Abstract
Objective
To investigate the possible association between plasma proprotein convertase subtilisin/kexin type 9 (PCSK9) and the incidence and severity of calcific aortic valve disease (CAVD).
Methods
This prospective, cross sectional study involved patients with and without (controls) aortic valve calcification diagnosed by transthoracic echocardiography and dual source computed tomography (DSCT) scan. Aortic valves calcification scores were calculated from DSCT scans and patients were graded: grade 1, no calcification; grade 2, mildly calcified; grade 3, moderately calcified; grade 4, heavily calcified. Plasma PCSK9 levels were measured using an enzyme-linked immunosorbent assay.
Results
Forty patients were grade 1 (controls), 32 were grade 2, 48 were grade 3 and 32 were grade 4. Plasma levels of PCSK9 were significantly different between the four groups and the highest value was observed in the patients with grade 2 calcification. Only low-density lipoprotein cholesterol and lipoprotein (Lp)(a) were associated with the severity of CAVD. Regression analysis showed that age, Lp(a) and PCSK9 were independent predictors of CAVD.
Conclusion
Data from this cross sectional study in a small sample of patients showed that plasma PCSK9 was correlated with the presence of CAVD but not its severity.
Keywords: Calcific aortic valve disease, proprotein convertase subtilisin/kexin type 9, lipoprotein (a), LDL cholesterol, dual-source computed tomography
Introduction
Calcific aortic valve disease (CAVD) is associated with increased risk of major cardiovascular disease events and is the most common cardiac valvular disorder in the elderly.1 Aortic valve calcification, which has long been considered a passive degenerative process, is the most frequent aetiological factor of aortic stenosis in western countries.1 However, recent data have challenged this concept and CAVD is now believed to be a complex process. Atherogenesis and inflammation are thought to trigger biologically active and progressive processes that result in aortic valve calcification.2 The early stages of aortic valve degeneration are similar to atherothrombotic coronary disease and large longitudinal studies have shown that factors such as smoking, age, hypertension and hypercholesterolaemia are closely related to the incidence of CAVD.3
The pathological mechanisms involved in the development of CAVD are not fully understood and a fundamental question is what drives the calcific remodelling of the aortic valve. Retention of lipids, oxidized lipid species, and lipoprotein (Lp)(a) have been shown to be associated with CAVD.3 Mutations in the gene, proprotein convertase subtilisin/kexin type 9 (PCSK9), have been associated with familial hypercholesterolaemia.4 By binding to the epidermal growth factor-like repeat A domain of the low-density lipoprotein (LDL) receptor, PCSK9 promotes its subsequent degradation.4 The inhibition of PCSK9 reduces a number of atherogenic lipoproteins, including apolipoprotein B (Apo B), LDL cholesterol (LDL-C), non high-density lipoprotein cholesterol (non-HDL-C) and Lp(a).4 To our knowledge, the possible association between PCSK9 and the process of aortic valve calcification has not previously been investigated. Therefore, the purpose of this current study was to investigate the correlation between circulating PCSK9 levels and the incidence and severity of CAVD.
Patients and methods
Study population
For this cross sectional study, patients with and without (controls) aortic valve calcification were prospectively enrolled at the Tianjin Chest Hospital between March 2015 and December 2015. Patients had initially attended the clinic because of chest pain or chest tightness and those included were aged ≥ 60 years and had been evaluated by transthoracic echocardiography and dual-source computed tomography (DSCT) within the previous 2 months. Exclusion criteria were: use of statins; confirmed coronary artery disease; bicuspid aortic valve; severe mitral valve stenosis (i.e. valve area less than 1 cm2); mitral valve calcification; severe mitral or aortic regurgitation; rheumatic valvular heart disease; cardiac failure; a planned aortic valve replacement; infectious disease; moderate-to-severe renal dysfunction; current or chronic history of hepatic illness; a history of alcohol or drug addiction.
The study was approved by the Ethical Review Board of Tianjin Chest Hospital (approval no. 2015KY-006-01) and written informed consent was obtained from all study participants. The study adhered strictly to the Declaration of Helsinki principle 2008 and was registered at http://www.chictr.org.cn/ (ChiCTR-COC-15006428).
Echocardiographic assessment
At study entry, all eligible patients had a two-dimensional echocardiogram (Somatom Definition Flash; Siemens Medical Solutions, Forchheim, Germany) performed in the left lateral decubitus position. Images were obtained using a S5-1 transducer and reviewed by two experienced observers (X.G. and C.L.) blinded to the other laboratory results. According to results from the transthoracic echocardiogram, patients were divided into those with and without CAVD. Aortic valve calcification was defined as focal areas of increased echogenicity and aortic valve leaflets thickness > 2 mm in the absence of aortic stenosis (i.e. velocity across the valve less ≤ 2.5 m/sec).5 Left ventricular ejection fraction was calculated using the biplane Simpson’s rule.6 Aortic valve morphology was evaluated at the parasternal short axis view.
DSCT scan assessment
Aortic valve images were also obtained using a DSCT system (Somatom Definition Flash; Siemens Medical Solutions) within 2 months of study entry. Aortic valves were scanned (detector collimation 2 × 32 × 0.75 mm, gantry rotation time 330 ms, tube voltage 100–120 kV, and tube current 320mA). The slice thickness of reconstruction was 0.75 mm and reconstruction interval was 0.5 mm. A remote workstation (VE36A, Syngo MultiModality Workplace; Siemens Medical Solutions) was used for post processing and image analysis. The CT scans were interpreted by two experienced medical radiologists (H.Z. and Y.M.) who were blinded to the echocardiographic and clinical data. The double oblique transverse view of the aortic valve was obtained by the correct orientation of the reconstructed coronal and single oblique sagittal view through the aortic valve.
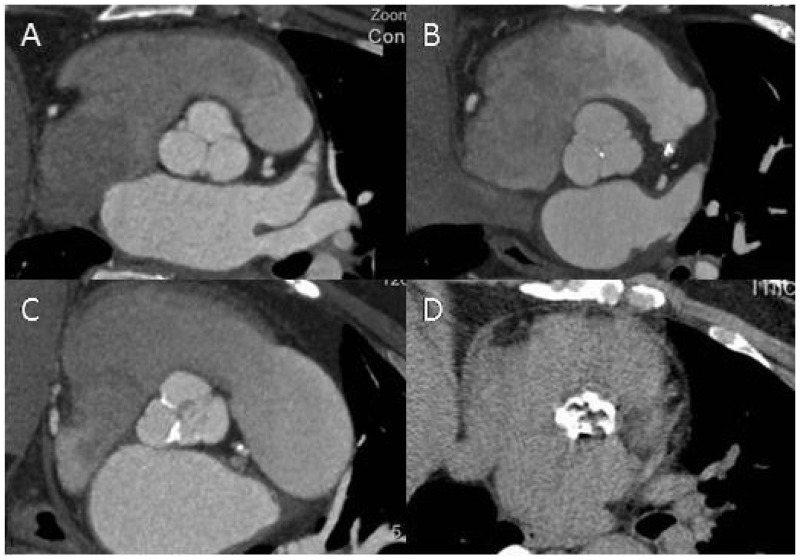
The presence of aortic valve calcification was defined as any calcified pathological changes detected in the aortic valve leaflet area or extending to the root of the aorta. Mean phantom adjusted calcification scores were used. The calcification score of each lesion was acquired by multiplying lesion area by a density factor that was derived from maximal Hounsfield units (HUs), as reported previously.7 A plaque of at least three contiguous pixels with a density of more than 130 HUs was termed as calcification lesion. A total calcification score was created by summing lesion scores of each calcification lesion. Aortic valve calcification was further assessed by DSCT quantitative scoring system:8 grade 1, no calcification (i.e. controls); grade 2, mildly calcified (i.e. small isolated calcification spots in the aortic valve); grade 3, moderately calcified (i.e. multiple larger spots); grade 4, heavily calcified (i.e. extensive calcification of all aortic cusps) (Figure 1).
Figure 1.
Assessment of aortic valve calcification by dual source computed tomography. The degree of aortic valve calcification was graded as follows: grade 1, no calcification (a); grade 2, mildly calcified, small isolated spots (b); grade 3, moderately calcified, multiple larger spots (c); grade 4, heavily calcified, extensive calcification on all cusps (d).
Laboratory analyses
Fasting venous blood samples were taken at study entry and collected in pre-cooled ethylenediaminetetra-acetic acid tubes after centrifugation at 1000 g for 15 min at 4℃ within 30 min of collection; all plasma aliquots were stored at −80℃. Total cholesterol (TC), LDL-C, HDL cholesterol (HDL-C), triglycerides (TG), ApoA1 and B, Lp(a), fasting glucose and high sensitivity C-reactive protein (hs-CRP) were assessed using commercial kits (Roche Diagnostics, Mannheim, Germany) and a Roche c701 analyser. Plasma PCSK9 levels were measured using an enzyme-linked immunosorbent assay (ELISA) with a commercial PCSK9 ELISA Kit (Human Proprotein Convertase 9/PCSK9 Quantikine ELISA Kit; R&D Systems, Abingdon, UK).
Statistical analyses
Following results from a pilot study assessing PCSK9 levels, the statistical power was set as 0.9, the type I error rate as 1% and estimated the minimum sample size to be 30 patients in each group. All data were analysed using the SPSS® statistical package, version 20.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Continuous variables were presented as mean ± SD or interquartile range in cases of non-Gaussian distribution and categorical variables were presented by frequencies and percentages. Analysis of variance using the Bonferroni correction for multiple comparisons was used for continuous variables and the χ2 test was used in the comparison of categorical variables. Data that were not normally distributed (i.e. TG, Lp(a), hs-CRP) were transformed logarithmically. To characterize the relationship between aortic valve calcification score and laboratory parameters, Pearson’s correlation coefficients (r) were calculated. Independent risk factors of CAVD were analysed using binary logistic regression. A P value < 0.05 was considered to indicate statistical significance.
Results
This study enrolled 152 patients and their clinical characteristics are shown in Table 1. According to the DSCT scan, 40 patients were grade 1 (controls), 32 were grade 2, 48 were grade 3 and 32 were grade 4. Overall, 86 of 152 (56.6%) of the population was male, 74 of 152 (48.7%) had hypertension, 64 of 152 (42.1%) were smokers and 43 of 152 (28.3%) had diabetes mellitus. No significant differences between the four groups were found in the proportion of males, patients who smoked, had hypertension or diabetes mellitus. There were also no significant differences between groups in terms of medication usage, left ventricular ejection fraction (LVEF) and left ventricular end diastolic diameter (LVDD). There were significant differences between the four groups in age (P < 0.05) with grades 2, 3 and 4 patients being slightly older than controls and in aortic valve calcification scores (P < 0.01).
Table 1.
Clinical characteristics of the 152 patients involved in the study to investigate the correlation between plasma proprotein convertase subtilisin/kexin type 9 levels and the incidence and severity of calcific aortic valve disease. Patients were graded according to results from a dual source computed tomography (DSCT) scan.
| Clinical characteristic | Grade of aortic valve calcification |
Statistical significancea | |||
|---|---|---|---|---|---|
| Grade 1 (controls) n = 40 | Grade 2 n = 32 | Grade 3 n = 48 | Grade 4 n = 32 | ||
| Age, years | 67.8 ± 4.9 | 70.4 ± 5.0 | 70.0 ± 6.3 | 72.2 ± 4.7 | P < 0.05 |
| Male | 18 (45.0) | 21 (65.6) | 31 (64.6) | 16 (50.0) | NS |
| Smoker | 17 (42.5) | 14 (43.8) | 18 (37.5) | 15 (46.9) | NS |
| Hypertension | 15 (37.5) | 14 (43.8) | 25 (52.1) | 20 (62.5) | NS |
| Diabetes mellitus | 9 (22.5) | 8 (25.0) | 11 (22.9) | 15 (46.9) | NS |
| Medication use | |||||
| ACEI/ARB | 8 (20.0) | 14 (43.8) | 15 (31.3) | 12 (37.5) | NS |
| β blocker | 5 (12.5) | 9 (28.1) | 7 (14.6) | 5 (15.6) | NS |
| CCB | 6 (15.0) | 9 (28.1) | 10 (20.8) | 5 (15.6) | NS |
| Diuretic | 5 (12.5) | 5 (15.6) | 6 (12.5) | 7 (21.9) | NS |
| Aspirin | 10 (25.0) | 10 (31.3) | 13 (27.1) | 7 (21.9) | NS |
| Echocardiography parameters | |||||
| LVDD, mm | 50.7 ± 3.0 | 51.0 ± 2.2 | 51.6 ± 2.4 | 51.9 ± 3.1 | NS |
| LVEF, % | 61.2 ± 5.1 | 61.0 ± 4.2 | 60.3 ± 4.7 | 58.3 ± 6.3 | NS |
| DSCT parameters | |||||
| AVC score | 0 | 42.6–63.4 | 140.6–182.2 | 324.7–572.0 | P < 0.01 |
Values are shown as mean ± SD, n (%) or interquartile range.
Analysis of variance using the Bonferroni correction for multiple comparisons was used for continuous variables and the χ2 test was used in the comparison of categorical variables.
Grade 1, no calcification (controls); grade 2, mildly calcified; grade 3, moderately calcified; grade 4, heavily calcified.
ACEI/ARB, angiotensin-converting enzyme inhibitors or angiotensin receptor blocker; CCB, calcium channel blockers; LVDD, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; AVC, aortic valve calcification; NS, no significant between-group difference (P ≥ 0.05).
Among the laboratory variables, plasma levels of TC, LDL-C, Apo B and Lp(a) were significantly higher in the grade 2, 3 and 4 groups compared with the control (grade 1) group (P < 0.05 for all comparisons; Table 2). There were no statistically significant differences between groups in plasma levels of TG, HDL-C, Apo A1, hs-CRP and glucose. Plasma levels of PCSK9 were significantly different between the four groups and the highest value was observed in patients with grade 2 aortic valve calcification (P < 0.01).
Table 2.
Comparison of selected laboratory variables among the 152 patients involved in the study to investigate the correlation between plasma proprotein convertase subtilisin/kexin type 9 (PCSK9) levels and the incidence and severity of calcific aortic valve disease.
| Laboratory variable | Grade of aortic valve calcification |
Statistical significance | |||
|---|---|---|---|---|---|
| Grade 1 (controls) n = 40 | Grade 2 n = 32 | Grade 3 n = 48 | Grade 4 n = 32 | ||
| TC, mmol/l | 4.48 ± 0.45 | 4.92 ± 0.68† | 5.15 ± 0.69‡ | 5.06 ± 0.53§ | P < 0.01 |
| HDL-C, mmol/l | 1.10 ± 0.14 | 1.08 ± 0.18 | 1.12 ± 0.20 | 1.02 ± 0.15 | NS |
| LDL-C, mmol/l | 2.91 ± 0.47 | 3.44 ± 0.60† | 3.44 ± 0.63‡ | 3.65 ± 0.44§ | P < 0.01 |
| Apo B, g/l | 0.86 ± 0.22 | 0.97 ± 0.25† | 1.05 ± 0.26‡ | 1.06 ± 0.21§ | P < 0.05 |
| TG, mmol/l* | 0.10 ± 0.22 | 0.18 ± 0.18 | 0.15 ± 0.17 | 0.19 ± 0.17§ | NS |
| Apo A1, g/l | 1.09 ± 0.25 | 1.02 ± 0.20 | 1.05 ± 0.23 | 0.96 ± 0.27 | NS |
| Lp(a), nmol/l* | 1.21 ± 0.30 | 1.41 ± 0.32† | 1.61 ± 0.34‡ | 1.63 ± 0.38§ | P < 0.01 |
| hs-CRP, mg/l* | 0.23 ± 0.37 | 0.25 ± 0.36 | 0.32 ± 0.33 | 0.39 ± 0.32 | NS |
| PCSK9, ng/ml | 260.2 ± 40.7 | 352.4 ± 59.3† | 334.0 ± 66.4‡ | 312.4 ± 66.4§ | P < 0.01 |
| Glucose, mmol/l | 5.48 ± 0.88 | 5.29 ± 0.94 | 5.31 ± 0.94 | 5.44 ± 0.96 | NS |
Data are expressed as mean ± SD.
Grade 1, no calcification (controls); grade 2, mildly calcified; grade 3, moderately calcified; grade 4, heavily calcified.
Log transformed data.
TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Apo, apolipoprotein; TG, triglyceride; Lp(a), lipoprotein(a); hs-CRP, high sensitivity C-Reactive Protein; NS, no significant between-group difference (P ≥ 0.05).
Grade 2 compared with grade 1, P < 0.05; ‡grade 3 compared with grade 1, P < 0.05; §grade 4 compared with grade 1, P < 0.05; analysis of variance using the Bonferroni correction for multiple comparisons was used for continuous variables.
Across all patients, aortic valve calcification scores evaluated by DSCT were statistically significantly correlated with TC (P < 0.01), LDL-C (P < 0.01), Apo B (P < 0.01), Lp(a) (P < 0.01) and PCSK9 (P < 0.01) (Table 3). In patients with CAVD (n = 112), only LDL-C and Lp(a) were significantly correlated with aortic valve calcification scores (P < 0.01 for both comparisons). Plasma levels of PCSK9 in patients with CAVD were not correlated with aortic valve calcification scores.
Table 3.
Pearson’s correlation coefficient analysis of the possible correlation between aortic valve calcification scores and laboratory variables.
| Laboratory variable | All patients n = 152 | CAVD group n = 112 |
|---|---|---|
| TC | 0.296† | 0.135 |
| LDL-C | 0.395† | 0.257† |
| Apo B | 0.263† | 0.154 |
| Lp(a)* | 0.408† | 0.286† |
| PCSK9* | 0.214† | −0.010 |
| hs-CRP* | 0.150 | 0.129 |
Log transformed data.
P < 0.01.
TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; Apo B, apolipoprotein B; Lp(a), lipoprotein(a); PCSK9, proprotein convertase subtilisin/kexin type 9; hs-CRP, high sensitivity C-reactive protein.
Binary logistic regression analysis showed that of the risks factors examined, age (P = 0.017), Lp(a) (P = 0.005) and PCSK9 (P = 0.006) were independent predictors of CAVD in all patients (Table 4).
Table 4.
Binary logistic regression analysis of risk factors possibly associated with calcific aortic valve disease (n = 152).
| Variable | Odds ratio | 95% CI | Statistical significance |
|---|---|---|---|
| Age | 1.121 | 1.021, 1.231 | P = 0.017 |
| Sex | 0.926 | 0.337, 2.542 | NS |
| Diabetes mellitus | 0.996 | 0.299, 3.328 | NS |
| Hypertension | 0.498 | 0.516, 3.907 | NS |
| Smoker | 1.878 | 0.629, 5.610 | NS |
| TC | 0.419 | 0.331, 14.223 | NS |
| LDL-C | 0.798 | 0.190, 8.666 | NS |
| Lp(a) | 1.038 | 1.011, 1.064 | P = 0.005 |
| PCSK9 | 1.014 | 1.004, 1.024 | P = 0.006 |
CI, confidence interval; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; Lp(a), lipoprotein(a); PCSK9, proprotein convertase subtilisin/kexin type 9; NS, no significant difference (P ≥ 0.05).
Discussion
In this cross sectional study, plasma PCSK9 levels were significantly higher in patients with CAVD at all grades compared with control patients without CAVD. Although regression analysis showed that plasma PCSK9 was significantly associated with the presence of CAVD, no correlation was observed between plasma PCSK9 and aortic valve calcification scores in patients with CAVD. In contrast, in patients with coronary heart disease (CHD), plasma PCSK9 levels were correlated with the severity of CHD and with an increased CHD risk.9 In addition, the effects of plasma PCSK9 levels on CHD were shown to be mediated by lipid (approximately 20%) and inflammation (approximately 15%).9 We suggest that the reason for the lack of correlation between plasma PCSK9 and the severity of CAVD in the present study is because elevated plasma PCSK9 may only be part of the complex processes involved in early stage CAVD.
In agreement with previous studies,10,11 this present found that plasma levels of LDL-C, TC, Apo B and Lp(a) were higher in patients with aortic valve calcification compared with control patients without CAVD. Further analysis showed that aortic valve calcification scores were correlated with plasma TC, LDL-C, Apo B and Lp(a). However, in patients with CAVD, the severity of the condition was not correlated with TC or Apo B. Regression analysis showed that age, Lp(a) and PCSK9 were independent risk factors for CAVD. Studies have established that elevated Lp(a) is involved causally with CAVD and the requirement for aortic valve surgery.12 Furthermore, a strong association has been established between a single nucleotide polymorphism in the locus of Lp(a) and the incidence of CAVD.13 Therefore, we believe that the ability of Lp(a) lowering therapies to modify CAVD progression is an area of potential investigation.
Research has confirmed that Lp(a) catabolism is regulated by PCSK9 through the LDL receptor;14 Lp(a) internalization by primary human fibroblasts and hepatic HepG2 cells was significantly decreased by PCSK9. In addition, upregulated expression of the LDL receptor in HepG2 cells improved the internalization of Lp(a) and the process was reduced following intervention with a LDL receptor monoclonal antibody.14 Therefore, the LDL receptor is involved in Lp(a) catabolism and the course can be modified by PCSK9. However, the significance of the role of LDL receptor is controversial. Another study found that LDL receptor-deficient fibroblasts did not alter the catabolism of Lp(a).15 The Lp(a) clearance by hepatocytes appeared to depend on very low density lipoprotein receptor (VLDLR) expression. Therefore, we postulate that the possible modulation of VLDLR by PCSK9 will be of great significance. Moreover, perhaps reducing PCSK9 expression or receptor binding activity may mediate the reduction of Lp(a). Indeed, anti-PCSK9 monoclonal antibodies have been shown to lower Lp(a) by 20–40%.16
The apparent link between lipid levels and aortic valve calcification as well as the pathological similarities with atherosclerosis has led to the hypothesis that statins might be beneficial in patients with aortic stenosis. This theory has been supported by nonrandomized clinical trials17 and experiments in hypercholesterolaemic animal models.18 However, predominantly negative, even conflicting results, have been reported when statins were formally tested in three independent randomized controlled clinical trials in patients with moderate-to-severe aortic stenosis.19–21 The discrepancy in these findings may be due to the pathophysiological mechanisms underlying CAVD. For example, although lipid deposition and inflammation may be important in the initiation phase of CAVD, the later calcification and mineralization stages are characterized by a self-perpetuating cycle of calcium formation and ossification.22 Lipid-lowering medications are probably ineffective once this propagation phase is established. Therefore, the inchoate stages of CAVD, when valve stiffness and obstruction to blood flow have not yet developed, may provide us with a possible opportunity for lipid-lowering therapies to inhibit the development of CAVD.
In this present study, hs-CRP levels did not correlate with aortic valve calcification scores in all subjects nor in patients with CAVD. Therefore, CRP was not correlated with either the presence or severity of CAVD. Previous findings on the relationship between CRP and CAVD are inconsistent. In one study, CRP levels in patients with advanced symptomatic aortic sclerosis awaiting aortic valve replacement were higher than that in patients with aortic valve regurgitation.23 For those patients who underwent the valve surgery, CRP levels declined after valve replacement. In contrast, the Cardiovascular Health Study found that the early inflammatory stages of CAVD were not associated with CRP values and elevated CRP levels did not precede the development of incident calcific aortic stenosis.24 We suggest a possible explanation for these contradictory results is that CRP might be an excellent biomarker of severe aortic stenosis, but cannot predict the early stages of aortic sclerosis or CAVD.
Clinic trials of statins in CAVD patients have shown negative results19–21 and so we believe that it is necessary to identify a more targeted and non-statin lipid intervention to halt disease progression in CAVD. Certainly, PCSK9 inhibition and the resulting increase in LDL receptors probably have synergistic advantages.4,25,26 Furthermore, anti-PCSK9 monoclonal antibodies have been shown to have a significant effect on reducing LDL-C even in patients on statin treatment.16 In addition, blocking PCSK9 might be expected to increase the clearance of other atherogenic lipoproteins and may have a therapeutic effect beyond that generated by the lowering of LDL-C.27
This present study had several limitations. Firstly, cross sectional data do not provide evidence of causality. Prospective, case–control studies would be necessary to assess whether PCSK9 can indeed predict progression of aortic valve calcification to aortic stenosis. Secondly, although the analyses were adjusted for the most pertinent variables that may have affected aortic valve calcification, the possibility exists that some confounding factors may have been omitted. Finally, the present study used a small patient population and so more studies with larger populations are required to confirm these preliminary findings.
In conclusion, data from this cross sectional study involving a small group of patients showed plasma PCSK9 was correlated with the presence of CAVD but not its severity. Further case–controlled studies involving more patients are required to confirm these findings.
Acknowledgements
We would like to thank the staff of Tianjin Cardiovascular Disease Research Institute and Nankai University, especially Professors Jihong Han and Yajun Duan for their excellent technical assistance. We would also like to thank the staff of the Department of Cardiology, Tianjin Chest Hospital for their help in patient recruitment.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the Tianjin Municipal Science and Technology Commission of China (grant number: 12ZCZDSY03200) and China Postdoctoral Science Foundation (grant number: 2015M581308).
References
- 1.Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013; 62: 1002–1012. [DOI] [PubMed] [Google Scholar]
- 2.Rajamannan NM, Evans FJ, Aikawa E, et al. Calcific aortic valve disease: not simply a degenerative process. A review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Executive summary: calcific aortic valve disease-2011 update. Circulation 2011; 124: 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thanassoulis G, Massaro JM, Cury R, et al. Associations of long-term and early adult atherosclerosis risk factors with aortic and mitral valve calcium. J Am Coll Cardiol 2010; 55: 2491–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKenney JM. Understanding PCSK9 and anti-PCSK9 therapies. J Clin Lipidol 2015; 9: 170–186. [DOI] [PubMed] [Google Scholar]
- 5.Gotoh T, Kuroda T, Yamasawa M, et al. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS cardiac echo and cohort study). Am J Cardiol 1995; 76: 928–932. [DOI] [PubMed] [Google Scholar]
- 6.Thavendiranathan P, Grant AD, Negishi T, et al. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol 2013; 61: 77–84. [DOI] [PubMed] [Google Scholar]
- 7.Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15: 827–832. [DOI] [PubMed] [Google Scholar]
- 8.Tops LF, Wood DA, Delgado V, et al. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging 2008; 1: 321–330. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Zhang Y, Xu RX, et al. Proprotein convertase subtilisin-kexin type 9 as a biomarker for the severity of coronary artery disease. Ann Med 2015; 47: 386–393. [DOI] [PubMed] [Google Scholar]
- 10.Parisi V, Leosco D, Ferro G, et al. The lipid theory in the pathogenesis of calcific aortic stenosis. Nutr Metab Cardiovasc Dis 2015; 25: 519–925. [DOI] [PubMed] [Google Scholar]
- 11.Smith JG, Luk K, Schulz CA, et al. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA 2014; 312: 1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capoulade R, Chan KL, Yeang C, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol 2015; 66: 1236–1246. [DOI] [PubMed] [Google Scholar]
- 13.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013; 368: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romagnuolo R, Scipione CA, Boffa MB, et al. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J Biol Chem 2015; 290: 11649–11662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reblin T, Niemeier A, Meyer N, et al. Cellular uptake of lipoprotein[a] by mouse embryonic fibroblasts via the LDL receptor and the LDL receptor-related protein. J Lipid Res 1997; 38: 2103–2110. [PubMed] [Google Scholar]
- 16.Desai NR, Kohli P, Giugliano RP, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C assessment with proprotein convertase subtilisin kexin type 9 monoclonal antibody inhibition combined with statin therapy (LAPLACE)-Thrombolysis in myocardial infarction (TIMI) 57 trial. Circulation 2013; 128: 962–969. [DOI] [PubMed] [Google Scholar]
- 17.Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 2007; 49: 554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss RM, Ohashi M, Miller JD, et al. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation 2006; 114: 2065–2069. [DOI] [PubMed] [Google Scholar]
- 19.Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid lowering therapy in calcific aortic stenosis. N Engl J Med 2005; 352: 2389–2397. [DOI] [PubMed] [Google Scholar]
- 20.Chan KL, Teo K, Dumesnil JG, et al. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010; 121: 306–314. [DOI] [PubMed] [Google Scholar]
- 21.Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008; 359: 1343–1356. [DOI] [PubMed] [Google Scholar]
- 22.New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res 2011; 108: 1381–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber IL, Stewart RA, Hammett CJ, et al. Effect of aortic valve replacement on c-reactive protein in nonrheumatic aortic stenosis. Am J Cardiol 2003; 92: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 24.Novaro GM, Katz R, Aviles RJ, et al. Clinical factors, but not C-reactive protein, predict progression of calcific aortic valve disease: the cardiovascular health study. J Am Coll Cardiol 2007; 50: 1992–1998. [DOI] [PubMed] [Google Scholar]
- 25.Giugliano RP, Sabatine MS. Are PCSK9 Inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol 2015; 65: 2638–2651. [DOI] [PubMed] [Google Scholar]
- 26.Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370: 1809–1819. [DOI] [PubMed] [Google Scholar]
- 27.Vogel RA. PCSK9 inhibition: the next statin? J Am Coll Cardiol 2012; 59: 2354–2355. [DOI] [PubMed] [Google Scholar]