Abstract
Objective
To explore the feasibility of high-throughput massively parallel genomic DNA sequencing technology for the noninvasive prenatal detection of fetal sex chromosome aneuploidies (SCAs).
Methods
The study enrolled pregnant women who were prepared to undergo noninvasive prenatal testing (NIPT) in the second trimester. Cell-free fetal DNA (cffDNA) was extracted from the mother’s peripheral venous blood and a high-throughput sequencing procedure was undertaken. Patients identified as having pregnancies associated with SCAs were offered prenatal fetal chromosomal karyotyping.
Results
The study enrolled 10 275 pregnant women who were prepared to undergo NIPT. Of these, 57 pregnant women (0.55%) showed fetal SCA, including 27 with Turner syndrome (45,X), eight with Triple X syndrome (47,XXX), 12 with Klinefelter syndrome (47,XXY) and three with 47,XYY. Thirty-three pregnant women agreed to undergo fetal karyotyping and 18 had results consistent with NIPT, while 15 patients received a normal karyotype result. The overall positive predictive value of NIPT for detecting SCAs was 54.54% (18/33) and for detecting Turner syndrome (45,X) was 29.41% (5/17).
Conclusion
NIPT can be used to identify fetal SCAs by analysing cffDNA using massively parallel genomic sequencing, although the accuracy needs to be improved particularly for Turner syndrome (45,X).
Keywords: High-throughput sequencing, sex chromosomal aneuploidies, noninvasive prenatal testing, prenatal diagnosis, prenatal screening, cffDNA
Introduction
Birth defects have become a major public health problem for children’s health and they have affected the quality of life of the affected population of newborns.1 Chromosomal abnormalities are one of the most serious birth defects. Due to the lack of effective treatment, they often cause serious damage to the fetus. For example, it is well known that aneuploidies are the most common chromosomal abnormalities, including trisomy 21 (Down’s syndrome), trisomy 18 (Edwards’ syndrome), trisomy13 (Patau’s syndrome), and sex chromosome aneuploidies (SCA).2 SCAs are caused by the presence of an abnormal number of sex chromosomes (X or Y) in a cell, and they include 45,X (Turner syndrome), 47,XXX (Triple X syndrome), 47,XXY (Klinefelter syndrome) and 47,XYY. The main features of 45,X, 47,XXY and some cases of 47,XXX are sex development retardation or abnormality, and infertility. Some SCAs, especially 45,X and 47,XXX may show intellectual disability.3 Once the patient has clinical symptoms, there are no effective treatments available. Early intervention services such as physical therapy, occupational therapy, and individualized education plans can make a huge difference in the outcome of patients with SCAs.3,4 Additionally, early hormonal therapy and hormonal replacement therapy have been shown to improve the outcomes for babies with 45,X syndrome or 47,XXY syndrome.5 Having the correct diagnostic information about SCAs in the newborn baby is critical for the introduction of early therapy. Earlier screening leads to a better prognosis for many SCA cases.6
Currently, the most common prenatal screening method for determining the risk of trisomy 21 (Down’s syndrome) is based on measuring the maternal serum levels of alpha-fetoprotein (AFP), unconjugated estriol (uE3), and the free beta subunit of human chorionic gonadotropin (fβhCG) combined with the maternal age in the second trimester.7,8 The rate of detection of trisomy 21 is 75% with a 5% false-positive rate using this screening programme.9 The detection rate is much lower in some developing countries if there is not good quality control.9–11 Therefore, screening efficiency is currently unsatisfactory.
Recently, noninvasive prenatal testing (NIPT) for fetal aneuploidies has been shown to be a better prenatal screening method than blood biochemical screening; with NIPT detecting cell-free fetal DNA (cffDNA) obtained from the maternal plasma using massively parallel sequencing technology.12 Currently, NIPT is widely used to screen for trisomy 21, trisomy 18, and trisomy 13 fetal aneuploidies because of its high accuracy and sensitivity. For example, a detection rate of 99.2% with a false-positive rate of 0.09% was reported for trisomy 21, a detection rate of 96.3% and a false-positive rate of 0.13% for trisomy 18, and a detection rate of 91.0% and a false-positive rate of 0.13% for trisomy 13.13 Some professional medical organizations have issued guidelines about NIPT, such as the American College of Obstetricians and Gynecologists, the International Society for Prenatal Diagnosis, and the American College of Medical Genetics.14–16 NIPT is regarded to be the best technology for screening for the commonest autosomal trisomies, such as trisomy 21, trisomy 18, and trisomy 13, at present.17 Research has also investigated the use of NIPT to screen for fetal SCAs.13 However, there are few publications on the use of NIPT for SCAs and little clinical experience.18
The present study investigated the feasibility of using NIPT to screen for fetal SCAs using cffDNA in maternal plasma.
Patients and methods
Patient population
This retrospective study recruited consecutive pregnant women who attended Changzhou Woman and Children Health Hospital affiliated with Nanjing Medical University, Changzhou City, Changzhou, Jiangsu Province, China for prenatal screening and diagnosis between October 2012 and October 2016. The inclusion criteria were as follows: (i) pregnant women aged 18–50 years; (ii) gestational age of 13–27 weeks. Gestational age was determined using the date of the last menstrual period and data from the first ultrasound. After prenatal screening in the second trimester, women were recruited to undergo NIPT.
The study was approved by the Institutional Review Board of Changzhou Woman and Children Health Hospital and each study participant provided written informed consent prior to participation.
Routine prenatal screening in the second trimester
After the demographic characteristics and medical history were recorded, a maternal blood sample (5 ml) was withdrawn from the cubital vein and collected into a BD Vacutainer sample tube (Becton, Dickinson & Co., Franklin Lakes, NJ, USA) containing spray-coated silica and a polymer gel for serum separation. The serum was separated by centrifugation as soon as possible and stored at −20℃. The concentrations of AFP, uE3, and fβhCG were measured using DELFIA® time-resolved fluorescence assays (PerkinElmer, Gaithersburg, MD, USA). Combined with maternal age, gestational age, body weight, and presence of type 2 diabetes mellitus, the risk of trisomy 21 and trisomy 18 was calculated using Lifecycle 4.0 software (PerkinElmer). A high-risk score for trisomy 21 was ≥ 1/300 and for trisomy 18 was ≥ 1/350; an intermediate risk score for trisomy 21 was 1/301–1/1000 and for trisomy 18 was 1/351–1/1000. A risk value less than 1/300 or 1/350 was considered as low risk for trisomy 21 and trisomy 18, respectively. Advanced maternal age was defined as ≥ 35 years.
Laboratory methodology for NIPT
A sample of whole blood (5 ml) was collected from all study participants into EDTA-K2 spray-dried Vacutainers (EDTA tubes; Becton, Dickinson & Co.). Whole blood samples were refrigerated or stored on wet ice and were processed to plasma within 6 h of collection. The maternal blood samples were centrifuged using an Eppendorf 5810R centrifuge (Eppendorf, Hamburg, Germany) at 1600 g for 10 min at 4℃, and the plasma was collected. The plasma was then centrifuged in an Eppendorf 5424 centrifuge (Eppendorf) at 1600 g for 10 min at 4℃ and immediately stored frozen at −70℃ until DNA extraction. The plasma DNA was extracted from 1 ml plasma for each study participant using a QIAamp Circulating Nucleic Acid Kit (Qiagen, Valencia, CA, USA). If the concentration of total free DNA was > 0.7 ng/µl, then it would not be possible to construct DNA libraries, so a second blood sample was collected. The resulting plasma DNA was used to make DNA libraries for sequencing using the modified ChIP-Seq protocol as described previously.19 DNA libraries from 12 plasma samples were indexed using 6 nt barcodes and quantified with a KAPA SYBR® FAST qPCR kit (Kapa Biosystems, Wilmington, MA, USA). These libraries were then pooled and loaded. One lane of an Illumina NextSeq 500 flow cell (Illumina China, Shanghai, China) was used to perform the sequencing using a single-ended 43-base pair sequencing protocol following the manufacturer’s instructions.
The sequences from each library were split according to their unique indexes. The split sequences were then mapped to the unmasked human genome sequence (hg19). SOAP2 mapping algorithm was used to obtain the results as previously described.20 The sequences of each sample that were mapped to each chromosome were counted, and the guanine-cytosine (GC) content was calculated. Normalized chromosome representation and GC correction were used to generate a Z-score as previously described.20 Each pair of chromosomes was defined as increased if their Z-score was > 3 and decreased if its Z-score was < –3. Samples with a fetal fraction < 4% of the total cell-free DNA were considered inappropriate for further analysis as there were insufficient data to analyse.
Fetal chromosome karyotype analyses
Patients identified as having pregnancies associated with SCAs were offered prenatal fetal chromosomal karyotyping. All prenatal samples were cultured following standard protocols.21 Amniocytes were cultured with BIO-AMF™-2 medium (Biological Industries, Kibbutz Beit-Haemek, Israel) and Chang Medium® D (Irvine Scientific, Santa Ana, CA, USA). Cord blood cells were cultivated with peripheral blood lymphocyte medium (Xiangya Gene Technology, Hunan, China). At least 20 G-banded metaphases from each sample were analysed using the Wright’s staining method.22
Statistical analyses
All statistical analyses were performed using the SPSS ® statistical package, version 18.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Data are presented as mean ± SDs. Analysis of variance was used to compare the differences between different groups. A P-value < 0.05 was considered statistically significant.
Results
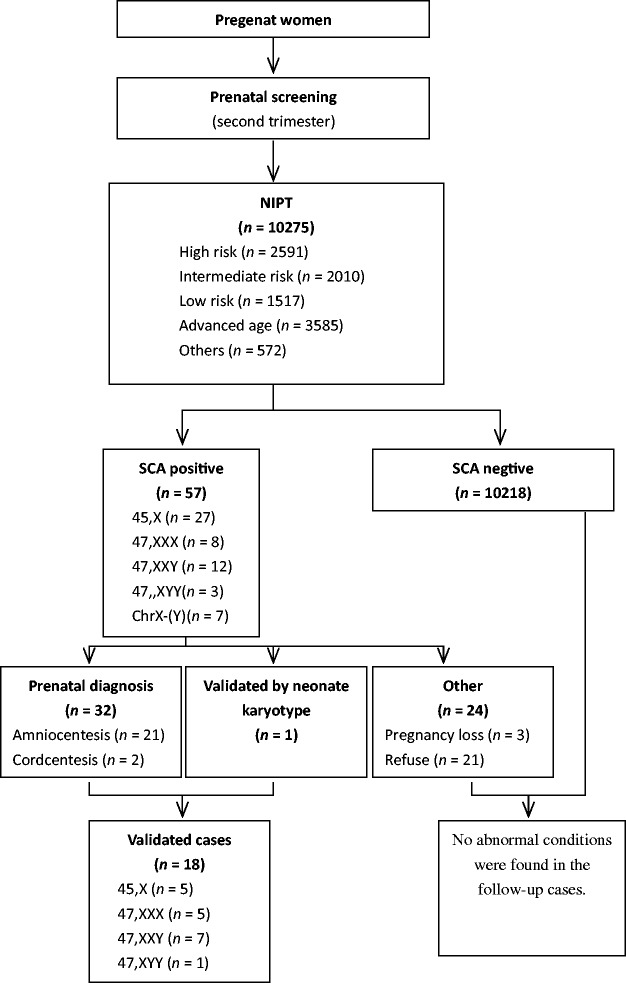
After prenatal screening during the second trimester, a total of 10 275 pregnant women agreed to undergo NIPT. Of these, 6118 pregnant women agreed to prenatal biochemical screening prior to NIPT and 4157 agreed to undergo NIPT without prior prenatal biochemical screening. Figure 1 shows the flow of study participants through this study. The baseline demographic and clinical characteristics of the patients are shown in Table 1. Blood was collected from all patients at gestational ages of 13–27 weeks. A total of 3585 of 10 275 (34.89%) patients were of advanced maternal age (≥ 35 years). A total of 2591 of 10 275 (25.22%) patients were considered at high risk of second trimester prenatal screening and 2010 of 10 275 (19.56%) were at intermediate risk.
Figure 1.
Flow diagram showing patient numbers at various stages of a study that investigated the use of noninvasive prenatal testing (NIPT) for screening for fetal sex chromosome aneuploidies.
Table 1.
Demographic and clinical characteristics of the pregnant women (n = 10 275) enrolled in a study that investigated the use of noninvasive prenatal testing (NIPT) for screening for fetal sex chromosome aneuploidies.
| Characteristic | n (%) |
|---|---|
| Chinese | 10 275 (100.00) |
| Singleton pregnancy | 10 275 (100.00) |
| Gestational age at NIPT | |
| 13–27 weeks | 10 275 (100.00) |
| Routine prenatal screening results | |
| High risk | 2591 (25.22) |
| Intermediate risk | 2010 (19.56) |
| Low risk | 1517 (14.76) |
| Maternal age, years | |
| <35 | 6690 (65.11) |
| ≥35 | 3585 (34.89) |
Using massively parallel sequencing technology, 57 patients (0.55%) demonstrated positive NIPT results for fetal SCA, including 27 patients positive for 45,X (Turner syndrome), eight for 47,XXX (Triple X syndrome), 12 for 47,XXY (Klinefelter syndrome) and three for 47,XYY (Figure 1). As a result of these genetic findings, 33 of 57 pregnant women underwent fetal karyotyping, which included 30 patients who underwent amniocentesis, two who underwent cordocentesis and one newborn baby who underwent neonatal blood karyotyping. The remaining women either refused to undergo any further tests (n = 21) or the pregnancy was lost (n = 3). A comparison of the outcome of the NIPT with the karyotyping results demonstrated that the fetal SCA of 18 of 33 patients was validated by karyotyping and the newborns of 15 patients were considered to have normal karyotypes. The positive predictive value (PPV) of NIPT in SCA was 54.54% (Table 2).
Table 2.
Comparison of the positive noninvasive prenatal testing (NIPT) results for fetal sex chromosome aneuploidies (SCA) compared with karyotyping in pregnant women (n = 57) enrolled in a study that investigated the use of NIPT for screening for fetal SCAs.
| NIPT positive SCA | Karyotype validateda |
Without karyotype validated | |||
|---|---|---|---|---|---|
| n | True positive | False positive | Positive predictive value (%) | ||
| 45,X | 27 | 5 | 12 | 5/17 (29.41) | 10 |
| 47,XXY | 12 | 7 | 2 | 7/9 (77.78) | 3 |
| 47,XXX | 8 | 5 | 0 | 5/5 (100.00) | 3 |
| 47,XYY | 3 | 1 | 0 | 1/1 (100.00) | 2 |
| ChrX-(Y) | 7 | 0 | 1 | – | 6 |
| Total | 57 | 18 | 15 | 18/33 (54.54) | 24 |
Tests included amniocentesis (n = 30), cordocentesis (n = 2) and neonatal karyotyping (n = 1).
A comparison of the positive NIPT results for SCAs compared with karyotyping in pregnant women stratified according to the demographic characteristics of prenatal screening risk and advanced age demonstrated that of the 2591 high-risk pregnant women, 13 (0.50%) were NIPT positive (Table 3). After karyotyping validation, seven patients were confirmed as being true positives, giving a PPV of 77.78%. For the 12 out of 2010 intermediate-risk patients who received NIPT positive results, the PPV was 37.50%. Of the 3585 women aged ≥ 35 years, 19 had a positive NIPT result and the PPV was 45.45%.
Table 3.
Comparison of the positive noninvasive prenatal testing (NIPT) results for fetal sex chromosome aneuploidies (SCA) compared with karyotyping in pregnant women stratified according to the demographic characteristics of prenatal screening risk and advanced age.
| Characteristic | n | NIPT positive | Karyotype validateda |
Without karyotype validated | ||
|---|---|---|---|---|---|---|
| True positive | False positive | Positive predictive value (%) | ||||
| Prenatal screening risk | ||||||
| High risk | 2591 | 13 | 7 | 2 | 7/9 (77.78) | 4 |
| Intermediate risk | 2010 | 12 | 3 | 5 | 3/8 (37.50) | 4 |
| Low risk | 1517 | 8 | 2 | 2 | 2/2 (50.00) | 4 |
| Advanced age | 3585 | 19 | 5 | 6 | 5/11 (45.45) | 8 |
Tests included amniocentesis, cordocentesis and neonatal karyotyping.
When the individual SCAs were analysed, the PPVs were as follows: Turner syndrome (45,X; PPV: 29.41% [5/17]); Klinefelter syndrome (47,XXY; PPV: 77.78% [7/9]), Triple X syndrome (47,XXX; 100% [5/5]) and XYY syndrome (PPV: 100% [1/1]).
Discussion
In the past few years, NIPT has been widely applied to screen for trisomy 21, trisomy 18 and trisomy 13 using cffDNA in maternal plasma.23 Clinicians consider NIPT to be the best way to screen for trisomy 21, trisomy 18 and trisomy 13 in the fetus during the second trimester, being better than prenatal screening in the first or second trimesters.24 According to a 2015 meta-analysis, the detection rates and false-positive rates of NIPT in singleton pregnancies were 99.2% and 0.09%, for trisomy 21, respectively; 96.3% and 0.13% for trisomy 18; and 91.0% and 0.13% for trisomy 13.13 Other studies have yielded similar results.25,26
In addition to screening for the common fetal aneuploidies described above, some research has demonstrated that NIPT could be used to identify SCA. For example, a meta-analysis showed that the detection rate was 90.3% for monosomy X and 93.0% for SCAs other than monosomy X.13 However, the detection rates were different in the individual studies included in the meta-analysis and the detection rate for monosomy X ranged from 66.7% to 100%.13 Another report showed that the analysis of maternal plasma cffDNA using a targeted assay could detect fetal SCA with a reasonably high sensitivity (92.6%) and a combined false-positive rate of less than 1%.27 The positive predictive rate for the SCAs was reported to be 48.4% and the negative predictive value was 100% in a study using massively parallel genomic sequencing of DNA.28 Another study using the same sequencing technique reported a PPV of 54.17% for fetal SCAs.29 In present study, a high-throughput massively parallel genomic sequencing technique was used to screen for fetal SCAs as part of an investigation to determine the potential value of NIPT in detecting fetal SCAs in the second trimester. The overall PPV of NIPT in the present study was 54.54%, which when categorized by individual SCAs was 29.41% for Turner syndrome (45,X), 77.78% for Klinefelter syndrome (47,XXY), 100% for Triple X syndrome (47,XXX) and 100% for XYY syndrome (47,XYY). In this present study, there were 24 patients at a high risk SCAs as determined by NIPT who refused to undergo further karyotyping analysis. If these 24 patients had yielded true positive results, then the upper limit PPV of NIPT would have been 73.68 % (42/57); and the lower limit PPV of NIPT would have been 31.58 % (18/57), if these 24 patients were regarded as false positive.
Circulating cell-free DNA is derived from both maternal and placental tissues, so intrinsic biological factors such as maternal somatic mosaicism, undiagnosed maternal SCA and maternal copy-number imbalance can influence the accuracy of NIPT.30 Cell-free fetal DNA mainly originates from the placental trophoblasts, which are often discovered to be mosaic. In a mosaic, the degree of mosaicism will impact the performance of the test because it will reduce the effective fetal fraction.31–33 This potential for mosaicism should be considered as a limitation of NIPT. Secondly, the strongest factor associated with the fetal fraction is maternal weight; the false negative rate and rate of low fetal fractions are highest for women with high maternal weights.31–33 These are well-known reasons for discordant results between NIPT and fetal karyotyping.
In the present study, the PPV for Turner syndrome (45,X; 29.41%) was lower than for the other SCAs. There are several reasons that might account for this: (i) there are 1098 genes on the X chromosome and 78 genes on the Y chromosome; of which 58 genes are homologous genes on both sex chromosomes, and the majority of these (29 genes) are at the ends of the X and Y chromosomes; (ii) at present, the detection length is only 36 bases, which could easily lead to sequencing positioning dislocation between the X and Y chromosomes; (iii) the non-random inactivation of the X chromosome in placental tissue might be the reason for the low PPV of Turner syndrome, with the paternal X chromosome tending to inactivate in XX female trophoblasts.34,35
This study had a number of limitations. First, the sensitivity, specificity and negative predictive value were not calculated due to the difficulties in screening every newborn baby by karyotype analysis. Newborns with SCA can appear normal without physical or intellectual disability, so it is difficult to confirm the presence of the SCA syndrome without karyotype analysis before adolescence. Secondly, a relatively small number of pregnant women in a single centre were enrolled into the study. Larger multicentre studies are warranted to corroborate these findings. Thirdly, there was a small number of patients with SCAs, which has an incidence of 1 in 400 newborns.
In conclusion, this present study demonstrated that NIPT can be used to identify fetal SCAs by analysing cffDNA from the mother’s plasma using massively parallel genomic sequencing, although the accuracy needs to be improved particularly for Turner syndrome (45,X).
Acknowledgements
We thank all of the project participants for their contributions.
Declaration of conflicting interests
The authors declare that there are no conflicts of interests.
Funding
The study was supported by funding for the training of high level health professionals in Changzhou (no. 2016CZLJ013) and by a grant from the Social Development Fund of Changzhou (no. CE20155055).
References
- 1.Mai CT, Kirby RS, Correa A, et al. Public health practice of population-based birth defects surveillance programs in the United States. J Public Health Manag Pract 2016; 22: E1–E8. [DOI] [PubMed] [Google Scholar]
- 2.Fauret AL, Bilan F, Patri S, et al. Molecular biology usefulness for rapid diagnosis of Down's syndrome and common aneuploidies. Gynecol Obstet Fertil 2009; 37: 611–619. [Article in French, English abstract]. [DOI] [PubMed] [Google Scholar]
- 3.Pieters JJ, Kooper AJ, van Kessel AG, et al. Incidental prenatal diagnosis of sex chromosome aneuploidies: health, behavior, and fertility. ISRN Obstet Gynecol 2011. 2011:807106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girardin CM, Lemyre E, Alos N, et al. Comparison of adolescents with Klinefelter syndrome according to the circumstances of diagnosis: amniocentesis versus clinical signs. Horm Res 2009; 72: 98–105. [DOI] [PubMed] [Google Scholar]
- 5.Warren MP, Chua A. Appropriate use of estrogen replacement therapy in adolescents and young adults with Turner syndrome and hypopituitarism in light of the Women's Health Initiative. Growth Horm IGF Res 2006; 16(Suppl A): S98–S102. [DOI] [PubMed] [Google Scholar]
- 6.Abramsky L, Hall S, Levitan J, et al. What parents are told after prenatal diagnosis of a sex chromosome abnormality: interview and questionnaire study. BMJ 2001; 322: 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu B, Zhang B, Shi Y, et al. Bioinformatics characterization of differential proteins in serum of mothers carrying Down syndrome fetuses: combining bioinformatics and ELISA. Arch Med Sci 2012; 8: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus-Braun N, Birk O, Manor E, et al. Dependence of maternal serum [AFP]/[hCG] median ratios on age of gestation: comparison of trisomy 21 to euploid pregnancies. Prenat Diagn 2009; 29: 1130–1134. [DOI] [PubMed] [Google Scholar]
- 9.Dey M, Sharma S, Aggarwal S. Prenatal screening methods for aneuploidies. N Am J Med Sci 2013; 5: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dash P, Puri RD, Kotecha U, et al. Using noninvasive prenatal testing for aneuploidies in a developing country: lessons learnt. J Fetal Med 2014; 1: 131–135. [Google Scholar]
- 11.Campaña H, Ermini M, Aiello HA, et al. Prenatal sonographic detection of birth defects in 18 hospitals from South America. J Ultrasound Med 2010; 29: 203–212. [DOI] [PubMed] [Google Scholar]
- 12.Kotsopoulou I, Panagiota Tsoplou P, Mavrommatis K, et al. Non-invasive prenatal testing (NIPT): limitations on the way to become diagnosis. Diagnosis 2015; 2: 141–158. [DOI] [PubMed] [Google Scholar]
- 13.Gil MM, Quezada MS, Revello R, et al. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol 2015; 45: 249–266. [DOI] [PubMed] [Google Scholar]
- 14.Committee Opinion Summary No. 640. Cell-free DNA screening for fetal aneuploidy. Obstet Gynecol 2015; 126: 691–692. [DOI] [PubMed] [Google Scholar]
- 15.Benn P, Borell A, Chiu R, et al. Position statement from the Chromosome Abnormality Screening Committee on behalf of the Board of the International Society for Prenatal Diagnosis. Prenat Diagn 2015; 35: 725–734. [DOI] [PubMed] [Google Scholar]
- 16.Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med 2016; 18: 1056–1065. [DOI] [PubMed] [Google Scholar]
- 17.Greeley ET, Kessler KA, Vohra N. Clinical applications of noninvasive prenatal testing. J Fetal Med 2015; 2: 1–7. [Google Scholar]
- 18.Manotaya S, Xu H, Uerpairojkit B, et al. Clinical experience from Thailand: noninvasive prenatal testing as screening tests for trisomies 21, 18 and 13 in 4736 pregnancies. Prenat Diagn 2016; 36: 224–231. [DOI] [PubMed] [Google Scholar]
- 19.Chiu RW, Chan KC, Gao Y, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A 2008; 105: 20458–20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Huang S, Zhou X, et al. Non-invasive prenatal testing for fetal aneuploidies in the first trimester of pregnancy. Ultrasound Obstet Gynecol 2015; 45: 55–60. [DOI] [PubMed] [Google Scholar]
- 21.Caspersson T, Farber S, Foley GE, et al. Chemical differentiation along metaphase chromosomes. Exp Cell Res 1968; 49: 219–222. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Duan C, Shen C, et al. Detection of complex deletions in chromosomes 13 and 21 in a fetus by noninvasive prenatal testing. Mol Cytogenet 2016; 9: 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mardy A, Wapner RJ. Confined placental mosaicism and its impact on confirmation of NIPT results. Am J Med Genet C Semin Med Genet 2016; 172: 118–122. [DOI] [PubMed] [Google Scholar]
- 24.Fairbrother G, Johnson S, Musci TJ, et al. Clinical experience of noninvasive prenatal testing with cell-free DNA for fetal trisomies 21, 18, and 13, in a general screening population. Prenat Diagn 2013; 33: 580–583. [DOI] [PubMed] [Google Scholar]
- 25.Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med 2015; 372: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Gao Y, Jiang F, et al. Non-invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146,958 pregnancies. Ultrasound Obstet Gynecol 2015; 45: 530–538. [DOI] [PubMed] [Google Scholar]
- 27.Hooks J, Wolfberg AJ, Wang ET, et al. Non-invasive risk assessment of fetal sex chromosome aneuploidy through directed analysis and incorporation of fetal fraction. Prenat Diagn 2014; 34: 496–499. [DOI] [PubMed] [Google Scholar]
- 28.Porreco RP, Garite TJ, Maurel K, et al. Noninvasive prenatal screening for fetal trisomies 21, 18, 13 and the common sex chromosome aneuploidies from maternal blood using massively parallel genomic sequencing of DNA. Am J Obstet Gynecol 2014; 211 365.e1–12. [DOI] [PubMed] [Google Scholar]
- 29.Yao H, Jiang F, Hu H, et al. Detection of fetal sex chromosome aneuploidy by massively parallel sequencing of maternal plasma DNA: initial experience in a Chinese hospital. Ultrasound Obstet Gynecol 2014; 44: 17–24. [DOI] [PubMed] [Google Scholar]
- 30.Cheung SW, Patel A, Leung TY. Accurate description of DNA-based noninvasive prenatal screening. N Engl J Med 2015; 372: 1675–1677. [DOI] [PubMed] [Google Scholar]
- 31.Sekizawa A, Farina A, Okai T. Cell-free fetal DNA in plasma of pregnant women: clinical potential and origin. Taiwanese Journal of Obstetrics & Gynecology 2005; 44: 116–122. [Google Scholar]
- 32.Canick JA, Palomaki GE, Kloza EM, et al. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn 2013; 33: 667–674. [DOI] [PubMed] [Google Scholar]
- 33.Choi H, Lau TK, Jiang FM, et al. Fetal aneuploidy screening by maternal plasma DNA sequencing: ‘false positive' due to confined placental mosaicism. Prenat Diagn 2013; 33: 198–200. [DOI] [PubMed] [Google Scholar]
- 34.Clerc P, Avner P. Reprogramming X inactivation. Science 2000; 290: 1518–1519. [DOI] [PubMed] [Google Scholar]
- 35.Eggan K, Akutsu H, Hochedlinger K, et al. X-Chromosome inactivation in cloned mouse embryos. Science 2000; 290: 1578–1581. [DOI] [PubMed] [Google Scholar]