Abstract
Objective
This study evaluated the risks and outcomes of capsule retention during capsule endoscopy (CE) for investigating small bowel disease. Capsule retention is the most serious complication of CE.
Methods
Before CE, the gastrointestinal tract was evaluated for blockages with computerized tomography. Analysis of CE was made retrospectively.
Results
Capsule endoscopy was used to investigate obscure bleeding (90.2%; n = 324) or other symptoms (9.8%; n = 35). The capsule retention rate was 11/359 (3.1%); it was retained in a malignant lesion area (adenocarcinoma or melanoma) in two patients (18.2%), in the small bowel in an ulcerated area in five patients (45.5%), and in the oesophagus/stomach in four patients (36.4%) due to dysmotility. None of the patients had symptoms of obstruction.
Conclusions
Scanning patients before CE did not predict capsule retention. Retention is a complication of CE, but occurs as a result of the underlying disease. The risk of retention is increased in patients with motility disorders, suspected small bowel ulcers or malignancies.
Keywords: Capsule endoscopy, capsule retention, small bowel, patency capsule
Introduction
Capsule endoscopy (CE) is the technique of preference for evaluating small bowel disease.1 The main contraindications for CE are obstruction and dysmotility. The most serious complication is capsule retention, although the rate of capsule retention varies depending on the clinical indication for CE. The retention risk is increased in patients with Crohn’s disease (CD), malignant lesions and motility disorders.2 Dysmotility, in particular, is underdiagnosed during routine clinical assessment. Radiological evaluations, such as contrast computed tomography (CT), magnetic resonance imaging, enterography and enteroclysis may be used to try and pre-empt retention, however, these tools cannot rule out the possibility of an intestinal stricture.3
A retained capsule is usually asymptomatic but may be associated with symptoms of partial or complete bowel obstruction.3 Symptomatic bowel obstruction requiring surgical or endoscopic removal of the retained capsule can also occur. The current study investigated the outcomes of patients who developed retention complications.
Patients and methods
Study design
This retrospective study examined CE outpatient procedures performed between January 2005 and December 2013. Patients were specifically referred to the Department of Gastroenterology Endoscopy unit, Istanbul Medical Faculty, Istanbul University for CE, and presented with obscure gastrointestinal bleeding (OGIB) or other indications, such as abdominal pain or suspicion of CD. The medical records of all patients with retention complications during CE were analysed retrospectively. Before CE, the gastrointestinal passage was checked by CT, and CE was not performed in patients with strictures or obstructions. The local ethics committee of Istanbul University Medical Faculty approved this retrospective study (no. 2013/429-987), and all participants signed an informed consent form before the CE procedure.
Capsule endoscopy methods
The PillCam SB2 (Given Diagnostic Imaging Systems, Yoqneam, Israel), a vitamin-sized, wireless camera swallowed by the patient, was used for the procedure. Bowel preparation was performed using 4 l polyethylene glycol solution 1 day before the procedure. The patient swallowed the PillCam SB2 capsule in the outpatient clinic. Eating and drinking were permitted 4 h after the initial administration of the capsule. All patients were allowed to continue their usual daily life. After 8 h, the sensor array and the recording device were removed, and results analysed using Given Imaging (Given Imaging Ltd, Yoqneam, Israel) software. The same experienced endoscopist (F.A.) analysed the CE results of all patients.
Patients were instructed to check their stools for the capsule and to notify the endoscopy unit if it was not excreted. Capsule retention was defined as the presence of the capsule in the gastrointestinal tract for at least 2 weeks after ingestion.4
Results
This retrospective study examined 359 CE outpatient procedures. Of these, 324 patients (90.2%) presented with OGIB and 35 patients (9.8%) presented with abdominal pain or suspicion of CD. The capsule retention rate in this study was 11/359 (3.1%). Of these 11 cases, the capsule was retained in the malignant lesion area (adenocarcinoma [ileum] or melanoma [jejunum]) in two patients (18.2%), in an ulcerated area in five patients (45.5%) and in the oesophagus/stomach due to a motility disorder in four patients (36.4%). Demographic data and details of complications and outcomes of the 11 patients who developed retention complications are presented in Table 1.
Table 1.
Demographic data, complications and outcomes of patients (n = 11) with capsule retention during capsule endoscopy.
| Age/sex | CE findings | Lesion localization | Acute obstruction | DBE | Outcome |
|---|---|---|---|---|---|
| 22/M | Ulcer | Ileum (proximal) | None | X | Medical |
| 32/F | Ulcer (CD) | Ileum (proximal) | None | X | Died (sepsis) |
| 31/M | Diaphragm disease | Ileum (distal) | None | Capsule extraction | Capsule extraction using DBE |
| 41/F | Gluten enteropathy-ulcerative jejunoileitis | Ileum (proximal) | None | X | Surgery (small bowel resection) |
| 62/M | Malignancy | Ileum (proximal) | None | Lesion biopsy | Surgery (adenocarcinoma) |
| 25/F | Ulcer | Jejunum (distal) | None | Lesion could not be reached | Surgery (nonspecific ulcer – volvulus) |
| 32/F | Malignancy | Jejunum (proximal) | None | X | Surgery (melanoma) |
| 75/M | Motility disorder | None | X | Achalasia | |
| 38/M | Motility disorder | None | X | Gastroparesis | |
| 38/M | Motility disorder | None | X | MNGIE syndrome (capsule passed in stools after 2 months) | |
| 20/F | Motility disorder Capsule retained in oesophagus | None | X | MNGIE syndrome (capsule extracted using gastroscope) |
CE, capsule endoscopy; DBE, double-balloon enteroscopy; M, male; X, not performed; F, female; CD, Crohn’s disease; MNGIE syndrome, mitochondrial neurogastrointestinal encephalopathy.
The capsule was retained in the upper gastrointestinal tract in two patients, both of whom presented with OGIB. In both patients, retention prevented study of the rest of the gastrointestinal tract. In the first patient, the capsule was retained within the oesophagus for 3–4 h following CE. This patient was diagnosed with achalasia by oesophageal manometry after CE and the capsule was passed in the stools 3 weeks later. The second patient with CE retention within the upper gastrointestinal tract was diagnosed with a motility disorder and scintigraphy studies showed this to be caused by diabetic gastroparesis. The capsule was passed 3 weeks later, spontaneously, in the stools. No further interventions were made as these two patients showed no signs of obstruction.
Mitochondrial neurogastrointestinal encephalopathy (MNGIE) syndrome was detected in two of the four patients diagnosed with dysmotility. One of the patients with MNGIE syndrome excreted the capsule spontaneously after 2 months; this patient later died of respiratory arrest in the intensive care unit 4 months after the CE procedure. In the second patient with MNGIE syndrome, the capsule was retained in the oesophagus and extracted with a gastroscope 1 day later.
Small bowel ulcers were present in five patients with retention complications. The ulcers were due to CD (n = 1), idiopathic diaphragm disease (n = 1), ulcerous jejunoileitis (n = 1) or were nonspecific (n = 2). The patient with CD showed no signs of obstruction or complications related to capsule retention and so no action was taken to retrieve the capsule. This patient died due to acute renal failure and septicaemia 1 year after the CE procedure. The cause of the renal deficiency was not found.
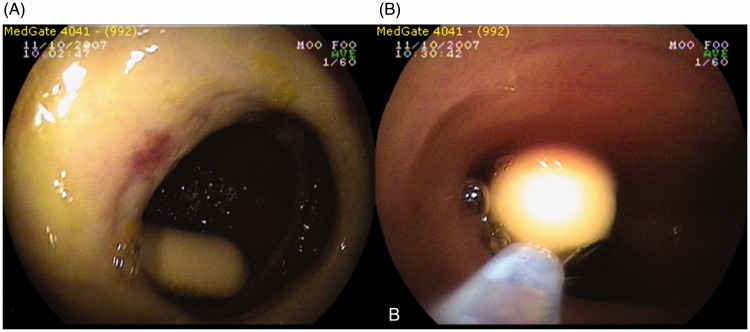
The retained capsule in the patient with diaphragm disease was extracted by double-balloon enteroscopy (DBE; Figures 1A and 1B). This patient had no symptoms suggesting obstruction. Two patients, referred for CE due to OGIB, had small bowel ulcers (one with gluten enteropathy-ulcerative jejunoileitis and one with a nonspecific small bowel ulcer); both underwent surgery to treat the underlying condition, during which the retained capsules were also removed. The patient with ulcerative jejunoileitis needed stricturoplasty and resection for the large number of strictures detected during surgery. Volvulus was diagnosed in the patient with a nonspecific ulcer, which had not been detected by CT before surgery. Neither patient showed symptoms of obstruction.
Figure 1.
Capsule retained in a 31-year-old patient with diaphragm disease undergoing capsule endoscopy for obscure gastrointestinal bleeding (A); with right image showing the extraction of the capsule with double-balloon enteroscopy (B).
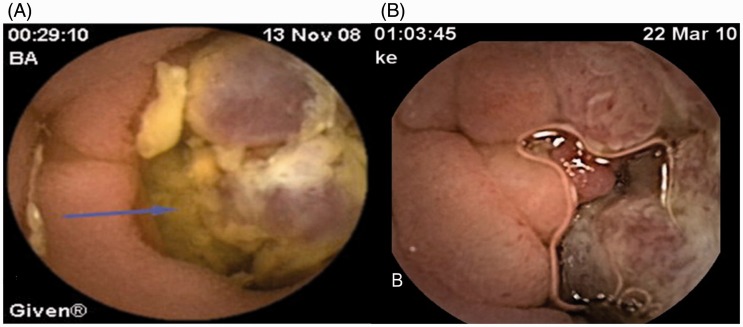
In the other two patients, capsules were retained in areas of tumour lesions (Figures 2A and 2B). These patients had no symptoms of obstruction but underwent surgery because of the underlying disease based on the CE findings. Melanoma was detected in one of these patients and small bowel adenocarcinoma in the other.
Figure 2.
Images of tumour lesions revealed during capsule endoscopy. Left image (A), adenocarcinoma: blue arrow points to tumour lesion; right image (B), melanoma.
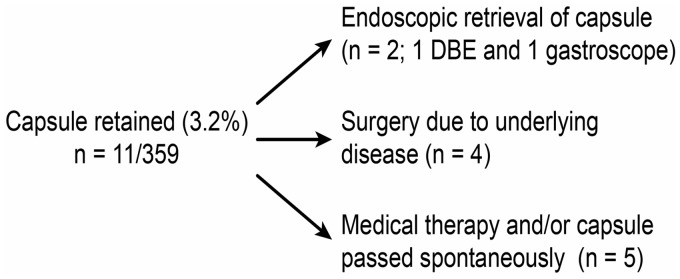
No deaths occurred due to retention complications following CE. Outcomes of those with capsule retention during CE are shown in Figure 3.
Figure 3.
Outcomes of patients (n = 11) with a retained capsule during capsule endoscopy. DBE, double-balloon enteroscopy.
Discussion
Capsule endoscopy is a widely accepted, safe and simple, noninvasive procedure, which is well tolerated by patients, does not require sedation, surgery or unnecessary radiation exposure.4 It can facilitate the diagnosis and treatment of underlying disease. There is a risk of capsule retention with CE; however, this is a rare complication, which is not usually serious. The rate of capsule retention depends on the indication for CE: 1.4% for OGIB, 1.5% for suspected CD, 5–13% for known CD and 21% for suspected small bowel obstruction.5–7 In the current case series, retention complications occurred in 3.1% of patients.
Of the patients in the present case series with capsule retention, 36.4% (n = 4) had dysmotility. Achalasia was detected in one of these patients, and diabetic gastroparesis in another. The patient with achalasia had been evaluated endoscopically at another centre before CE, and the results had been normal. Questioning the patient in detail before the CE examination, and performing further endoscopy prior to CE might have prevented capsule retention.
Diabetic gastroparesis is a motility disorder in which capsule retention can occur due to prolongation of the gastrointestinal transit time. Use of prokinetics does not improve gastric transit times.8–10 In cases of known gastroparesis in patients unable to swallow, the video capsule can be administered endoscopically. In the present study, no symptoms of gastroparesis were detected in the patient with diabetic gastroparesis, so the capsule was administered orally. Neither of the two patients with dysmotility developed obstruction complications with CE, and for both, the capsule was excreted spontaneously 3 weeks after the CE procedure.
In the present study, in two patients undergoing CE for OGIB, the CE results were examined at the end of the procedure as dysmotility was suspected. Both patients were diagnosed with MNGIE. Neither patient had symptoms of obstruction prior to undergoing CE. MNGIE is a rare autosomal recessive, multi-systemic disorder characterized by cachexia, gastrointestinal dysmotility, leukoencephalopathy and peripheral neuropathy.11 The main cause of the gastrointestinal dysmotility is the synergistic effect of neuromuscular and autonomic dysfunctions. The major gastrointestinal symptoms are nausea and vomiting, which are caused by intestinal pseudo-obstruction. MNGIE is a life-threatening condition with high morbidity rates. Diagnosis is difficult, as it mimics the clinical outcomes of mechanical obstruction, with symptoms such as marked gut propulsive motility dysfunction.
The risk of capsule retention has been reported to be higher in patients with definite or suspected small bowel ulcers, CD, neoplastic lesions, enteropathy, stenosis or adhesions and in those taking non-steroidal anti-inflammatory drugs.6,7,12 Consistent with this, in the present study, small intestinal ulcers or malignancies were detected in 63.6% of patients with retention complications after CE. CE is a valuable tool for the diagnosis of CD and can also be used to monitor disease activity, detect complications and evaluate therapeutic response. However, the risk of retention is increased in patients with small bowel CD. This risk can be significantly decreased by routine utilization of a dissolvable patency capsule prior to ingestion of the diagnostic capsule.13 Patency capsules were not available at our centre at the time and so were not used in the current study.
Removal of retained capsules by DBE has been reported previously,14 and was used successfully to extract the retained capsule from the patient with diaphragm disease in this present study.
In the present study, one patient with small intestinal ulcers and capsule retention but no obstruction symptoms underwent surgery for her underlying disease. During surgery, volvulus, which had not been seen in CT images taken before CE, was detected. When retrospectively reviewed, no significant dilatation of the intestine or typical volvulus findings, such as the ‘northern exposure sign’, ‘coffee bean sign’, ‘three-line sign’ or ‘white-stripe sign’ were seen. It was thought that the lack of these signs in the CT images was probably due to the disease being in the early stage.
The presence of a tumour lesion is also a risk factor for capsule retention. In one large study, small bowel tumours were shown to be associated with capsule retention in 19% of subjects.15 In this present study, the rate of retention in patients with tumours was 18.2%.
In routine practice, contrast CT to evaluate the gastrointestinal passage is undertaken on all patients before CE. In the present study, the CT results of all patients with retention complications were normal, underlining the fact that this imaging method cannot predict retention complications.16–19
In the present study, when patients who developed capsule retention complications were retrospectively reviewed, the lesions causing the retention were not seen with CT, and the gastrointestinal passage was open. However, the present study used abdominal CT to both review the passage and to prevent retention complications before capsule administration. In the literature, studies on the diagnostic power of CE used either CT enterography versus CE or MR enterography versus CE.20–23
In the current study, patients with retention complications but without symptoms suggesting obstruction were followed-up medically. The capsule was eventually passed spontaneously in these patients. In our experience, nonsurgical management is the best initial option in patients without symptoms of acute intestinal obstruction.
Four patients underwent surgery following CE due to their underlying disease. This facilitated both diagnosis and treatment of the underlying conditions that caused the retention. It is important to be aware of the possibility of capsule retention, especially in patients with known or suspected small bowel ulcers, malignant lesions or motility disorders. As radiological studies have a low diagnostic yield and tend to underestimate small bowel strictures, careful patient selection is required to avoid complications. Patients should be questioned regarding the symptoms of motility disorders, including defaecation problems, bloating and dysphagia.
In conclusion, CE is a safe procedure, and capsule retention is not a serious problem in routine practice. Screening with CT before CE is not predictive of capsule retention. Motility disorders are risk factors for capsule retention, although dysmotility is underdiagnosed in routine clinical evaluation. Patients’ symptoms should be evaluated carefully to decrease the risk of retention complications.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Wang A, Banerjee S, Barth BA, et al. Wireless capsule endoscopy. Gastrointest Endosc 2013; 78: 805–815. [DOI] [PubMed] [Google Scholar]
- 2.Mata A, Llach J, Bordas JM. Wireless capsule endoscopy. World J Gastroenterol 2008; 14: 1969–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rondonotti E, Villa F, Mulder CJ, et al. Small bowel capsule endoscopy in 2007: indications, risks and limitations. World J Gastroenterol 2007; 13: 6140–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cave D, Legnani P, de Franchis R, et al. ICCE consensus for capsule retention. Endoscopy 2005; 37: 1065–1067. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Gurudu SR, De Petris G, et al. Retention of the capsule endoscope: a single-center experience of 1000 capsule endoscopy procedures. Gastrointest Endosc 2008; 68: 174–180. [DOI] [PubMed] [Google Scholar]
- 6.Liao Z, Gao R, Xu C, et al. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc 2010; 71: 280–286. [DOI] [PubMed] [Google Scholar]
- 7.Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol 2006; 101: 2218–2222. [DOI] [PubMed] [Google Scholar]
- 8.de Franchis R, Avgerinos A, Barkin J, et al. ICCE consensus for bowel preparation and prokinetics. Endoscopy 2005; 37: 1040–1045. [DOI] [PubMed] [Google Scholar]
- 9.Postgate A, Tekkis P, Patterson N, et al. Are bowel purgatives and prokinetics useful for small-bowel capsule endoscopy? A prospective randomized controlled study. Gastrointest Endosc 2009; 69: 1120–1128. [DOI] [PubMed] [Google Scholar]
- 10.Rokkas T, Papaxoinis K, Triantafyllou K, et al. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: A meta-analysis. Am J Gastroenterol 2009; 104: 219–227. [DOI] [PubMed] [Google Scholar]
- 11.Hirano M, Silvestri G, Blake DM, et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): clinical, biochemical, and genetic features of an autosomal recessive mitochondrial disorder. Neurol 1994; 44: 721–727. [DOI] [PubMed] [Google Scholar]
- 12.Rondonotti E, Pennazio M, Toth E, et al. European Capsule Endoscopy Group, Italian Club for Capsule Endoscopy (CICE) and Iberian Group for Capsule Endoscopy. Small-bowel neoplasms in patients under going video capsule endoscopy: a multicenter European study. Endoscopy 2008; 40: 488–495. [DOI] [PubMed] [Google Scholar]
- 13.Herrerias JM, Leighton JA, Costamagna G, et al. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc 2008; 67: 902–909. [DOI] [PubMed] [Google Scholar]
- 14.Kameda N, Higuchi K, Shiba M, et al. A prospective, single-blind trial comparing wireless capsule endoscopy and double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. J Gastroenterol 2008; 43: 434–440. [DOI] [PubMed] [Google Scholar]
- 15.Höög CM, Bark LÅ, Arkani J, et al. Capsule retentions and incomplete capsule endoscopy examinations: an analysis of 2300 examinations. Gastroenterol Res Pract 2012; 2012: 518718–518718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karagiannis S, Faiss S, Mavrogiannis C. Capsule retention: a feared complication of wireless capsule endoscopy. Scand J Gastroenterol 2009; 44: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 17.Rondonotti E, Herrerias JM, Pennazio M, et al. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc 2005; 62: 712–716. [DOI] [PubMed] [Google Scholar]
- 18.Sidhu R, Sanders DS, McAlindon ME, et al. Capsule endoscopy for the evaluation of nonsteroidal anti-inflammatory drug-induced enteropathy: United Kingdom pilot data. Gastrointest Endosc 2006; 64: 1035–1035. [DOI] [PubMed] [Google Scholar]
- 19.Ho KK, Joyce AM. Complications of capsule endoscopy. Gastrointest Endosc Clin N Am 2007; 17: 169–178. [DOI] [PubMed] [Google Scholar]
- 20.Van Weyenberg SJ, Bouman K, Jacobs MA, et al. Comparison of MR enteroclysis with video capsule endoscopy in the investigation of small-intestinal disease. Abdom Imaging 2013; 38: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He B, Gong S, Hu C, et al. Obscure gastrointestinal bleeding: diagnostic performance of 64-section multiphase CT enterography and CT angiography compared with capsule endoscopy. Br J Radiol 2014; 87: 20140229–20140229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huprich JE, Fletcher JG, Fidler JL, et al. Prospective blinded comparison of wireless capsule endoscopy and multiphase CT enterography in obscure gastrointestinal bleeding. Radiology 2011; 260: 744–751. [DOI] [PubMed] [Google Scholar]
- 23.Boriskin HS, Devito BS, Hines JJ, et al. CT enterography vs. capsule endoscopy. Abdom Imaging 2009; 34: 149–155. [DOI] [PubMed] [Google Scholar]