Abstract
Objective
To investigate the regulation mechanism of T cell immunoglobulin and mucin domain-3 (Tim-3) combined with toll-like receptor 3 (TLR3) or TLR4 on antiviral immune and inflammatory response in patients with chronic hepatitis C virus (HCV) infection.
Methods
Patients with chronic HCV infection and healthy control subjects were recruited. Patients received interferon (IFN)-α based therapy. Plasma galectin-9 (Gal-9) was quantitated. Peripheral blood mononuclear cells (PBMCs) were cultured with TLR3 or TLR4 agonists, alone or in combination with Tim-3 antagonist. Levels of IFN-α, TNF-α, and 2′-5′ oligoadenylate synthetase (2′-5′OAS), myxovirus resistance protein A (MxA) and suppressor of cytokine 1 (SOCS1) RNA in PBMC cultures were evaluated.
Results
Plasma Gal-9 levels were increased in patients (n = 52) compared with controls (n = 20) and significantly declined at treatment week 12 and 24 weeks post-treatment. IFN-α, 2′-5′OAS, MxA, TNF-α and SOCS1 were upregulated by TLR3 and TLR4 agonists. TNF-α and SOCS1 levels were suppressed by the addition of Tim-3 antagonist.
Conclusions
Tim-3 blockade in combination with TLR activation induces the expression of antiviral molecules without a significant increase in TNF-α or SOCS1.
Keywords: Hepatitis C virus, T cell immunoglobulin and mucin domain-3, Galectin-9, toll-like receptors, peripheral blood mononuclear cells, antiviral immunological response
Introduction
Hepatitis C virus (HCV) infection affects around 185 million people worldwide. Chronic HCV infection can lead to liver fibrosis with occult progression – a leading cause of cirrhosis and liver cancer, and an indication for liver transplantation.1 Direct-acting antiviral agents (DAAs) have improved the rate of sustained virological response, but patients remain at risk of disease progression.2–3 Enhanced understanding of HCV–host interactions is required to combat this virus and to develop improved therapies.4
The activation of the sentinel toll-like receptor (TLR) system is an important triggering event in the initial immune defense, inducing both innate and adaptive immunity by recognizing pathogen-associated molecular patterns (PAMPs).5–6 TLR signaling pathways are strictly co-ordinated by several mechanisms to regulate adequate innate immune responses.7–9 Agonists can induce type I interferon (IFN) and antiviral molecules such as 2′-5′ oligoadenylate synthetase (2′-5′OAS) via the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway. Suppressor of cytokine signaling (SOCS) family proteins, originally identified as negative-feedback regulators in cytokine signaling, are known to be involved in regulation of TLR-mediated immune responses,10 and SOCS1, a negative regulator of the JAK/STAT pathway, is induced by HCV core protein.9,11,12 In addition, T cell immunoglobulin and mucin domain-3 (Tim-3), a key inhibitory receptor, is expressed on eosinophils, T lymphocytes, dendritic cells, macrophages and lymphoid cells,13 and is overexpressed on T cells in patients with HCV infection or HCV/HIV coinfection.14–17 It has been suggested that Tim-3, which is constitutively expressed by cells of the innate immune system, may synergize with the TLR system to influence a range of inflammatory conditions by promoting or terminating cell immunity.13 Tim-3 may therefore serve opposing roles, and induce distinct signaling in innate and adaptive immune cells.18 The interaction between Tim-3 and its ligand, Galectin-9 (Gal-9), can inhibit the T cell response and induce apoptosis,19–22 suggesting that the Tim-3/Gal-9 pathway may be adopted to escape immune surveillance during chronic viral infection. Blockade of the Tim-3/Gal-9 pathway might help to reinvigorate antiviral immunity and thereby improving the clinical efficacy of current immunotherapies.
Both innate and adaptive immunity have a profound impact on status of HCV infection, and impairment of host immunity may lead to chronic infection.23,24 We have previously shown that TLR3-expressing CD14+ monocyte levels are correlated with treatment response in patients with HCV infection.7 The aim of the present study was to evaluate the role of Tim-3/Gal-9 signaling in chronic HCV infection, and virological response in patients with chronic HCV infection treated with IFN-based therapies. In addition, we aimed to clarify whether TLR3/TLR4 activation and Tim-3 inhibition could induce production of antiviral molecules in human peripheral blood mononuclear cells (PBMCs), which might be beneficial for HCV clearance.
Patients and methods
Study population
Patients aged 18 to 65 years with chronic HCV infection were recruited from Third Hospital of Hebei Medical University, the Fifth Hospital of Shijiazhuang City and Bethune International Peace Hospital (Shijiazhuang, China) between December 2011 and November 2012, with follow-up concluded in May 2014. Diagnosis were made using European Association for the Study of the Liver (EASL) Clinical Practice Guidelines: Management of hepatitis C virus infection25 and the diagnostic criteria of The Guidelines for Prevention and Treatment of Hepatitis C issued by the Chinese Society of Hepatology and Chinese Society of Infectious Diseases and Parasitology of Chinese Medical Association.26 Exclusion criteria were: presence of decompensated cirrhosis; coinfection with HIV, hepatitis A, B or D virus; other causes of chronic liver disease; or comorbidities precluding interferon therapy. A group of age- and sex-matched healthy volunteers who were attending regular health screening and were free from HAV, HBV, HCV, HIV and any other cause of chronic liver disease were recruited as the control group.
The study protocol conformed to the guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of Third Hospital of Hebei Medical University, Shijiazhuang, China. All study participants provided written informed consent. All ongoing and related trials for this drug/intervention are registered (ChiCTR-TNRC-10001090).
Blood sampling
Peripheral blood (5 ml) was collected into sterile tubes containing 10.8 mg potassium–EDTA from all participants at baseline, and from patients at treatment week 12 and 24 weeks post-treatment. Blood was initially centrifuged at 1200 × g for 10 min at room temperature, then the supernatant was transferred into a new microcentrifuge tube and centrifuged at 12 000 × g for 15 min at 4℃ to remove cellular debris. The resulting plasma was stored at −80℃ until further use.
HCV detection
Plasma HCV antibodies were detected using a commercial enzyme linked immunosorbent assay (ELISA) kit (Anti-HCV Rapid Test Kit, Livzon diagnostics INC, Zhuhai, China). Plasma HCV RNA was quantified using a qualitative reverse transcriptase polymerase chain reaction (RT–PCR) kit (Cobas Taqman HCV Test, Roche Diagnostics, Indianapolis, IN, USA; lowest limit of detection 15 IU/ml) according to the manufacturer’s instructions. HCV genotypes were identified using an HCV genotyping oligochip (Tianjin Third Central Hospital, China).27
Biochemical assays
Blood (3 ml) was collecting from participants after an overnight fast, centrifuged at 1200 × g for 30 min and immediately divided into aliquots. Serum was then frozen and stored at −80℃ until use. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were quantified using an Olympus AU5400 automatic chemical analyser.
Treatment
Patients with chronic hepatitis C were treated with pegylated IFNα-2a (PegIFNα-2a; 135µg/week for body weight < 60 kg and 180µg/week for body weight ≥ 60 kg, subcutaneously; Pegasys, Roche, Basel, Switzerland) plus weight-based RBV (13–15 mg/kg per day; Zhejiang Chengyi Pharmaceutical Co., Ltd, Zhejiang, China). Treatment continued for 44 weeks after HCV RNA was undetectable. Standard definitions of responses were used. Complete early virological response (cEVR) was defined as undetectable plasma HCV RNA at week 12 during therapy, and sustained virological response (SVR) was defined as undetectable HCV RNA at 24 weeks after planned end of treatment.
PBMC culture
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood (15 ml) from 15 patients and 15 healthy control subjects using Ficoll density gradient centrifugation (Hanyang Biologicals Technology, Tianjin, China). PBMCs were plated in duplicate at 1.0 × 106 cells/well in 24-well plates and divided into five groups: control (untreated); TLR4 agonist group (1µg/ml lipopolysaccharide [LPS]); Tim-3 antagonist + TLR4 agonist group (10µg/ml anti-Tim-3 antibody [Biolegend, San Diego, CA, USA] for 30 min, supplemented with 1µg/ml LPS); TLR3 agonist group (20µg/ml polyinosinic–polycytidylic acid, [Poly(I:C)]) group; Tim-3 antagonist + TLR3 agonist group (10µg/ml anti-Tim-3 antibody for 30 min, supplemented with 20µg/ml Poly[I:C]). All cells were incubated at 37℃ with 5% carbon dioxide for 24 h in serum free medium (Botna Biological Technology, Beijing, China) containing 100µg/ml penicillin–streptomycin (North China Pharmaceutical Group Corporation, Hebei, China) and 300µg/ml l-glutamine (Bio-high Technology, Hebei, China). The supernatant was removed and used for cytokine assays, and the remaining PBMCs were used for RT–PCR for 2′-5′OAS, myxovirus resistance protein A (MxA) and SOCS1.
Cytokine assays
Plasma Gal-9 was quantified at baseline (all participants), and treatment week 12 and 24 weeks post-treatment (patients)(Human Galectin 9 ELISA kit, R&D Systems Inc. Minneapolis, MN, USA). Concentrations of IFN-α and tumor necrosis factor-α (TNF-α) in supernatants from PBMC culture were determined using ELISA kits (Human IFN-α and TNF-α ELISA kits, R&D Systems Inc.) according to the manufacturer’s instructions. Limits of detection were 0.3 pg/ml for Gal-9, 15 pg/ml for IFN-α and 8 pg/ml for TNF-α. The plates were read on a microplate reader (ELX800; Bio-Tek Instruments, Inc., Winooski, VT).
qRT–PCR
Total RNA was isolated from PBMCs using TRIzol® (Tiangen Biotech, Beijing, China), according to the manufacturer’s instructions. The mRNA concentrations of 2′-5′OAS, MxA and SOCS1 were determined by qRT–PCR using an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster, CA, USA) with SYBR Green Reagent (Tiangen Biotech). Expression levels of the target genes were normalized against the endogenous reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primer sequences (Sangon Biotech, Shanghai, China) are listed in Table 1. All data were obtained using Sequence Detector Software (Applied Biosystems).
Table 1.
Primers used for quantitative reverse transcription polymerase chain reaction.
| Gene | Product length | Primer sequences |
|---|---|---|
| 2′-5′OAS | 279 bp | Forward 5′-CTGGCGGCTATAAACCTAACC-3′ |
| Reverse 5′-GGGCTGTGTTGAAATGTGTTT-3′ | ||
| MxA | 331 bp | Forward 5′-CCACCCATATTTCAGGGATCT-3′ |
| Reverse 5′-ACTCCATTTGTGGAACTCGTG-3′ | ||
| SOCS1 | 347 bp | Forward 5′-ATGGTAGCACACAACCAGGTG-3′ |
| Reverse 5′-CTAAGGGCGAAAAAGCAGTTC-3′ | ||
| GAPDH | 87 bp | Forward 5′-GGCATGGACTGTGGTCATGAG-3′ |
| Reverse 5′-TGCACCACCAACTGCTTAGC-3′ |
2′-5′OAS, 2′-5′ oligoadenylate synthetase; MxA, myxovirus resistance protein A; SOCS1, suppressor of cytokine 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analyses
Sample size estimation was determined by PASS software (NCSS Statistical Software, Kaysville, UT, USA) based on references and prelimitary experiments. To detect a minimal 71.16 difference in plasma Gal-9 levels (between healthy controls and patients) with α = 0.05, two-tailed and a power of 80%, 11 patients per group were required (n = 22 in total). To increase the reliability of the results, 52 patients were recruited. Sample size analysis for IFN-α, 2′-5′OAS, MxA, TNF-α and SOCS1 quantification indicated 15 subjects were required per group for this part of the study.
Data were expressed as mean ± SD or median (range). Statistical analyses were performed with independent t-test, one-way analysis of variance (ANOVA) or Kruskal–Wallis H test, with the least significant difference-t (LSD-t) test or Mann–Whitney U-test for post-hoc comparison. The χ2-test was used to compare categorical data. Correlations between variables were calculated using Spearman rank order correlations. All P-values were two-tailed, and were considered significant when < 0.05. Data were analysed using SPSS® version 16.0 (SPSS Inc., Chicago, IL, USA) for Windows®.
Results
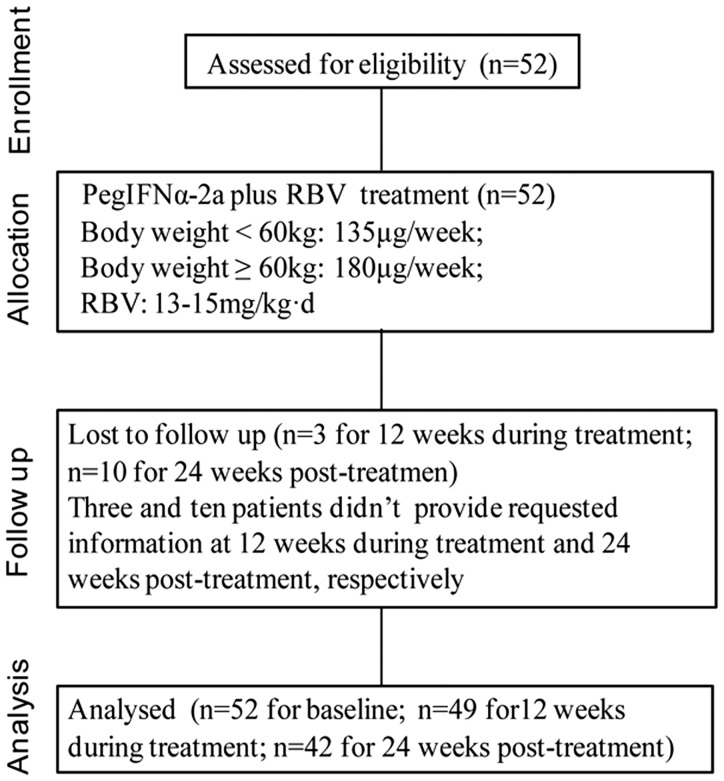
The study included 52 patients (22 males/30 females; mean age 45.1 ± 13.8 years; age range 18 – 65 years) and 20 control subjects (9 males/11 females; mean age 44.9 ± 11.7 years; age range 25 – 60 years). Demographic and clinical characteristics of the study population are given in Table 2. Serum ALT and AST were significantly higher in patients than in controls (P = 0.001 and P = 0.01, respectively; Table 1). There were no significant between group differences in gender, age, or body mass index. A flowchart indicating progression through the study is shown in Figure 1. A total of three patients were lost to follow-up at week 12 of treatment, and a total of ten patients were lost to follow-up at 24 weeks post-treatment. In total, 81.6% (40/49) of patients achieved cEVR, and 73.8% (31/42) achieved SVR. There were no statistically significant between-group differences in any baseline demographic or clinical characteristic when patients were stratified according to cEVR/non cEVR and SVR/non SVR (Table 3).
Table 2.
Baseline demographic and clinical characteristics of patients with chronic hepatitis C virus (HCV) infection and healthy control subjects included in a study investigating the role of Tim-3/Gal-9 signaling in chronic HCV infection, and virological response in patients with chronic HCV infection treated with interferon-based therapies.
| Characteristic | Control group n = 20 | Patient group n = 52 | Statistical significancea |
|---|---|---|---|
| Gender, male/female | 9/11 | 22/30 | NS |
| Age, years | 44.9 ± 11.7 | 45.1 ± 13.8 | NS |
| BMI, kg/m2 | 23.4 ± 3.3 | 22.3 ± 2.5 | NS |
| Serum ALT, IU/l | 21.1 ± 10.6 | 57.1 ± 49.2 | P = 0.001 |
| Serum AST, IU/l | 23.3 ± 9.7 | 43.6 ± 29.8 | P = 0.01 |
| HCV RNA, IU/ml | – | 9.1 × 105 (128 – 2.5 × 107) | |
| Mode of infection | |||
| Transfusion | – | 36 (69.2) | |
| Previous surgery | – | 4 (7.7) | |
| Stomatological treatment | – | 3 (5.8) | |
| Other/unknown | – | 9 (17.3) | |
| HCV genotype, 1b/2a | – | 46/6 |
Data presented as n (%), mean ± SD, or median (range).
BMI, body mass index; ALT, alanine transaminase; AST, aspartate transaminase.
Independent t-test or χ2-test.
NS, not statistically significant (P ≥ 0.05).
Figure 1.
Flow diagram of the progress through the phases of the study.
Table 3.
Baseline demographic and clinical characteristics of patients with chronic hepatitis C virus (HCV) infection, stratified according to attainment of complete early virological response (cEVR) at treatment week 12 and sustained virological response (SVR) at 24 weeks post-treatment with interferon-based therapy.
| Characteristic | Treatment week 12 |
24 weeks post-treatment |
||
|---|---|---|---|---|
| cEVR n = 40 | non-cEVR n = 9 | SVR n = 31 | non-SVR n = 11 | |
| Gender, male/female | 19/21 | 4/5 | 13/18 | 4/7 |
| Age, years | 44.5 ± 13.9 | 49.5 ± 10.0 | 46.0 ± 14.2 | 41.6 ± 10.2 |
| BMI, kg/m2 | 22.0 ± 2.30 | 23.5 ± 2.7 | 22.4 ± 2.8 | 21.7 ± 1.1 |
| Serum ALT, IU/l | 57.5 ± 49.9 | 64.8 ± 40.8 | 70.9 ± 51.5 | 46.3 ± 40.6 |
| Serum AST, IU/l | 43.2 ± 30.9 | 49.5 ± 27.9 | 51.6 ± 33.6 | 42.7 ± 24.1 |
| HCV RNA, IU/ml | 128–1.1 × 107 | 7.5 × 103 − 2.5 × 107 | 128–2.5 × 107 | (5.6 − 98.6) × 105 |
Data presented as n (ratio), mean ± SD, or range.
BMI, body mass index; ALT, alanine transaminase; AST, aspartate transaminase.
No statistically significant between-group differences (P ≥ 0.05; independent t-test or χ2-test).
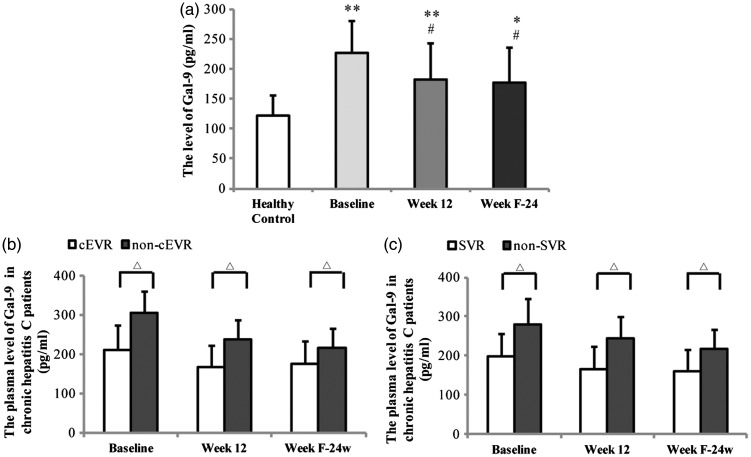
Plasma Gal-9 levels were significantly higher in patients than controls at each time point (P < 0.01 for each comparison; Figure 2(a)). In patients, Gal-9 levels were significantly lower than baseline at treatment week 12 and at 24 weeks post-treatment (P < 0.01 for each comparison; Figure 2(a)). Plasma Gal-9 levels were significantly lower in patients who achieved cEVR than those who did not, at baseline, treatment week 12, and 24 weeks post-treatment (P < 0.05 for each comparison; Figure 2(b)). Similarly, Plasma Gal-9 levels at each time point were significantly lower in patients who achieved SVR than those who did not (P < 0.05 for each comparison; Figure 2(c)). There was no correlation between plasma Gal-9 level and baseline HCV load (data not shown). Regression analysis showed that there was no relationship between Gal-9 and HCV RNA (data not shown).
Figure 2.
Plasma concentration of Gal-9 in (a) healthy control subjects and patients with chronic hepatitis C virus (HCV) infection at baseline, treatment week 12 and 24 weeks post-treatment with interferon-based therapy. (b) Patients stratified according to attainment of complete early virological response (cEVR). (c) Patients stratified according to attainment of sustained virological response (SVR). *P < 0.01 and **P < 0.001 vs healthy control; #P < 0.01 vs baseline; △P < 0.05; one-way analysis of variance with least significant difference-t test for post hoc comparisons.
Data regarding levels of IFN-α, TNF-α, and 2′-5′OAS, MxA and SOCS1 RNA in PBMC cultures are shown in Table 4. Treatment with TLR3 or TLR4 agonists alone and in combination with Tim-3 antagonist significantly upregulated IFN-α, 2′-5′OAS RNA and MxA RNA compared with untreated control cells, in PBMCs from both patients and healthy control subjects (P < 0.05 for each comparison; Table 4). IFNα levels were significantly lower in patient-derived PBMCs treated with combined TLR4 agonist and Tim-3 antagonist compared with the same treatment in control subject-derived cells (P < 0.05; Table 4). Levels of 2′-5′OAS RNA were significantly lower in patient-derived PBMCs treated with combined TLR4 agonist and Tim-3 antagonist as well as combined TLR3 agonist and Tim-3 antagonist compared with the same treatments in control subject-derived cells (P < 0.05 for each comparison; Table 4). There were no significant differences between patient-derived and control-derived cells in levels of MxA RNA.
Table 4.
Levels of immune modulators in peripheral blood mononuclear cell (PBMC) cultures from patients with chronic hepatitis C virus infection and healthy controls (n = 15/group), cultured in the presence or absence of toll-like receptor (TLR3) and TLR4 agonists (polyinosinic–polycytidylic acid [Poly(I:C)] and lipopolysaccharide, respectively) and a T-cell immunoglobulin and mucin domain 3 (Tim-3) antagonist (anti-Tim-3 antibody).
| Parameter | Control group |
Patient group |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | TLR4 agonist | Tim-3 antagonist + TLR4 agonist | TLR3 agonist | Tim-3 antagonist + TLR3 agonist | Control | TLR4 agonist | Tim-3 antagonist + TLR4 agonist | TLR3 agonist | Tim-3 antagonist + TLR3 agonist | |
| IFN-α, pg/ml | 25.5 ± 1.4 | 306.4 ± 63.4a | 377.7 ± 69.9a | 326.3 ± 54.0a | 665.2 ± 129.4abcd | 33.3 ± 1.9 | 258.4 ± 56.2a | 292.9 ± 72.8ae | 312.0 ± 56.9a | 623.5 ± 101.6abcd |
| 2′-5′OAS RNA | 1.0 ± 0.2 | 3.2 ± 0.8a | 6.7 ± 0.9ab | 4.8 ± 1.4abc | 8.5 ± 1.7abcd | 1.0 ± 0.3 | 2.4 ± 0.6a | 4.4 ± 0.7abe | 4.2 ± 0.9ab | 6.3 ± 1.2abcde |
| MxA RNA | 1.0 ± 0.1 | 3.2 ± 0.5a | 4.5 ± 0.8ab | 3.4 ± 0.6ac | 6.7 ± 0.7abcd | 1.1 ± 0.4 | 3.3 ± 1.0a | 4.4 ± 0.7ab | 3.8 ± 0.7a | 5.7 ± 0.2abcd |
| SOCS1 RNA | 1.1 ± 0.2 | 1.7 ± 0.4a | 1.7 ± 0.2a | 2.2 ± 0.5abc | 1.4 ± 0.2 | 2.5 ± 0.6e | 4.0 ± 0.8ae | 2.5 ± 0.2be | 4.0 ± 0.8ace | 1.5 ± 0.4abe |
| TNF-α, pg/ml | 15.9 ± 3.2 | 29.5 ± 5.4a | 25.9 ± 7.2a | 22.1 ± 6.8a | 18.8 ± 4.3 | 16.3 ± 4.1 | 29.7 ± 5.1a | 26.7 ± 6.9a | 25.7 ± 6.0a | 22.7 ± 6.9 |
Data presented as mean ± SD
IFN, interferon; TNF, tumour necrosis factor; 2′-5′OAS, 2′-5′ oligoadenylate synthetase; MxA, myxovirus resistance protein A; SOCS1, suppressor of cytokine 1.
P < 0.05 vs control cells in same group; bP < 0.05 vs LPS-treated cells in same group; cP < 0.05 vs Anti-Tim-3 + LPS-treated cells in same group; dP < 0.05 vs Poly(I:C)-treated cells in same group; eP < 0.05 vs same treatment in control group; one-way analysis of variance with least significant difference-t test for post hoc comparison.
In both patient-derived and control-derived cells, levels of IFN-α, 2′-5′OAS RNA and MxA RNA were significantly upregulated by combined TLR4 agonist and Tim-3 antagonist treatment compared with TLR4 agonist alone, and by combined TLR3 agonist and Tim-3 antagonist treatment compared with TLR3 agonist alone (P < 0.05 for each comparison; Table 4).
Treatment of both patient-derived and control-derived cells with TLR3 or TLR4 agonists significantly upregulated both SOCS1 RNA and TNF-α compared with untreated cells (P < 0.05 for each comparison; Table 4). In patient-derived cells, the addition of Tim-3 antagonist to TLR4 or TLR3 agonist treated cultures significantly reversed the increase in SOCS1 RNA (P < 0.05 for each comparison; Table 4). In control-derived cells, the addition of Tim-3 antagonist significantly reversed the increase in SOCS1 RNA in TLR3 treated cultures (P < 0.05; Table 4) but not in TLR4 agonist treated cultures. In both patient- and control-derived cells, the addition of Tim-3 antagonist had no effect on TNF-α levels in TLR4 agonist treated cultures, but significantly reversed the increase in TLR3 agonist treated cells (P < 0.05; Table 4).
In all treatment groups except Tim-3 antagonist/TLR3 combined treatment, levels of SOCS1 RNA were significantly higher in patient-derived cells compared with control-derived cells (P < 0.05 for each comparison; Table 4). There were no significant differences between patient-derived and control-derived cells in levels of TNF-α.
Discussion
The initial aim of this study was to investigate the dynamic changes in plasma Gal-9 levels in patients with chronic HCV infection undergoing PegIFNα-2/RBV treatment. Plasma Gal-9 levels were significantly elevated in patients with chronic HCV infection compared with healthy control subjects in the present study, suggesting that persistent high Gal-9 levels might contribute to refractory chronic hepatitis C. Antiviral treatment significantly reduced plasma Gal-9 levels compared with baseline in our patient cohort, particularly in those patients who achieved cEVR or SVR. Gal-9 may play an important role in the immune response.28 This indicated that the antiviral therapies could down-regulate the expressions of inhibitory molecule Gal-9 and related to the restoration of immune modulatory activity of Tim-3/Gal-9 pathway, which might improve outcomes of IFNα-based therapy for HCV infection.
Our previous study demonstrated that TLR3 expressing CD14 + monocytes were present in significantly lower numbers in patients who achieved cEVR than those who did not.4 TLR3 and TLR4 agonists have been shown to have significant efficacy against HCV,7 and both can induce antiviral molecules such as 2′-5′OAS.9 In the present study, TLR3 and TLR4 agonists increased the levels of IFN-α, and 2′-5′OAS and MxA RNAs. The combination of TLR3/TLR4 agonist and Tim-3 antagonist further stimulated expression of these antiviral proteins in the current study, with TLR3 agonist + Tim-3 antagonist shown to have the strongest inducer of anti-HCV molecules. These findings suggest that activation of TLR3 alone or in combination with Tim-3 suppression may produce an effective antiviral response and represent a possible approach for immune therapy in patients with chronic HCV infection.
The induction of the proinflammatory cytokine TNF-α was evaluated in the present study in order to determine the potential side effects of the TLR agonists and Tim-3 antagonist. TNF-α is a key proinflammatory cytokine, high levels of which have been shown to aggravate liver injury and result in a low response rate to IFN-α based antiviral therapy in patients with HCV infection.29 Blocking of Tim-3 on the surface of monocytes has been shown to inhibit TNF-α expression.30 Combination treatment of PBMCs with TLR3/TLR4 agonist and Tim-3 antagonist significantly reduced TNF-α levels compared with TLR3/TLR4 agonist alone in the present study. Interestingly, the addition of Tim-3 antagonist to TLR3 agonist treated cells completely ameliorated the increase in TNF-α levels seen with TLR3 agonist treatment alone. These findings suggest that comodulation of TLR3 and Tim-3 may provide a treatment option for HCV infection, suppressing inflammatory response while enhancing antiviral activity.
The negative immune-regulator SOCS1 was upregulated by treatment with TLR3 or TLR4 agonist in the present study. This may be due to induction of type 1 IFN by TLR, activating the JAK/STAT pathway and inducing SOCS1 expression.11 It has been reported that Tim-3 and SOCS1 communicate with each other and co-ordinately inhibit cell signalling transduction, resulting in impaired innate immune response.12,20 Culture with a combination of TLR3 agonist and Tim-3 antagonist did not induce SOCS1 expression in the present study, suggesting that blockade of Tim-3 could suppress SOCS1 expression induced by TLR
The present study had several limitations. In order to gain insight into the underlying mechanism and therapeutic role of modulation of TLRs and the Tim-3/Gal-9 pathway, large-scale studies and further research are required. In addition, T cells should be isolated from PBMCs for in vitro experiments, and the activation of effector T cell subsets and expression of related cytokines should be determined in patients with chronic HCV infection.
In conclusion, plasma Gal-9 levels were significantly upregulated in patients with chronic HCV infection, and were significantly reduced by antiviral treatment with IFN-based therapy. Low plasma Gal-9 levels before, during, and after antiviral therapy were crucial to achieve early and sustained virological responses. Blockade of Tim-3 in combination with TLR activation, especially TLR3, induces the expression of antiviral molecules without a significant increase in the proinflammatory TNF-α or the negative immune-regulator SOCS1, indicating a possible therapeutic approach for patients with chronic HCV infection.
Acknowledgements
We thank all subjects who generously provided blood samples for this study.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Key Program for Science and Technology Development of Hebei Province named Study on Prevention and Control of Viral Hepatitis (10276102D). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Geneva: World Health Organization. Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection. 2014. [PubMed]
- 2.Qian XJ, Zhu YZ, Zhao P, et al. Entry inhibitors: new advances in HCV treatment. Emerg Microbes Infect 2016; 5: e3–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paolucci S, Fiorina L, Mariani B, et al. Development and persistence of DAA resistance associated mutations in patients failing HCV treatment. J Clin Virol 2015; 72: 114–118. [DOI] [PubMed] [Google Scholar]
- 4.Horner SM. Activation and evasion of antiviral innate immunity by hepatitis C virus. J Mol Biol 2014; 426: 1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gay NJ, Gangloff M. Structure and function of toll receptors and their ligands. Annu Rev Biochem 2007; 76: 141–165. [DOI] [PubMed] [Google Scholar]
- 6.Hong B, Lee SH, Song XT, et al. A super TLR agonist to improve efficacy of dendritic cell vaccine in induction of anti-HCV immunity. PLoS One 2012; 7: e48614–e48614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su SS, He H, Kong LB, et al. Regulatory phenotype, PD-1 and TLR3 expression in T cells and monocytes from HCV patients undergoing antiviral therapy: a randomized clinical trial. PLoS One 2014; 9: e93620–e93620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson AC, Rossiter JP, Lafon M. Expression of toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J Neurovirol 2006; 12: 229–234. [DOI] [PubMed] [Google Scholar]
- 9.Thomas A, Laxton C, Rodman J, et al. Investigating toll-like receptor agonists for potential to treat hepatitis C virus infection. Antimicrob Agents Chemother 2007; 51: 2969–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Chen S, Guan Y, et al. Type III interferon induces distinct SOCS1 expression pattern that contributes to delayed but prolonged activation of Jak/STAT signaling pathway: implications for treatment non-response in HCV patients. PLoS One 2015; 10: e0133800–e0133800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto M, Naka T. SOCS1, a negative regulator of cytokine signals and TLR responses, in human liver diseases. Gastroenterol Res Pract 2010; 2010: 470–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Ma CJ, Wang JM, et al. Tim-3 negatively regulates IL-12 expression by monocytes in HCV infection. PLoS One 2011; 6: e19664–e19664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida Y, Ke B, Freitas MC, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology 2010; 139: 2195–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Che KF, Shankar EM, Muthu S, et al. p38 Mitogen-activated protein kinase/signal transducer and activator of transcription-3 pathway signaling regulates expression of inhibitory molecules in T cells activated by HIV-1-exposed dendritic cells. Mol Med 2012; 18: 1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elahi S, Niki T, Hirashima M, et al. Galectin-9 binding to Tim-3 renders activated human CD4+ T cells less susceptible to HIV-1 infection. Blood 2012; 119: 4192–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barathan M, Mohamed R, Vadivelu J, et al. Peripheral loss of CD8+ CD161(++) TCRVα7 2(+) mucosal-associated invariant T cells in chronic hepatitis C virus-infected patients. Eur J Clin Invest 2016; 46: 170–180. doi: 10.1111/eci.12581. [DOI] [PubMed] [Google Scholar]
- 17.Larsson M, Shankar EM, Che KF, et al. Molecular signatures of T-cell inhibition in HIV-1 infection. Retrovirolog 2013; 10: 31–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koguchi K, Anderson DE, Yang L, et al. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med 2006; 203: 1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005; 6: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 20.Hastings WD, Anderson DE, Kassam N, et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol 2009; 39: 2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mengshol JA, Golden-Mason L, Arikawa T, et al. A crucial role for kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS one 2010; 5: e9504–e9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin HT, Anderson AC, Tan WG, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 2010; 107: 14733–14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marukian S, Jones CT, Andrus L, et al. Cell culture-produced hepatitis C virus does not infect peripheral blood mononuclear cells. Hepatology 2008; 48: 1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology 2008; 47: 385–395. [DOI] [PubMed] [Google Scholar]
- 25.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2011; 55: 245–264. [DOI] [PubMed] [Google Scholar]
- 26.Wei L. Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline for prevention and treatment of hepatitis C: a 2015 update. Zhonghua Gan Zang Bing Za Zhi 2015; 23: 906–923. [in Chinese, English Abstract]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun ZH, Yang HL, Wei M, et al. Preparation and application of oligo microarrays for hepatitis virus detection and genotyping. Zhonghua Gan Zang Bing Za Zhi 2007; 15: 816–820. [in Chinese, English Abstract]. [PubMed] [Google Scholar]
- 28.Anderson AC, Anderson DE, Bregoli L, et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 2007; 318: 1141–1143. [DOI] [PubMed] [Google Scholar]
- 29.Akcam FZ, Tigli A, Kaya O, et al. Cytokine levels and histopathology in chronic hepatitis B and chronic hepatitis C. J Interferon Cytokine Res 2012; 32: 570–574. [DOI] [PubMed] [Google Scholar]
- 30.Frazier AD, Zhang CL, Ni L, et al. Programmed death-1 affects suppressor of cytokine signaling-1 expression in T cells during hepatitis C infection. Viral Immunol 2010; 23: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]