± 14 | −18.1, 37.1 | 22 | 0.85 |
| Diastolic BP | 5 ± 11 | −17.6, 27.6 | 33 | 0.63 |
| Mean arterial pressure | 13 ± 13 | −12.7, 38.4 | 30 | 0.70 |
Figure 2.
Pearson correlation plots between non-invasively measured blood pressure (NIBP) and mean values for invasively measured BP (IBP) for (a) systolic (syst), (b) diastolic (diast), and (c) mean arterial blood pressure (map) values. Bland–Altman plots between NIBP and mean values for IBP for (d) systolic (syst), (e) diastolic (diast), and (f) mean arterial blood pressure (map) measurements.
Measurements by the NIBP method correlated well with the IBP measurements as indicated by the Pearson correlation coefficients (Table 3). The Pearson correlation coefficient between IBP/NIBP was 0.70. The NIBP measurements markedly underestimated systolic BP, diastolic BP and MAP as shown by positive mean differences (bias) (Table 3). Only the PE of systolic BP was within the clinically accepted range (i.e. <30%).
Regression analysis showed that BMI and weight/height had no impact on the mean difference (bias) of MAPNIBP with respect to MAPIBP (BMI univariate, P = 0.7; BMI adjusted for age, P = 0.41; weight and height adjusted for age, P = 0.48 and P = 0.71).
MAP measured by the three different methods at different time-points
Values for the differences in MAP assessed by the three different methods at different time-points are presented in Table 4. As shown by PE, Nexfin® always correlated well with IBP (i.e. <30%) except at T3. These findings were confirmed by Pearson correlation coefficients. Measurements by NIBP correlated with IBP measurements at T0 and T15 but at none of the other time-points (Table 4).
Table 4.
Comparison of mean arterial blood pressure (MAP) measurements taken by the three methods (i.e. Nexfin®/invasive blood pressure [IBP]/non-invasive pressure [NIBP]) at different time-points.
| Time | Comparison | Data from Bland–Altman plots |
|||
|---|---|---|---|---|---|
| Mean ± SD difference (bias) (mmHg) | Limits of agreement (mmHg) | Percentage error (%) | Pearson correlation coefficient analysis | ||
| T0 | IBP – Nexfin® | −1 ± 14 | −27, 26 | 26 | 0.71 |
| IBP – NIBP | 13 ± 12 | −11, 37 | 24 | 0.76 | |
| T3 | IBP – Nexfin® | 1 ± 15 | −28, 30 | 34 | 0.30 |
| IBP – NIBP | 15 ± 14 | −12, 42 | 35 | 0.52 | |
| T15 | IBP – Nexfin® | 0 ± 13 | −26, 26 | 27 | 0.67 |
| IBP – NIBP | 13 ± 12 | −11, 37 | 28 | 0.69 | |
| T20 | IBP – Nexfin® | −1 ± 9 | −19, 17 | 19 | 0.84 |
| IBP – NIBP | 12 ± 13 | −13, 37 | 30 | 0.57 | |
| T30 | IBP – Nexfin® | 0 ± 9 | −18, 18 | 21 | 0.86 |
| IBP – NIBP | 11 ± 13 | −14, 36 | 31 | 0.60 | |
| TEnd | IBP – Nexfin® | −5 ± 11 | −27, 17 | 27 | 0.83 |
| IBP – NIBP | 8 ± 12 | −16, 32 | 33 | 0.79 | |
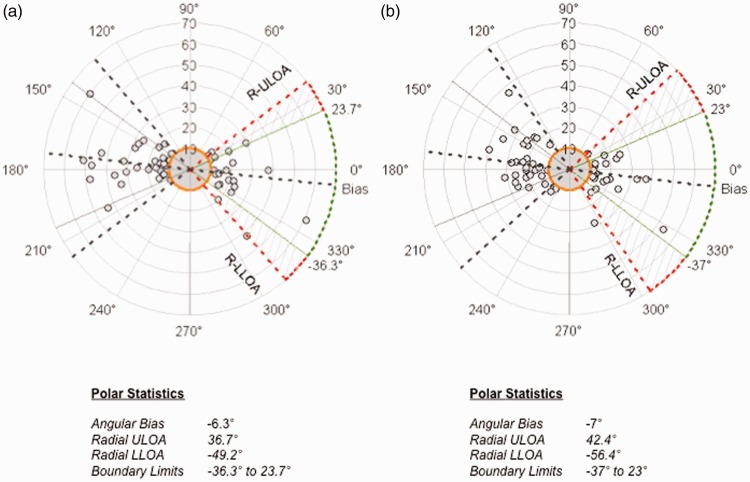
The trending ability of IBP with Nexfin® and IBP with NIBP are presented in polar plots in Figure 3. The trending ability of Nexfin® was comparable with NIBP using IBP as a reference (i.e. measurements were within the accepted range of angle/angular bias ±30°).
Figure 3.
Polar plots displaying the trending ability of (a) mean IBP and Nexfin® values and (b) mean IBP values and NIBP for mean arterial pressure (MAP) measurements. Radial ULOA, radial upper limit of agreement; Radial LLOA, radial lower limit of agreement.
Discussion
The usefulness of haemodynamic monitoring in surgery has long been acknowledged.14–16 For example, prolonged intraoperative decreases of 20 mmHg or more in MAP were associated with a significant increase in risk for postoperative ischaemic complications in patients undergoing elective non-cardiac surgery.26 In another study, there were more complications among hypertensive and diabetic patients whose MAP decreased to less than 70 mmHg.27 Moreover, intraoperative arterial hypotension is associated with prolonged length of hospital stay and increased mortality.14,28,29 In addition, an intraoperative systolic BP variability outside a targeted BP range (e.g. BP > 135 mmHg or < 90 mmHg) has been shown to predict 30-day postoperative mortality.30 Nevertheless, the placement of an indwelling arterial catheter is not without risk.2 The results of this present study show that the non-invasive Nexfin® monitor provides the clinician with continuous reconstructed arterial BP waveforms, which, from a clinical standpoint, could be used in moderate risk patients as an alternative to IBP.
This current prospective, observational pilot study involving 20 moderate risk patients scheduled for elective orthopaedic surgery, showed that continuous non-invasive BP measurements with Nexfin® were comparable with continuous IBP measurements and tended to be more precise than the traditional intermittent NIBP measurements. The current results showed that for MAP, there was sufficient correlation between Nexfin® and IBP measurements, which was better than the correlation between NIBP and IBP measurements. Nexfin® only underestimated the systolic BP whereas NIBP underestimated all arterial BP readings. In addition, the trending ability for MAPNexfin® was comparable with MAPIBP indicating that deviations from the gold standard (IBP) were consistently in the same direction. The current results are in agreement with previous studies,31,32 and suggest that Nexfin® is a reliable measure of procedure-related, minor-to-moderate haemodynamic variations in moderate risk patients.33 Only at one time-point did the PE of MAPNexfin® fall outside the clinically accepted range of <30%. This observation supports the possible interchangeability of IBP with Nexfin® for recording MAP. Consistent with these current results, accurate non-invasive BP measurements using another finger-clamp system (CNAP®) have been reported during vascular surgery.34 Previous studies have also suggested that the continuous Nexfin® monitor might improve the detection of BP changes compared with NIBP measurements.35,36
In a recent study, involving haemodynamically stable patients undergoing general anaesthesia for elective surgery, the agreement of Nexfin®-derived MAP with invasive MAP measurements was found to be non-inferior to conventional MAP measurements obtained with intermittent arm cuff oscillometry.16 In patients scheduled for elective coronary surgery, MAPNexfin® also correlated with both radial and femoral derived IBP before and after cardiopulmonary bypass and reflected percentage changes in MAP.31 In addition, Nexfin® monitoring reduced the length of intraoperative arterial hypo- and hypertension compared with NIBP monitoring.37 Moreover, in 45 critically ill patients, an excellent correlation between MAPNexfin® and femoral MAPIBP was preserved in a subgroup analysis of patients with severe hypotension, high systemic vascular resistance, low cardiac output, hypothermia and in patients supported by inotropic and/or vasopressive agents.32 Nevertheless, in a previous study in critically ill and postoperative cardiac surgery patients, Nexfin®-derived measurements did not correlate with traditional invasive measurements.11 This may partially be explained by a worse peripheral perfusion status in the second study and/or differences in patient-related factors, (e.g. obesity) in these patients.11 However, in this present study, patient related-factors (e.g. BMI, weight and height), did not influence the accuracy of Nexfin® measurements.
The correlation coefficients between IBP and Nexfin® devices observed during this study (i.e. 0.65 to 0.81) were not as high as those previously described in cardiac surgery patients (i.e. 0.96).9 The difference in these findings might be explained by the difference in the type of surgery (i.e. cardiac versus orthopaedic). In contrast to a more static patient positioning in cardiac surgery, at our centre, patient positioning in orthopaedic surgery is more dynamic due to the fact that one arm is continuously raised to gain an improved operative access to the ipsilateral side in hip surgery and to the surgical interventions (i.e. screwing, drilling and tilling). Nevertheless, in this present study, a review of mean differences (bias) and LOAs indicate that MAPNexfin® appeared to be as precise as MAPIBP. Furthermore, the percentage error for MAPNexfin® and MAPIBP was comparable with previous findings in non-cardiac surgery patients.16
Consistent with previous results, compared with Nexfin® values, NIBP values were lower than IBP values overall and at every given time-point.16 Although based on the same reference technique and cut-off values,16 the PE in this present study was outside the accepted range for NIBP values on four out of six time-points. Therefore, NIBP measurements were less precise than Nexfin® measurements and underestimated BP. This discrepancy has also been detected between NIBP and IBP measurements in critically ill patients.38,39 NIBP values have been shown to be especially inaccurate in overweight critically ill patients, which can lead to erroneous interpretations of BP.40 This may partially be explained by improper NIBP cuff size,41 cuff placement site42 and inconsistency of Korotkoff sounds.43 Nevertheless, in this present study, the trending ability of NIBP was comparable with that of Nexfin®.
The study had several limitations. For example, it was a pilot study and so the results were obtained from a small number of patients undergoing the same type of surgery (i.e. elective orthopaedic surgery). Therefore, it was not possible to determine the interchangeability of Nexfin® and IBP devices under different conditions as well as in emergency situations. More studies are required in other patient groups to demonstrate the clinical usefulness of the Nexfin® device. In addition, fingertip temperature measurements on the Nexfin® hand to monitor disturbances in microperfusion (e.g. cold-mediated vasoconstriction) were not performed, which might have affected the accuracy of the measurements. Moreover, inter-observer variability between the four assessors was not assessed, which may have also had an impact on the overall results.
In conclusion, BP monitoring with the Nexfin® device was feasible in this small group of patients undergoing orthopaedic surgery with general anaesthesia. It provided the clinician with a continuous, beat-to-beat, real-time BP monitoring that appeared to be comparable with IBP monitoring in these moderate risk patients. Furthermore, Nexfin® measurements tended to be more precise than NIBP measurements and had the advantage of being measured continuously. The absence of arterial cannulation-related side-effects renders non-invasive continuous BP monitoring a promising approach in the context of both patient safety and comfort. The results of this present study suggest that a high number of patients with a moderate perioperative risk could be optimized using the Nexfin® device. Given the comparability in accuracy and trending ability of non-invasive continuous BP monitoring versus the current gold standard of IBP, further studies should target the implications on patient outcome related to the application of this new technology. Furthermore, future investigations should focus on the interchangeability of Nexfin® and IBP devices under different situations.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest. Unrelated to this study, S.T. received funding for experimental research from B. Braun and honoraria for lectures from Edwards and Carinopharm. M.S. received funding for research from Edwards Life Sciences, The Medicines Company and Pulsion, and honoraria for lectures from Edwards and Pulsion.
Funding
This study was performed within an institutional grant from Charité-Universitätsmedizin Berlin. BMEYE B.V. (Amsterdam, Netherlands) provided the monitor and finger cuffs without charge for this study, but the company provided no further financial support.
References
- 1.Arora S, Singh PM, Goudra BG, et al. Changing trends of hemodynamic monitoring in ICU - from invasive to non-invasive methods: are we there yet? Int J Crit Illn Inj Sci 2014; 4: 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cousins TR, O’Donnell JM. Arterial cannulation: a critical review. AANA J 2004; 72: 267–271. [PubMed] [Google Scholar]
- 3.Penáz J. Criteria for set point estimation in the volume clamp method of blood pressure measurement. Physiol Res 1992; 41: 5–10. [PubMed] [Google Scholar]
- 4.Dorlas JC, Nijboer JA, Butijn WT, et al. Effects of peripheral vasoconstriction on the blood pressure in the finger, measured continuously by a new noninvasive method (the Finapres). Anesthesiology 1985; 62: 342–345. [DOI] [PubMed] [Google Scholar]
- 5.Wesseling KH, de Wit B, van der Hoeven GM. Physiocal, calibrating finger vascular physiology for Finapres. Homeostasis 1995; 36: 67–82. [Google Scholar]
- 6.Gizdulich P, Prentza A, Wesseling KH. Models of brachial to finger pulse wave distortion and pressure decrement. Cardiovasc Res 1997; 33: 698–705. [DOI] [PubMed] [Google Scholar]
- 7.Imholz BP, Wieling W, van Montfrans GA, et al. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology. Cardiovasc Res 1998; 38: 605–616. [DOI] [PubMed] [Google Scholar]
- 8.Eeftinck Schattenkerk DW, van Lieshout JJ, van den Meiracker AH, et al. Nexfin noninvasive continuous blood pressure validated against Riva-Rocci/Korotkoff. Am J Hypertens 2009; 22: 378–383. [DOI] [PubMed] [Google Scholar]
- 9.Martina JR, Westerhof BE, van Goudoever J, et al. Noninvasive continuous arterial blood pressure monitoring with Nexfin®. Anesthesiology 2012; 116: 1092–1103. [DOI] [PubMed] [Google Scholar]
- 10.Fischer MO, Avram R, Carjaliu I, et al. Non-invasive continuous arterial pressure and cardiac index monitoring with Nexfin after cardiac surgery. Br J Anaesth 2012; 109: 514–521. [DOI] [PubMed] [Google Scholar]
- 11.Stover JF, Stocker R, Lenherr R, et al. Noninvasive cardiac output and blood pressure monitoring cannot replace an invasive monitoring system in critically ill patients. BMC Anesthesiol 2009; 9: 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franz D, Roeder N. Mengendynamik in den Krankenhäusern: auch eine gesellschaftliche Frage. Dtsch Arztebl International 2012; 109: 2580–2584. [Google Scholar]
- 13.Molander L, Gustafson Y, Lovheim H. Low blood pressure is associated with cognitive impairment in very old people. Dement Geriatr Cogn Disord 2010; 29: 335–341. [DOI] [PubMed] [Google Scholar]
- 14.Sessler DI, Sigl JC, Kelley SD, et al. Hospital stay and mortality are increased in patients having a “triple low” of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology 2012; 116: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 15.Tassoudis V, Vretzakis G, Petsiti A, et al. Impact of intraoperative hypotension on hospital stay in major abdominal surgery. J Anesth 2011; 25: 492–499. [DOI] [PubMed] [Google Scholar]
- 16.Vos JJ, Poterman M, Mooyaart EA, et al. Comparison of continuous non-invasive finger arterial pressure monitoring with conventional intermittent automated arm arterial pressure measurement in patients under general anaesthesia. Br J Anaesth 2014; 113: 67–74. [DOI] [PubMed] [Google Scholar]
- 17.Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J 2014; 35: 2383–2431. [DOI] [PubMed] [Google Scholar]
- 18.White CJ, Gray WA. Endovascular therapies for peripheral arterial disease: an evidence-based review. Circulation 2007; 116: 2203–2215. [DOI] [PubMed] [Google Scholar]
- 19.Renner J, Cavus E, Meybohm P, et al. Stroke volume variation during hemorrhage and after fluid loading: impact of different tidal volumes. Acta Anaesthesiol Scand 2007; 51: 538–544. [DOI] [PubMed] [Google Scholar]
- 20.Reuter DA, Felbinger TW, Schmidt C, et al. Stroke volume variations for assessment of cardiac responsiveness to volume loading in mechanically ventilated patients after cardiac surgery. Intensive Care Med 2002; 28: 392–398. [DOI] [PubMed] [Google Scholar]
- 21.Arnold DM, Burns KE, Adhikari NK, et al. The design and interpretation of pilot trials in clinical research in critical care. Crit Care Med 2009; 37(1 Suppl): S69–S74. [DOI] [PubMed] [Google Scholar]
- 22.Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 1999; 15: 85–91. [DOI] [PubMed] [Google Scholar]
- 23.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 24.Critchley LA, Yang XX, Lee A. Assessment of trending ability of cardiac output monitors by polar plot methodology. J Cardiothorac Vasc Anesth 2011; 25: 536–546. [DOI] [PubMed] [Google Scholar]
- 25.Hunsicker O, Feldheiser A, Wernecke KD, et al. Assessment of agreement and trending between haemodynamic monitors is still challenging. Intensive Care Med 2014; 40: 767–767. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, MacKenzie CR, Gold JP, et al. The preoperative and intraoperative hemodynamic predictors of postoperative myocardial infarction or ischemia in patients undergoing noncardiac surgery. Ann Surg 1989; 210: 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, MacKenzie CR, Gold JP, et al. Intraoperative blood pressure. What patterns identify patients at risk for postoperative complications? Ann Surg 1990; 212: 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reich DL, Wood RK, Jr., Emre S, et al. Association of intraoperative hypotension and pulmonary hypertension with adverse outcomes after orthotopic liver transplantation. J Cardiothorac Vasc Anesth 2003; 17: 699–702. [DOI] [PubMed] [Google Scholar]
- 29.Davis MJ, Menon BK, Baghirzada LB, et al. Anesthetic management and outcome in patients during endovascular therapy for acute stroke. Anesthesiology 2012; 116: 396–405. [DOI] [PubMed] [Google Scholar]
- 30.Aronson S, Stafford-Smith M, Phillips-Bute B, et al. Intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients. Anesthesiology 2010; 113: 305–312. [DOI] [PubMed] [Google Scholar]
- 31.Broch O, Bein B, Gruenewald M, et al. A comparison of continuous non-invasive arterial pressure with invasive radial and femoral pressure in patients undergoing cardiac surgery. Minerva Anestesiol 2013; 79: 248–256. [PubMed] [Google Scholar]
- 32.Ameloot K, Van De Vijver K, Van Regenmortel N, et al. Validation study of Nexfin® continuous non-invasive blood pressure monitoring in critically ill adult patients. Minerva Anestesiol 2014; 80: 1294–1301. [PubMed] [Google Scholar]
- 33.Sipkens LM, Treskes K, Ariese-Beldman K, et al. Application of Nexfin noninvasive beat-to-beat arterial blood pressure monitoring in autonomic function testing. Blood Press Monit 2011; 16: 246–251. [DOI] [PubMed] [Google Scholar]
- 34.Biais M, Vidil L, Roullet S, et al. Continuous non-invasive arterial pressure measurement: evaluation of CNAP device during vascular surgery. Ann Fr Anesth Reanim 2010; 29: 530–535. [DOI] [PubMed] [Google Scholar]
- 35.Siebig S, Rockmann F, Sabel K, et al. Continuous non-invasive arterial pressure technique improves patient monitoring during interventional endoscopy. Int J Med Sci 2009; 6: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahmanian PB, Adams DH, Castillo JG, et al. Predicting hospital mortality and analysis of long-term survival after major noncardiac complications in cardiac surgery patients. Ann Thorac Surg 2010; 90: 1221–1229. [DOI] [PubMed] [Google Scholar]
- 37.Chen G, Chung E, Meng L, et al. Impact of non invasive and beat-to-beat arterial pressure monitoring on intraoperative hemodynamic management. J Clin Monit Comput 2012; 26: 133–140. [DOI] [PubMed] [Google Scholar]
- 38.Bur A, Hirschl MM, Herkner H, et al. Accuracy of oscillometric blood pressure measurement according to the relation between cuff size and upper-arm circumference in critically ill patients. Crit Care Med 2000; 28: 371–376. [DOI] [PubMed] [Google Scholar]
- 39.Bur A, Herkner H, Vlcek M, et al. Factors influencing the accuracy of oscillometric blood pressure measurement in critically ill patients. Crit Care Med 2003; 31: 793–799. [DOI] [PubMed] [Google Scholar]
- 40.Araghi A, Bander JJ, Guzman JA. Arterial blood pressure monitoring in overweight critically ill patients: invasive or noninvasive? Crit Care 2006; 10: R64–R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hersh LT, Sesing JC, Luczyk WJ, et al. Validation of a conical cuff on the forearm for estimating radial artery blood pressure. Blood Press Monit 2014; 19: 38–45. [DOI] [PubMed] [Google Scholar]
- 42.Schimanski K, Jull A, Mitchell N, et al. Comparison study of upper arm and forearm non-invasive blood pressures in adult emergency department patients. Int J Nurs Stud 2014; 51: 1575–1584. [DOI] [PubMed] [Google Scholar]
- 43.Zheng D, Di Marco LY, Murray A. Effect of respiration on Korotkoff sounds and oscillometric cuff pressure pulses during blood pressure measurement. Med Biol Eng Comput 2014; 52: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]