Abstract
Objective
A retrospective study to determine the relationships between platelet parameters and inflammation and fibrosis of the liver in patients with chronic hepatitis B virus infection (CHB).
Methods
Patients with liver biopsy-confirmed CHB were included in the study. Liver fibrosis and inflammation were assessed by histopathology of biopsied liver tissue. Platelet count (PLT), platelet distribution width (PDW) and mean platelet volume (MPV) were determined as part of routine blood tests. The relationship between inflammation and fibrosis and platelet parameters were analysed by multiple linear regression.
Results
The study included 677 patients. PLT and PDW accounted for 20.5% of liver inflammation. PLT and PDW accounted for 18.4% of liver fibrosis.
Conclusion
Platelet parameters can provide valuable information for the assessment of hepatic inflammation and fibrosis.
Keywords: Hepatitis B, chronic hepatitis, platelet, histology, correlation
Introduction
Hepatitis B virus (HBV) infection is a worldwide health problem. China has a large number of patients with HBV infection, and chronic hepatitis B (CHB) infection poses a serious public health threat. According to a survey by the Chinese health administration, 1317982 people were newly diagnosed with viral hepatitis in 2010, 80% of whom were infected with HBV. There are estimated 93 million HBV carriers and 20 million patients with CHB in China, and 350 million HBV carriers worldwide.1–3
Complications of HBV infection include inflammation, hepatofibrosis, cirrhosis and hepatocellular carcinoma.4 Timely assessment of the severity of liver inflammation and fibrosis is essential in the treatment of CHB, with pathological observation of liver biopsy specimens the gold standard diagnostic tool.5 However, since liver biopsy is invasive, its use is limited by the availability of equipment and pathologists, and by patient compliance.
Liver damage can be indirectly assessed via blood concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and glutamyl transpeptidase (GGT), while total bile acid (TBA), total bilirubin (TBIL) and cholinesterase (CHE) can reflect hepatic function. However, levels of transaminase enzymes are affected by the compensative capacity of the liver.6,7
Clinical experience suggests that platelet parameters may be useful in the assessment of liver inflammation and fibrosis, and platelet parameters are commonly evaluated during routine blood tests. The aim of the present study was to determine the relationships between platelet parameters and liver pathology, and whether these could provide a simple method for the assessment of liver damage in patients with CHB.
Patients and methods
Study population
The study retrospectively recruited patients with liver biopsy-confirmed CHB who attended the Department of Infectious Diseases, The First Affiliated Hospital of Xiamen University, Xiamen, China, between August 2007 and August 2014. Patients were required to have undergone blood tests both before and after biopsy. Diagnosis of CHB was made using the criteria of the Chinese Hematology Society: (i) blood HBsAg positive for > 6 months; (ii) serum HBV DNA positive; (iii) elevated serum ALT for > 3 months).8 Exclusion criteria were: (i) blood tests not performed within a week before and after biopsy; (ii) presence of other hepatitis-causing viral infections; (iii) non-HBV hepatitis (alcohol or autoimmune induced hepatitis); (iv) the presence of any disease that affects platelet count (aplastic anaemia, purpura haemorrhagica, primary thrombocytosis); (v) use of any medication that could affect platelet parameters during the previous 3 months.
The study was approved by the ethics committee of the First Affiliated Hospital of Xiamen University. As part of routine clinical procedure, written informed contest was acquired from each patient before liver biopsy.
Platelet parameters
Platelet parameters were evaluated using an automated haematology analyser (LHX750XE-2100; Beckman Coulter, Brea, CA, USA). Parameters included platelet count (PLT; normal range 125–350 × 109/l), platelet distribution width (PDW; normal range 9–17%) and mean platelet volume (MPV; normal range 7.66–13.26 × 10−15/l).
Liver histopathology
Liver biopsy was performed under local anaesthesia using 2% lidocaine, and samples were obtained using a Magnum 16 G disposable core tissue biopsy needle (MN1610; Bard, Dublin, Ireland) with a spring-loaded reusable instrument (Magnum reusable core biopsy instrument; Bard). Tissue (diameter 0.1 cm, length 1.5–2.0 cm) was stored in formalin prior to histopathological evaluation.
All liver specimens were assessed by the Department of Pathology, The First Affiliated Hospital of Xiamen University. After being dehydrated and embedded in paraffin, 4 -µm sections were cut using a microtome. Sections were stained with haemotoxylin and eosin, Masson’s trichrome and Masson–Fontana method for argentaffin cells, and inflammation and fibrosis were evaluated via light microscopy. Inflammation was graded from G0 (no inflammation) to G4 (severe interface hepatitis), and fibrosis was staged from S0 (no fibrosis) to S4 (early cirrhosis).8
Statistical analyses
Data were presented as mean ± SD. Between group comparisons were made using independent sample t-test. Correlations were evaluated using Spearman test, with multiple linear regression analysis used to determine the relationships between platelet parameters and degree of inflammation and fibrosis. Receiver operating characteristic (ROC) curves were generated for the use of platelet parameters in the diagnosis of liver inflammation and fibrosis. All data were analysed using SPSS® version 19.0 (SPSS Inc., Chicago, IL, USA) for Windows®, and EViews® version 7.0 (IHS Global Inc., Irvine, CA, USA). P-values < 0.05 were considered statistically significant.
Results
The study included 677 patients (520 male/157 female, mean age 32.0 ± 9.5 years; age range 9 – 70 years). There were no significant between-gender differences in severity of inflammation (males 2.59 ± 0.82; females 2.46 ± 0.83) or fibrosis (males 1.93 ± 1.03; females 1.82 ± 0.92). There were no significant between time point (before vs after biopsy) differences in any platelet parameter (data not shown).
There were significant negative correlations between PLT and MPV (r −0.280, P < 0.001), PLT and age (r −0.174, P < 0.001), and MPV and PDW (r −0.396, P < 0.001). There were no significant correlations between PDW and PLT, PDW and age, or MPV and age.
Regression analysis with degree of liver inflammation as the dependent variable and PLT, PDW, MPV and age as independent variables found that PLT and PDW were significantly negatively related to degree of liver inflammation (P < 0.05 for each relationship), together accounting for 20.5% of variation in the severity of inflammation. MPV and age were not related to the degree of liver inflammation (Table 1).
Table 1.
Multiple regression analysis of the diagnostic value of platelet parameters for grade of liver inflammation in Chinese patients with chronic hepatitis B virus infection (n = 677).
| Parameter | R2 | P-value | NSRC |
SRC |
T | P-value | |
|---|---|---|---|---|---|---|---|
| Coefficient B | SE | Coefficient B | |||||
| Intercept | 5.586 | 0.246 | |||||
| PLT | 0.108 | <0.001 | −0.006 | 0.001 | −0.341 | −9.932 | <0.001 |
| PDW | 0.205 | <0.001 | −0.126 | 0.014 | −0.312 | −9.075 | <0.001 |
| MPV | 0.207 | 0.294 | 0.022 | 0.020 | 0.042 | 1.078 | 0.281 |
| Age | 0.210 | 0.069 | 0.006 | 0.003 | 0.064 | 1.882 | 0.069 |
SRC, standardized regression coefficient; NSRC, nonstandardized regression coefficient; SE, standard error PLT, platelet count; MPV, mean platelet volume; PDW, platelet distribution width.
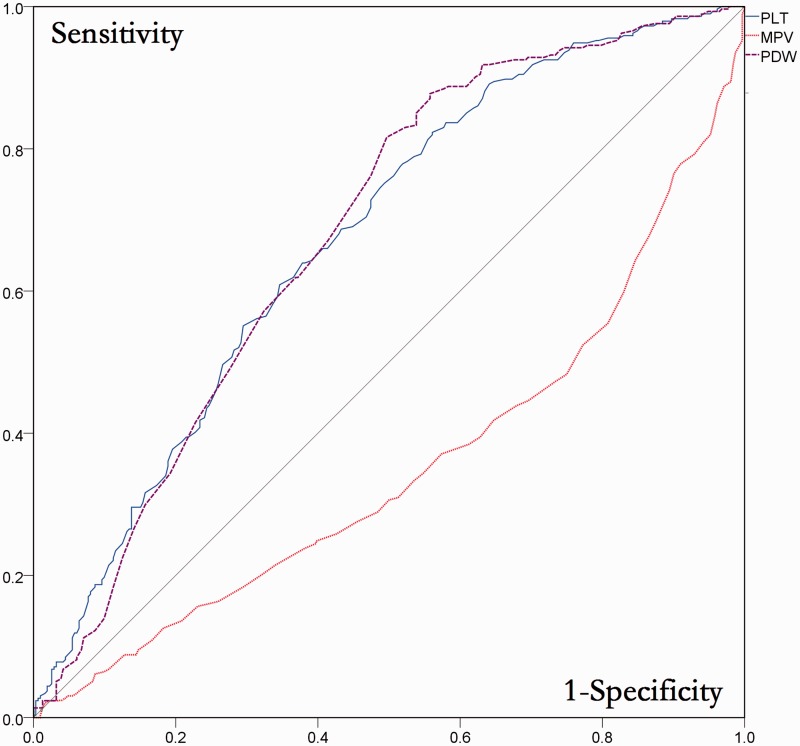
Patients were stratified according to degree of inflammation (none/mild vs moderate/severe) for ROC analysis. The area under the ROC curve (AUC) indicated that PLT (AUC 0.662, 95% confidence interval [CI] 0.621, 0.703) and PDW (AUC 0.666, 95% CI 0.624, 0.707) but not MPV (AUC 0.344, 95% CI 0. 303, 0.385) had predictive value for grade of liver inflammation (Figure 1).
Figure 1.
Receiver operating characteristic analysis of the diagnostic value of platelet parameters for grade of liver inflammation in Chinese patients with chronic hepatitis B virus infection (n = 677). PLT, platelet count; MPV, mean platelet volume; PDW, platelet distribution width.
Regression analysis with stage of liver fibrosis as dependent variable and PLT, PDW, MPV and age as independent variables showed that age was significantly positively related to stage of fibrosis, and PLT and PDW were significantly negatively related to stage of fibrosis (P < 0.05 for each relationship; Table 2). PLT and PDW together accounted for 18.4% of variation in fibrosis grade. There was no relationship between MPV and fibrosis grade.
Table 2.
Multiple regression analysis of the diagnostic value of platelet parameters for stage of liver fibrosis in Chinese patients with chronic hepatitis B virus infection (n = 677).
| Parameter | R2 | P-value | NSRC |
SRC |
T | P-value | |
|---|---|---|---|---|---|---|---|
| Coefficient B | SE | Coefficient B | |||||
| Intercept | 4.208 | 0.345 | |||||
| PLT | 0.136 | <0.001 | −0.007 | 0.001 | −0.351 | −9.866 | <0.001 |
| PDW | 0.170 | <0.001 | −0.088 | 0.017 | −0.180 | −5.155 | <0.001 |
| Age | 0.184 | 0.001 | 0.013 | 0.004 | 0.122 | 3.417 | 0.001 |
| MPV | 0.188 | 0.094 | 0.043 | 0.025 | 0.066 | 1.679 | 0.094 |
SRC, standardized regression coefficient; NSRC, nonstandardized regression coefficient; SE, standard error PLT, platelet count; MPV, mean platelet volume; PDW, platelet distribution width.
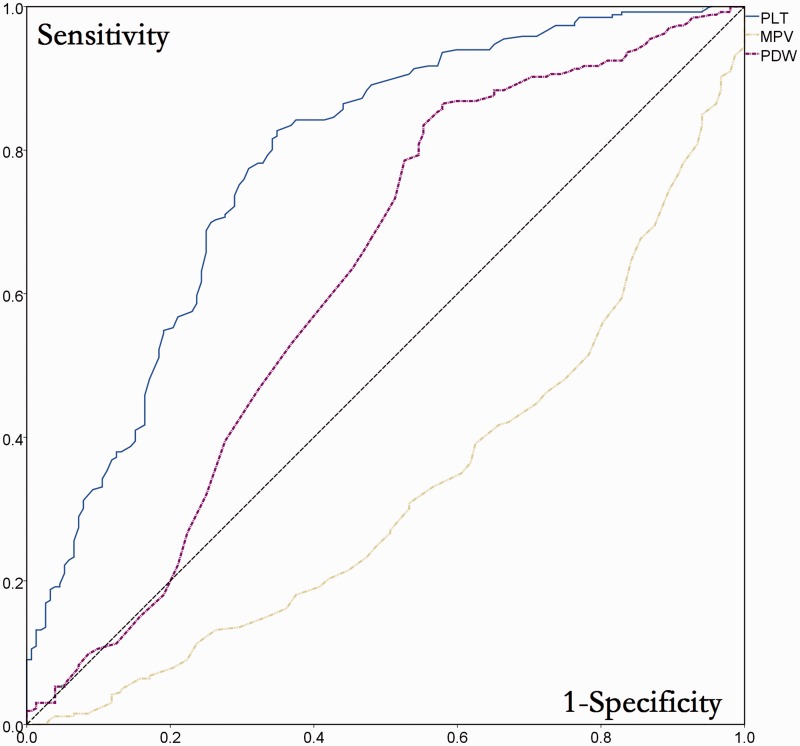
Patients were stratified according to grade of fibrosis (grade 0/1/2 vs grade 3/4) for ROC analysis. The AUC indicated that PLT (AUC 0.656, 95% CI 0.615, 0.696) and PDW (AUC 0.591, 95% CI 0.549, 0.633), but not MPV (AUC 0.397, 95% CI 0.354, 0.440) had predictive value for stage of liver fibrosis (Figure 2).
Figure 2.
Receiver operating characteristic analysis of the diagnostic value of platelet parameters for stage of liver fibrosis in Chinese patients with chronic hepatitis B virus infection (n = 677). PLT, platelet count; MPV, mean platelet volume; PDW, platelet distribution width.
Discussion
China has a high prevalence of CHB, and the city of Xiamen, where this study was carried out, has one of the highest CHB rates within China.9 Studying the diagnostic and treatment of CHB is extremely important for both medical and socioeconomical reasons. The 2010 chronic hepatitis B control guidelines8 state that the general purpose of CHB treatment is to maximally inhibit the viability of HBV, alleviate liver cell putrescence, inflammation and fibrosis, and to postpone and prevent liver cirrhosis, liver decompensation, liver carcinoma and other complications. Anticipating and treating early stage liver inflammation and fibrosis is vital to achieve this goal. However, the invasive nature of liver biopsy means that it cannot be used frequently enough to guide treatment. A noninvasive test using a small amount of peripheral blood to achieve a similar diagnostic accuracy would have tremendous clinical value. We investigated the relationship between peripheral blood platelet parameters and liver inflammation and fibrosis in the present study, in order to provide a simple and noninvasive method to guide treatment for patients with CHB.
The aetiology of chronic hepatic inflammation following HBV infection is not clear, although regulatory T cells are known to play an important role.9,10 Both PLT and PDW were significantly related to liver inflammation in the present study. Although these parameters accounted for only 20.5% of inflammation, when considering the complicated mechanisms of CHB development, this level of predictive value from a single routine blood test is satisfactory.
There are several noninvasive tests used to stage liver fibrosis, including serum aspartate aminotransferase to platelet ratio index (APRI),11 FibroTest (BioPredictive, Paris, France),12 Forns index13 and Fibro Index,14 all of which include platelet count. The aetiology of platelet count changes in patients with liver fibrosis is complex, but may include (i) portal hypertension leading to hypersplenism in patients with cirrhosis, resulting in the production of platelet antibodies and immunoglobulins; (ii) the presence of a platelet storage pool in patients with cirrhosis, which lowers the circulating platelet count; and (iii) HBV virus could affect bone marrow megakaryocytes and cause thrombocytopenia. In addition, liver fibrosis affects the production of thrombopoietin, but studies have found no significant relationships between platelet count and thrombopoietin concentrations.15,16
In accordance with the findings of others,17–21 PLT was negatively related to fibrosis stage in the present study. Our finding that PDW was negatively related to the stage of fibrosis is in contrast to others who have reported a positive relationship between these parameters,22–25 indicating a need for further studies. Overall, platelet parameters accounted for 18.4% of fibrosis stage in the present study, a satisfactory predictive value from a single noninvasive test.
The present findings are limited by the retrospective study design. In addition, all of the patients enrolled in this study were in the compensated stage of CHB, because it may be hazardous to perform liver biopsies in patients with decompensated cirrhosis. It is likely that our findings cannot are not applicable to patients with decompensated cirrhosis. CHB is a complex condition, and a combination of multiple should be used to predict the severity of liver inflammation and fibrosis.
In conclusion, platelet parameters can provide valuable information for the assessment of hepatic inflammation and fibrosis. They can be applied as alternative and complimentary methods for the prediction of hepatic pathology.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Yan YP, Su HX, Ji ZH, et al. Epidemiology of hepatitis B virus infection in China: current status and challenges. J Clin Transl Hepatol 2014; 2: 15–22. [DOI] [PMC free article] [PubMed]
- 2.Lavanchy D. Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention. J Clin Virol 2005; 34(Suppl 1): S1–S3. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Zhang H, Elizabeth A, et al. Epidemiology of hepatitis B and associated liver diseases in china. Chinese Medical Sciences Journal 2012; 27: 243–248. [DOI] [PubMed] [Google Scholar]
- 4.Yuen MF, Hui CK, Cheng CC, et al. Long-term follow-up of interferon alfa treatment in Chinese patients with chronic hepatitis B infection: The effect on hepatitis B e antigen seroconversion and the development of cirrhosis-related complications. Hepatology 2001; 34: 139–145. [DOI] [PubMed] [Google Scholar]
- 5.Zeremski M, Dimova RB, Benjamin S, et al. FibroSURE as a noninvasive marker of liver fibrosis and inflammation in chronic hepatitis B. BMC Gastroenterol 2014; 14: 118–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernaez R, Yeh HC, Lazo M, et al. Elevated ALT and GGT predict all-cause mortality and hepatocellular carcinoma in Taiwanese male: a case-cohort study. Hepatol Int 2013; 7: 1040–1049. [DOI] [PubMed] [Google Scholar]
- 7.Yin Z, Chen Y. Prognostic value of Gc-globulin in Chinese patients with acute-on-chronic hepatitis B liver failure. J Coll Physicians Surg Pak 2015; 25: 176–180. [PubMed] [Google Scholar]
- 8.Jia J. Hepatitis B in China: from guideline to practice. Virologica Sinica 2008; 23: 152–155. [Google Scholar]
- 9.Chen Q, Huang Y. Analysis on Virus Hepatitis Epidemic Situation in Tongan from 1992 to 2003. 2005.
- 10.Stoop JN, van der Molen RG, Baan CC, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005; 41: 771–778. [DOI] [PubMed] [Google Scholar]
- 11.Teshale E, Lu M, Rupp LB, et al. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). J Viral Hepat 2014; 21: 917–920. [DOI] [PubMed] [Google Scholar]
- 12.Castera L, Winnock M, Pambrun E, et al. Comparison of transient elastography (FibroScan), FibroTest, APRI and two algorithms combining these non-invasive tests for liver fibrosis staging in HIV/HCV coinfected patients: ANRS CO13 HEPAVIH and FIBROSTIC collaboration. HIV Med 2014; 15: 30–39. [DOI] [PubMed] [Google Scholar]
- 13.Alboraie M, Khairy M, Elsharkawy M, et al. Value of Egy-Score in diagnosis of significant, advanced hepatic fibrosis and cirrhosis compared to aspartate aminotransferase-to-platelet ratio index, FIB4 and Forns’ index in chronic hepatitis C virus. Hepatol Res 2015; 45: 560–570. [DOI] [PubMed] [Google Scholar]
- 14.Takaki S, Kawakami Y, Miyaki D, et al. Non-invasive liver fibrosis score calculated by combination of virtual touch tissue quantification and serum liver functional tests in chronic hepatitis C patients. Hepatol Res 2014; 44: 280–287. [DOI] [PubMed] [Google Scholar]
- 15.Cerutti A, Custodi P, Duranti M, et al. Thrombopoietin levels in patients with primary and reactive thrombocytosis. Br J Haematol 1997; 99: 281–284. [DOI] [PubMed] [Google Scholar]
- 16.Aledort LM, Hayward CP, Chen MG, et al. Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol 2004; 76: 205–213. [DOI] [PubMed] [Google Scholar]
- 17.Karagoz E, Ulcay A, Tanoglu A, et al. Clinical usefulness of mean platelet volume and red blood cell distribution width to platelet ratio for predicting the severity of hepatic fibrosis in chronic hepatitis B virus patients. Eur J Gastroenterol Hepatol 2014; 26: 1320–1324. [DOI] [PubMed] [Google Scholar]
- 18.Ekiz F, Yüksel O, Koçak E, et al. Mean platelet volume as a fibrosis marker in patients with chronic hepatitis B. J Clin Lab Anal 2011; 25: 162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceylan B, Fincanci M, Yardimci C, et al. Can mean platelet volume determine the severity of liver fibrosis or inflammation in patients with chronic hepatitis B? Eur J Gastroenterol Hepatol 2013; 25: 606–612. [DOI] [PubMed] [Google Scholar]
- 20.Han L, Han T, Nie C, et al. Elevated mean platelet volume is associated with poor short-term outcomes in hepatitis B virus-related acute-on-chronic liver failure patients. Clin Res Hepatol Gastroenterol 2015; 39: 331–339. [DOI] [PubMed] [Google Scholar]
- 21.Ceylan B, Fincanci M, Yardimci C, et al. Mean platelet volume in chronic viral hepatitis. Eur J Gastroenterol Hepatol 2014; 26: 240–241. [DOI] [PubMed] [Google Scholar]
- 22.Karagoz E, Ulcay A, Tanoglu A, et al. Clinical usefulness of mean platelet volume and red blood cell distribution width to platelet ratio for predicting the severity of hepatic fibrosis in chronic hepatitis B virus patients. Eur J Gastroenterol Hepatol 2014; 26: 1320–1324. [DOI] [PubMed] [Google Scholar]
- 23.Chen B, Ye B, Zhang J, et al. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS One 2013; 8: e68780–e68780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceylan B, Mete B, Fincanci M, et al. A new model using platelet indices to predict liver fibrosis in patients with chronic hepatitis B infection. Wien Klin Wochenschr 2013; 125: 453–460. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Xie G, Zhao A, et al. Serum bile acids are associated with pathological progression of hepatitis B-induced cirrhosis. J Proteome Res 2016; 15: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]