Abstract
Objective
To investigate the synergistic effects of combining erlotinib and RNA-interference downregulation of focal adhesion kinase (FAK) expression on the proliferation, apoptosis, invasion and migration of the human gastric adenocarcinoma cell line AGS.
Methods
Cells were divided into five experimental groups: Group A, nontransfected control; Group B, transfected with empty vector; Group C, transfected with FAK-shRNA; Group D, erlotinib treatment; Group E, combination erlotinib treatment and transfected with FAK-shRNA. FAK protein levels were confirmed via Western blotting. Cell proliferation (CCK-8 assay, apoptosis (flow cytometry), cell invasion (transwell assay) and migration (scratch assay) were evaluated.
Results
RNA interference significantly decreased FAK protein levels. Cell proliferation, invasion and migration were significantly lower in Groups C, D and E compared with Group A, and significantly lower in Group E than in Groups C and D.
Conclusions
RNA interference effectively silences FAK expression and inhibits malignant cell proliferation and invasion in gastric cancer cells. The effect of FAK inhibition is increased by co-treatment with erlotinib.
Keywords: RNA interference, FAK gene, erlotinib, gastric cancer
Introduction
Epidermal growth factor receptor (EGFR) is a tyrosine kinase that regulates the Ras/Raf/MAPK, PI3K/AKT and JAK/ STAT signal transduction pathways, leading to differentiation and proliferation of cancer cells.1,2 EGFR was shown to stimulate metastasis of pancreatic carcinoma and breast cancer by activating the Src-Focal adhesion kinase (FAK)–p130cas pathway, while an integrin antagonist had an inhibitory effect on carcinoma cell invasion and metastasis.3,4 Therefore, the Ras/Raf/MAPK, PI3K/AKT, JAK/STAT and Src/FAK/p130cas signal transduction pathways may interact with one another, possibly influencing the proliferation and invasion of gastric cancer cells.
The aim of the present study was to use RNA interference to downregulate the expression of FAK, combined with erlotinib to block the interaction between EGFR and the integrin/src/FAK/p130cas signal transduction pathway in a gastric cancer cell line. The effects on proliferation, apoptosis, invasion and migration were evaluated, in order to provide a theoretical foundation for targeted therapy of gastric cancer.
Materials and methods
Cell culture
The human gastric adenocarcinoma cell strain AGS was kindly provided by Professor Xu Lin (Key Laboratory of Ministry of Education for Gastrointestinal Cancer, Fuzhou, China). Cells were routinely cultured in DMEM (HyClone; Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone), 100 U/ml penicillin and 100 μg/ml streptomycin in a 5% carbon dioxide/95% air humidified incubator at 37 ℃. Cells were subcultured every 2–3 days.
shRNA design
Small hairpin (sh) RNA was designed against three sites in the FAK mRNA sequence. Oligonucleotides were synthesized (FAK-shRNA-1, FAK-shRNA-2, FAK-shRNA-3) as shown in Table 1.
Table 1.
Sequences of small hairpin (sh)RNAs targeting human focal adhesion kinase (FAK).
| Name | Sequence |
|---|---|
| FAK-shRNA-1 | CAGGAGTCAGTCACT GCCAACATAA |
| FAK-shRNA-2 | GAATGTTCTGGTGTC CTCAAATGAT |
| FAK-shRNA-3 | GCTTTCTCCCTTTG GTCTTCT |
Construction of shRNA expression vector
The lentiviral vector pHBLV-U6-ZsGreen-Puro was purchased from Hanheng Biotechnology (Shanghai, China). The vector was digested with BamHI and EcoRI, and the annealed oligonucleotides were ligated into the vector. All ligation products were transformed into DH5α competent cells, and plasmids were confirmed by DNA sequencing.
Experimental design
Cells were divided into five experimental groups: Group A, control group (nontransfected, no erlotinib); Group B, empty vector group (transfected with empty vector, no erlotinib); Group C, FAK group (transfected with FAK-shRNA, no erlotinib); Group D, erlotinib group (nontransfected, 40 µmol/l erlotinib); Group E, combination group (transfected with FAK-shRNA, 40 µmol/l erlotinib).
Transfection
Transfection was performed using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) when cells were at around 90% confluence in 24-well plates, according to the manufacturer's protocol. Transfection efficiency was determined by determining the percentage of GFP-positive cells via fluorescent microscopy. A stably transfected clone was used for further experiments.
Western blotting
Cells in each group were cultured for 72h, then total protein was extracted, quantified (via BCA protein assay) and normalized. Total protein was separated via 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis then transferred to a PVDF membrane. The membrane was blocked with 5% non-fat dry milk at room temperature for 2h, then incubated with rabbit-antihuman FAK (1:1000 dilution, Abcam, Shanghai, China) and rabbit-antihuman GAPDH (1:1000 dilution, Abcam), overnight at 4˚C on a rocking platform. The membrane was washed 3 times with tris buffered saline–Tween 20 (TBST), then incubated with HRP-conjugated goat-antirabbit IgG (1:5000 dilution) for 2 h at room temperature and washed three times with TBST. Protein bands were detected using SuperSignal West Pico Chemiluminescent substrate (Thermo Fisher Scientific, Waltham, MA, USA).
CCK-8 cell proliferation assay
Cells were plated in 96-well plates (six wells/group) and cultured for 24, 48, 72 and 96 h. CCK-8 reagent was then added (10 μl/well) and incubation continued for a further 2 h. The optical density (OD) at 450 nm was determined using a microplate reader and the growth inhibition rate (IR) was calculated as: IR (%) = (OD control−OD experiment) / (OD control−OD background) × 100%.
Flow-cytometry
Cells were cultured for 48 h, collected, and rinsed twice with cold phosphate buffered saline (PBS). Cells (5 × 104) were suspended in 500 μl binding buffer. Annexin V-PE and 7-aminoactinomycin D (7AAD) (Annexin V-PE/7AAD apoptosis kit; Becton Dickinson and Co., NY, USA) were added according to the manufacturer's instructions. Flow cytometry was performed following calibration with an unstained cell sample and AnnexinV-FITC and PI-mono labelled samples. The proportion of apoptotic cells in each sample was calculated using FlowJo version 7.6 (FlowJo, LLC, Ashland, OR, USA).
Cell invasion assay
Cells (5 × 105/ml) were suspended in serum-free DMEM medium and seeded in the upper chamber of a Transwell plate (6.5 mm; 24-well plate, Costar; Corning, NY, USA) at 200 μl/well (three wells/group). At the same time, 500 µl high glucose DMEM with 10% FBS was added into the lower chamber. After incubation for 24 h, the chambers were removed and rinsed twice with PBS, and the membrane was fixed with 4% paraformaldehyde for 30 min, stained with crystal violet for 20 min and rinsed with double distilled water. A total of five visual fields (upper, lower, left, right and centre) were captured via light microscopy (×400), and the mean number of cells that moved through the membrane to the lower chamber surface was determined.
Cell migration assay
Cells (5 × 105/ml) were seeded in 6-well plates (3 wells/group), and allowed to adhere to the well surface, at which time serum-free medium was added and culture continued for 24 h. The confluent cell monolayer was then scratched with a 10 µl pipette-tip in a standardized manner to create a cell-free zone in each well. Cell migration was documented by photography, and the residual gap between the cells was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analyses
Data were presented as mean ± SD and analysed using one-way analysis of variance (ANOVA). Homogeneity of variance was tested by the least significant difference method, and heteroscedasticity of variance was evaluated using Dunnett's T test. Statistical analyses were performed using SPSS® version 18.0 (SPSS Inc., Chicago, IL, USA) for Windows®, and P-values < 0.05 were considered statistically significant.
Results
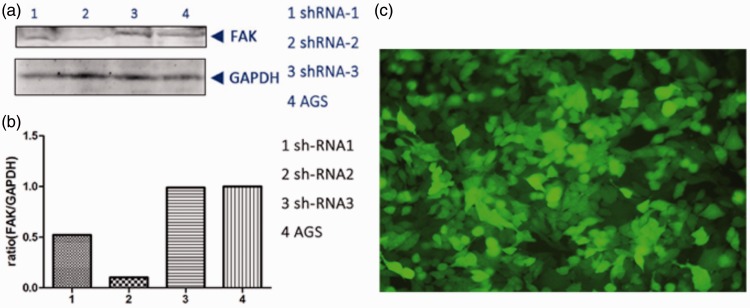
The inhibitory effect of FAK-shRNA-2 on FAK protein levels was visibly higher than that of FAK-shRNA-1 and FAK-shRNA-3 (Figure 1), with a transfection efficiency of >85% (data not shown). Cells transfected with FAK-shRNA-2 were therefore used for further experiments.
Figure 1.
Effect of transfection of the gastric adenocarcinoma cell line AGS with three different small hairpin (sh)RNAs targeting focal adhesion kinase (FAK). (a) Western blot analysis of FAK and GAPDH following transfection. (b) Densitometric analysis of FAK relative to GAPDH. ShRNA-2 was used for further experiments. (c) Presence of green fluorescent protein (GFP) in AGS cells at 48 h after transfection, indicating efficacy of transfection (original magnification × 200).
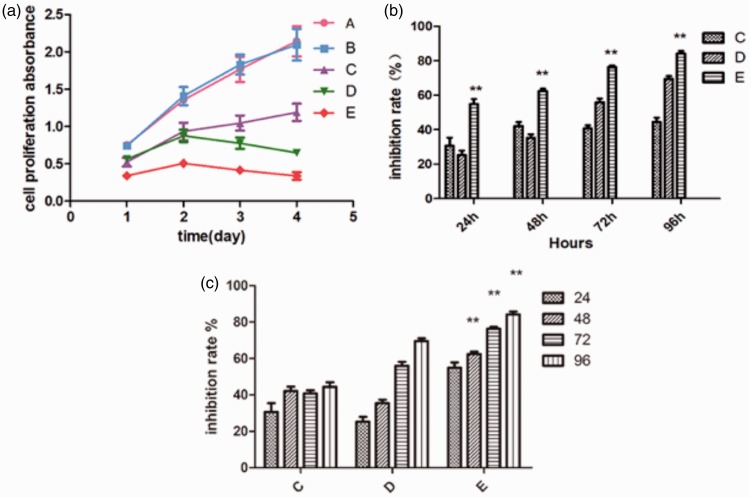
Cell proliferation assay data are shown in Figure 2 and Table 2. There were no significant differences in cell proliferation between control cells (Group A) and cells in the empty vector group (Group B) at any time (Figure 2 (a)). Cell proliferation was significantly lower in groups C, D and E than Group A at 96h (P < 0.01 for each comparison, Figure 2 (a)). The combination of erlotinib and FAK-shRNA-2 (Group E) resulted in significantly higher levels of growth inhibition than either FAK-shRNA-2 (Group C) or erlotinib (Group D) alone at each time point (P < 0.05 for each comparison; Figure 2(b)). The inhibition rate increased significantly at each time point in Group E (P < 0.05 for each comparison; Figure 2(c)).
Figure 2.
Cell proliferation in the gastric adenocarcinoma cell line AGS following erlotinib treatment and/or transfection with small hairpin (sh)RNA targeting focal adhesion kinase (FAK). Group A, control group (nontransfected, no erlotinib); Group B, empty vector group (transfected with empty vector, no erlotinib); Group C, FAK group (transfected with FAK-shRNA, no erlotinib); Group D, erlotinib group (nontransfected, 40 µmol/l erlotinib); Group E, combination group (transfected with FAK-shRNA, 40 µmol/l erlotinib). (a) Cell proliferation. (b) Rate of inhibition of proliferation. **P < 0.05 vs group C and D; one-way analysis of variance. (c) Rate of inhibition of proliferation; **P < 0.05 versus previous time point; one-way analysis of variance. Data are mean ± SD (n = 4 wells/group).
Table 2.
Cell proliferation (CCK-8 assay), apoptosis, cell invasion and cell migration (wound healing rate) in the gastric adenocarcinoma cell line AGS following erlotinib treatment and/or transfection with small hairpin (sh)RNA targeting focal adhesion kinase (FAK).
| Parameter | Group |
||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| CCK-8 assay | |||||
| 24h | 0.75 ± 0.03 | 0.74 ± 0.02 | 0.53 ± 0.06a | 0.56 ± 0.04a | 0.34 ± 0.04ab |
| 48h | 1.36 ± 0.01 | 1.41 ± 0.12 | 0.94 ± 0.12a | 0.88 ± 0.08a | 0.51 ± 0.03ab |
| 72h | 1.77 ± 0.17 | 1.83 ± 0.13 | 1.05 ± 0.10a | 0.78 ± 0.08a | 0.42 ± 0.03ab |
| 96h | 2.14 ± 0.20 | 2.10 ± 0.21 | 1.19 ± 0.12a | 0.65 ± 0.03a | 0.34 ± 0.05ab |
| Apoptosis Rate, % | 7.3 ± 3.2 | 7.3 ± 2.0 | 35.0 ± 4.7a | 45.2 ± 4.2a | 59.1 ± 6.5ab |
| Cell invasion | 65.8 ± 0.8 | 64.6 ± 0.9 | 51.2 ± 1.3a | 21.7 ± 0.6a | 13.7 ± 1.2ab |
| Wound healing rate, % | 21.1 ± 0.9 | 21.3 ± 0.4 | 15.2 ± 0.8a | 12.8 ± 0.6a | 5.8 ± 0.6ab |
Data presented as mean ± SD.
P < 0.05 vs Group A;
P < 0.05 vs Group C and D; one-way analysis of variance.
Group A, control group (nontransfected, no erlotinib); Group B, empty vector group (transfected with empty vector, no erlotinib); Group C, FAK group (transfected with FAK-shRNA, no erlotinib); Group D, erlotinib group (nontransfected, 40 µmol/l erlotinib); Group E, combination group (transfected with FAK-shRNA, 40 µmol/l erlotinib)
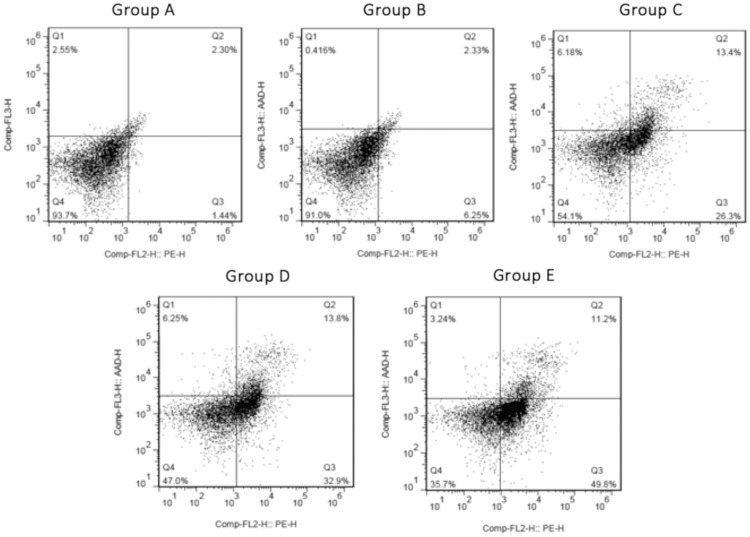
Data regarding apoptosis are shown in Figure 3 and Table 2. The apoptotic rate was significantly higher in Groups C, D and E than Group A (P < 0.05 for each comparison). There was no significant difference between Group A and Group B. In addition, the rate of apoptosis was significantly higher in Group E than either Group C or Group D (P < 0.05 for each comparison; Table 2).
Figure 3.
Apoptosis in the gastric adenocarcinoma cell line AGS following erlotinib treatment and/or transfection with small hairpin (sh)RNA targeting focal adhesion kinase (FAK). Group A, control group (nontransfected, no erlotinib); Group B, empty vector group (transfected with empty vector, no erlotinib); Group C, FAK group (transfected with FAK-shRNA, no erlotinib); Group D, erlotinib group (nontransfected, 40 µmol/l erlotinib); Group E, combination group (transfected with FAK-shRNA, 40 µmol/l erlotinib).
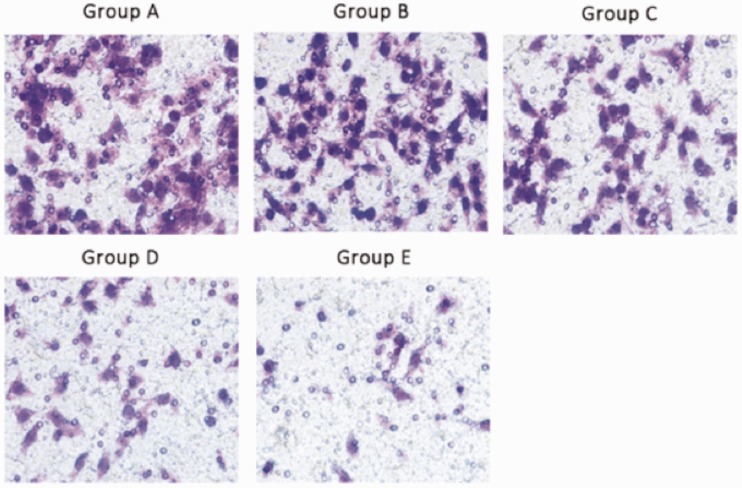
Representative light photomicrographs of the cell invasion (transwell) assay are shown in Figure 4, and cell invasion data are shown in Table 2. Cell invasion was significantly higher in Groups C, D and E than in Group A, and significantly higher in Group E compared with Groups C and D (P < 0.05 for each comparison; Table 2). There was no significant difference between groups A and B.
Figure 4.
Representative light photomicrographs of cell invasion (transwell assay) in the gastric adenocarcinoma cell line AGS following erlotinib treatment and/or transfection with small hairpin (sh)RNA targeting focal adhesion kinase (FAK). Group A, control group (nontransfected, no erlotinib); Group B, empty vector group (transfected with empty vector, no erlotinib); Group C, FAK group (transfected with FAK-shRNA, no erlotinib); Group D, erlotinib group (nontransfected, 40 µmol/l erlotinib); Group E, combination group (transfected with FAK-shRNA, 40 µmol/l erlotinib). Original magnification × 400.
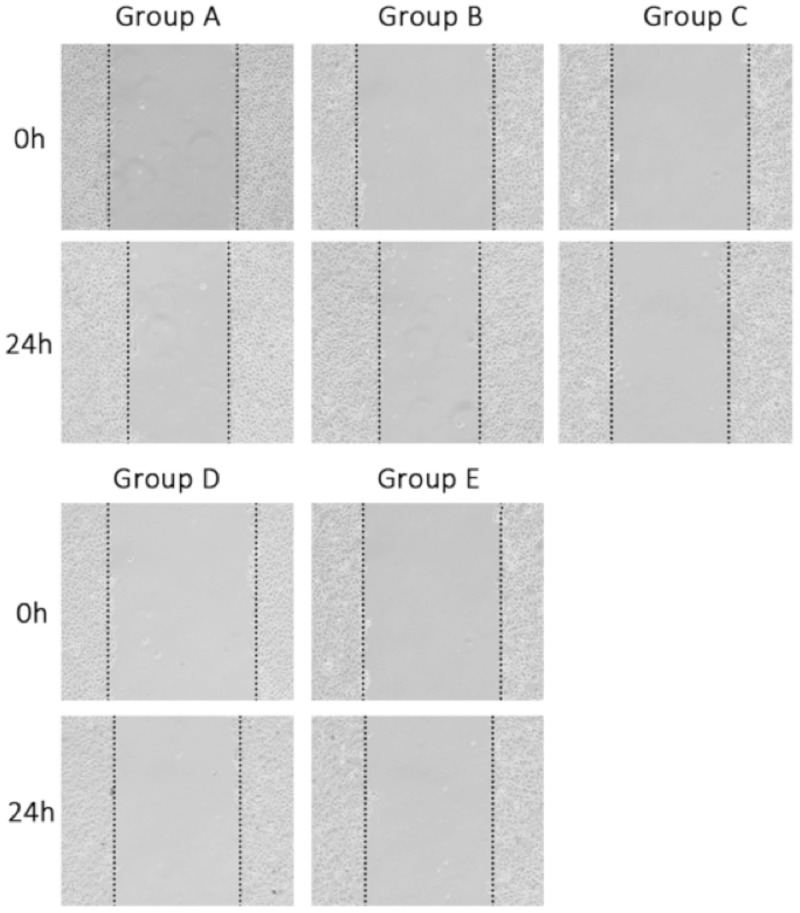
Representative photomicrographs of the cell migration (scratch) assay are shown in Figure 5, and cell migration data are given in Table 2. The wound healing (cell migration) rate was significantly lower in Groups C, D and E than in group A, and significantly lower in Group E compared with Groups C and D (P < 0.05 for each comparison; Table 2). There was no significant difference in wound healing between groups A and B.
Figure 5.
Representative light photomicrographs of cell migration (scratch assay) in the gastric adenocarcinoma cell line AGS following erlotinib treatment and/or transfection with small hairpin (sh)RNA targeting focal adhesion kinase (FAK). Group A, control group (nontransfected, no erlotinib); Group B, empty vector group (transfected with empty vector, no erlotinib); Group C, FAK group (transfected with FAK-shRNA, no erlotinib); Group D, erlotinib group (nontransfected, 40 µmol/l erlotinib); Group E, combination group (transfected with FAK-shRNA, 40 µmol/l erlotinib). Original magnification × 200.
Discussion
The proliferation, migration and invasion of gastric cancer AGS cells were significantly downregulated by FAK-shRNA, erlotinib, or both in the present study, while apoptosis was significantly increased. In addition, these effects were significantly greater in cells treated with both FAK-shRNA and erlotinib compared with those treated with one intervention only. This may be related to the interruption of the dual-signal channel, and is worthy of further investigation.
Gastric cancer is one of the most common cancers worldwide, with almost a million new cases diagnosed each year,5 of which approximately 65% are from developing countries and 42% are from China.6 Gastric cancer is the second most fatal cancer, with its death rate being the highest among malignancies in Japan, Korea, China, and other Asian countries.7 The pathogenesis of gastric cancer is not sufficiently understood, and effective measures for prevention are lacking.8 Molecular biology studies in oncology have shown that the occurrence and development of gastric cancer is extremely complex, involving polygenes and the actions of multiple factors over long periods.9 Fundamentally, gastric cancer is a genetic disease, and the activation of oncogenes or the inactivation of tumour suppressor genes may therefore cause abnormal cell proliferation and differentiation, which together with apoptosis, are critical factors in tumour occurrence and development. It has been shown that combination treatment with chemotherapy and trastuzumab improved the survival of patients with HER2-positive advanced gastric cancer compared with chemotherapy alone.10 Unfortunately, the rate of HER2-positivity is only 25% worldwide, and even lower in China.11 In addition, about half of individuals with HER2-overexpressing cancer do not respond to trastuzumab-based therapies, owing to various resistance mechanisms.12 Further studies are required to confirm whether HER2 is a useful predictive marker of trastuzumab efficacy, and whether targeting other molecules can treat patients who are HER2-negative13.
Integrin/Src/FAK/p130cas and the EGFR signal channel are known to be closely related to the occurrence and development of gastric cancer.14,15
RNA interference is a protection mechanism that exists widely in animals and plants to defend against viral infections and genomic instability caused by insertion mutations. Double-stranded small interference RNA can cause degradation of specifically targeted mRNA in vitro, and can knock out genes with high-efficiency, high-specificity, high-stability, transmissibility and heritability16. Western blot analysis showed that shRNA effectively inhibited FAK expression in AGS cells in the present study, and combined treatment with FAK-shRNA erlotinib affected cell proliferation, growth, migration and apoptosis. The significantly greater effect of combined treatment compared with either treatment alone suggests that there is cross-talk between the integrin signal channel and the EGFR channel, as supported by the findings of others.4 RNA interference designed to reduce FAK expression inhibited cell growth, metabolism and invasiveness in the gastric cancer cell line SGC7901.17 Tyrosine kinase inhibitors have been shown to inhibit tumour cell proliferation and induce apoptosis, and reduce VEGF expression, inhibiting angiogenesis.18 Erlotinib, in combination with radiotherapy, has been shown to inhibit and radiosensitize tumour cells via effects on the cell cycle.19 In addition, the inhibition of FAK activity significantly reduced the invasion ability of AGS cells.20 Therefore, prevention of FAK signal channel conduction together with EGFR tyrosine kinase inhibition (by erlotinib) may provide a new therapeutic strategy for patients with gastric cancer.
In conclusion, we have shown that RNA interference effectively silences FAK expression and inhibits malignant cell proliferation and invasion in gastric cancer cells. The effect of FAK inhibition is increased by co-treatment with erlotinib. The mechanism of action may be closely related to the dual signal channel, but it is not known whether this is a result of bypass activation or a dual synergistic effect. Identification of the specific mechanisms involved requires further studies. Our findings provide a foundation for gastric cancer treatment using combined inhibition of multiple signal channels, and is the focus of gene-targeted gastric cancer biotherapy.21,22
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This work was supported by Fujian Provincial Natural Science Foundation of China (Grant No.2011J01171) and Scientific Research Foundation of the Higher Education Institutions of Fujian Province, China (No. JK2011022).
References
- 1.Hida T, Ogawa S, Park JC, et al. Gefitinib for the treatment of non-small-cell lung cancer. Expert Rev Anticancer Ther 2009; 9: 17–35. PMID: 19105704. [DOI] [PubMed] [Google Scholar]
- 2.Boysen M, Longson C, Stevens A. Erlotinib for the treatment of non-small-cell lung cancer. Lancet Oncol 2009; 10: 15–16. PMID: 19115513. [DOI] [PubMed] [Google Scholar]
- 3.Ricono JM, Huang M, Barnes LA, et al. Specific cross-talk between epidermal growth factor receptor and integrin αvβ5 promotes carcinoma cell invasion and metastasis. Cancer Res 2009; 69: 1383–1391. PMID: 19208836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai YL, Chu PY, Lai IR, et al. An EGFR/Src-dependent β4 integrin/FAK complex contributes to malignancy of breast cancer. Sci Rep 2009; 69: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torre LA, Bray F, Siegel RL, et al. Global Cancer Statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 6.Bernard W, Christopher P. World Cancer Report 2014. IARC Nonserial Publication 2014, pp. 17–21. [Google Scholar]
- 7.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. PMID: 25220842. [DOI] [PubMed] [Google Scholar]
- 8.Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14: 490–499. PMID: 23594786. [DOI] [PubMed] [Google Scholar]
- 9.Gong J, Morishita A, Kurokohchi K, et al. Use of protein array to investigate receptor tyrosine kinases activated in gastric cancer. Int J Oncol 2010; 36: 101–106. [PubMed] [Google Scholar]
- 10.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-poshtive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376: 687–697. PMID: 20728210. [DOI] [PubMed] [Google Scholar]
- 11.Lordick F, Bang YJ, Kang YK, et al. 3541 POSTER HER2-positive advanced gastric cancer: similar HER2-positivity levels to breast cancer. EJC Suppl 2007; 5: 272. [Google Scholar]
- 12.Zhang S, Huang WC, Li P, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med 2011; 17: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng WQ, Huang D, Ying JM, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol 2013; 24: 2360–2364. PMID: 23788757. [DOI] [PubMed] [Google Scholar]
- 14.Weng X, Zhang H, Ye J, et al. Hypermethylated Epidermal growth factor receptor (EGFR) promoter is associated with gastric cancer. Sci Rep 2015; 5: 10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JH, Lee BL, Yoon J, et al. Focal adhesion kinase (FAK) gene amplification and its clinical implications in gastric cancer. Hum Pathol 2010; 41: 1664–1673. [DOI] [PubMed] [Google Scholar]
- 16.Castanotto D, Rossh JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009; 457: 426–433. PMID: 19158789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dan L, Jian D, Na L, et al. Crosstalk between EGFR and integrin affects invasion and proliferation of gastric cancer cell line, SGC7901. Onco Targets Ther 2012; 5: 271–277. PMID: 23109808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Solomon B, Hagekyriakou J, Trivett MK, et al. EGFR blockade with ZD1839 (“Iressa”) potentiates the antitumor effects of single and multiple fractions of ionizing radiation in human A431 squamous cell carcinoma. Epidermal growth factor receptor. Int J Radiat Oncol Biol Phys 2003; 55: 713–723. PMID: 12573759. [DOI] [PubMed] [Google Scholar]
- 19.Liu XQ, Qiao TK. Radiosensitizing effect of erlotinib on human lung adenocarcinoma cell line A549. Zhonghua Zhong Liu Za Zhi 2013; 35: 819–823. [in Chinese, English Abstract]. PMID: 24447478. [PubMed] [Google Scholar]
- 20.Lai IR, Chu PY, Lin HS, et al. Phosphorylation of focal adhesion kinase at Tyr397 in gastric carcinomas and its clinical significance. Am J Pathol 2010; 177: 1629–1637. PMID: 20724588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng L, Tan W, Zhang J, et al. Combining trastuzumab and cetuximab combats trastuzumab-resistant gastric cancer by effective inhibition of EGFR/ErbB2 heterodimerization and signaling. Cancer Immunol Immunother 2014; 63: 581–586. PMID: 24668364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita-Kashima Y, Iijima S, Yorozu K, et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res 2011; 17: 5060–5070. PMID: 21700765. [DOI] [PubMed] [Google Scholar]