Abstract
Objective
To investigate the effect of pretreatment with midazolam on myoclonus induced by etomidate injection.
Methods
A meta-analysis was performed using Review Manager software, version 5.2. Two researchers independently searched PubMed, Cochrane Library, and Embase® databases for randomized controlled trials involving patients who underwent etomidate induced general anaesthesia with or without midazolam pretreatment, published between 1990 and 2016. Outcome measures comprised overall myoclonus incidence rate and incidence rate classified by degree of myoclonus following etomidate injection. Data were assessed using a fixed effects model.
Results
Five studies, comprising 302 patients, were included for analysis. Overall incidence rate of etomidate injection-induced myoclonus was significantly lower in the pooled midazolam group versus controls (relative risk [RR] 0.34, 95% confidence interval [CI] 0.26, 0.44); Results subgrouped by degree of myoclonus showed significantly lower incidence in midazolam groups versus control groups for mild myoclonus (RR 0.56, 95% CI 0.39, 0.80); moderate myoclonus (RR 0.20, 95% CI 0.10, 0.41); and severe myoclonus (RR 0.12, 95% CI 0.04, 0.39).
Conclusion
Midazolam can effectively prevent etomidate-induced myoclonus, and alleviate the degree of etomidate-induced myoclonus.
Keywords: Midazolam, etomidate, myoclonus, meta-analysis
Introduction
Etomidate is a general anaesthesia drug that provides smooth anaesthesia induction conditions with little effect on the circulation and on breathing suppression.1,2 Etomidate is widely used in clinical anaesthesia, and is particularly suitable for use in patients with poor heart function.3 Without other interventions, however, incidence of etomidate-induced myoclonus in patients undergoing anaesthesia induction may be as high as 50–80%.4 Myoclonus can result in increased patient discomfort, for example, non-fasting patients tend to have a higher risk of reflux and aspiration;5 in patients with open-globe injuries, myoclonus can result in increased intraocular pressure, giving rise to serious complications such as vitreous prolapse;4 myoclonus may also induce epilepsy or increase focal epileptic activities;6 and in cases of electrical cardioversion, continuous electroencephalographic monitoring may also be disturbed by myoclonus, thus affecting the treatment.7
The effects of midazolam pretreatment on etomidate-induced myoclonus have been investigated in a number of clinical trials. The aim of the present study was to perform a review and meta-analysis of published data to analyse the effect of pretreatment with midazolam on myoclonus induced by etomidate injection.
Materials and methods
Study criteria and outcomes
To be included for analysis, studies were required to meet all of the following criteria: (1) randomized controlled trial design; (2) included patients aged ≥18 years who underwent general anaesthesia induced with etomidate; (3) and included a midazolam group and a placebo group. Animal studies, case reports, reviews and other publications, any publications with incomplete or repetitive data, and studies that were not randomized controlled trials were excluded.
Outcome measures comprised myoclonus incidence rate, and degree of myoclonus, following etomidate injection.
Search strategy
The following databases were searched for articles published between January 1990 and May 2016: PubMed, Cochrane Library and Embase®. The search was limited to English language articles and search terms comprised the following: midazolam, etomidate and its synonyms, myoclonus and its synonyms, randomized, controlled, and placebo.
Screening and data extraction
Search results were assessed by two researchers (CZ and YZ) who independently read titles and abstracts and excluded studies inconsistent with the inclusion criteria. Articles that met the inclusion criteria were then independently read in full by the two researchers, and the results were cross checked. Cases of disagreement were discussed between the two researchers and a third party (LR) if necessary until agreement was reached.
In cases of unclear or missing data, authors or corresponding authors were contacted by telephone or in writing to confirm or supplement the data as appropriate. For publications that met all inclusion criteria, the following information was extracted: (1) general information (including author, publication date, and other literature sources); (2) study setting and design (general information, interventions, and types of surgery undertaken); (3) outcome measures (overall myoclonus incidence rate, and degree of myoclonus following etomidate injection).
Quality assessment
Methodological quality of the included studies was evaluated according to the Jadad scale,8 which scores factors such as randomization method, concealment of allocation and blinding. Out of a maximum score of 7 points: ≥ 4 points equated to a high-quality publication; and <4 points equated to a low-quality publication. For trials that had been supplemented and published several times, the publication with the most complete data was selected.
Statistical analyses
Meta-analyses were performed using Review Manager software, version 5.2 (Cochrane Collaboration, London, UK). Data were evaluated as overall myoclonus incidence rate, and myoclonus incidence rate in patients divided into subgroups according to degree of myoclonus:4 mild, only mild fasciculation involving face and/or distal upper and/or lower extremities; moderate, marked movements of the face and/or limbs; or severe, involving limbs and trunk. Enumeration data are presented as relative risk (RR), and measurement data are presented as standardized mean difference (SMD), with a 95% confidence interval (CI), and test level of effect size α = 0.05. Heterogeneity was assessed using χ2-test. A fixed effects model was used to conduct the meta-analysis when P > 0.1 and I2 < 50%, indicating no heterogeneity. Following analysis of sources of heterogeneity, a random effects model was used to conduct combined effect analyses when P ≤ 0.1 and I2 ≥ 50%, indicating heterogeneity.
Results
Search results and study characteristics
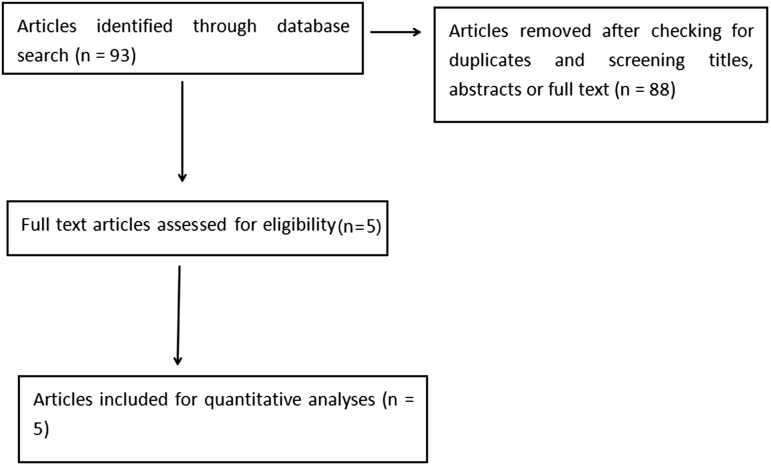
The literature search and review results are presented in Figure 1. The initial search resulted in 93 articles for screening. Following duplicate-checking and reading titles, abstracts, and original articles, five articles,9–13 with a total of 302 patients were included in the meta-analysis. The pooled study group comprised 151 midazolam-treated patients and 151 normal saline-treated controls. Basic characteristics of the included studies are shown in Table 1.
Figure 1.
Flow diagram showing results of the literature search and review of articles reporting randomized controlled trials of myoclonus associated with etomidate-induced general anaesthesia with or without midazolam pretreatment.
Table 1.
Characteristics of five articles included for meta-analysis that reported randomized controlled trials of etomidate induced general anaesthesia with or without use of midazolam.
| Publication detail | Study Country | Study participants, n | Time between midazolam and anaesthesia induction, s | Treatment group | Surgical setting | Jadad score |
|---|---|---|---|---|---|---|
| Patel et al. 20159 | India | 60 | 60 | Midazolam 0.05 mg/kg (n = 30) | elective surgery | 3 |
| Normal Saline 10 ml (n = 30) | ||||||
| Aktolga et al. 201010 | India | 102 | 90 | Midazolam 0.05 mg/kg (n = 51) | elective surgery | 4 |
| Normal Saline (n = 51) | ||||||
| Hwang et al. 200811 | Korea | 60 | 60 | Midazolam 0.05 mg/kg (n = 30) | elective surgery | 6 |
| Normal Saline (n = 30) | ||||||
| Huter et al. 200712 | Germany | 40 | 90 | Midazolam 0.015 mg/kg (n = 20) | cardiac surgery | 4 |
| Normal saline (n = 20) | ||||||
| Schwarzkopf et al. 200313 | Germany | 40 | 90 | Midazolam 0.015 mg/kg (n = 20) | elective gynaecological, ophthalmologic or urological surgery. | 4 |
| Normal saline (n = 20) |
All included studies followed the principle of randomization, and only one study9 did not mention the blinding method. Except for one study,11 results for all outcome measures were complete. None of the studies reported any patients leaving the study early, or lost to follow-up.
Meta-analysis results
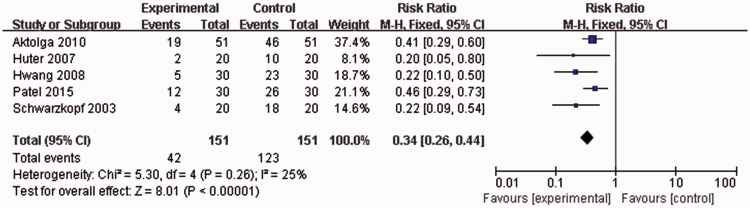
All included studies, with a total of 302 patients, reported incidence rates of myoclonus following etomidate injection. No statistically significant heterogeneity was found (P = 0.26, I2 = 25%), thus, a fixed effects model was used for meta-analysis. Overall myoclonus incidence rate following etomidate injection in patients pretreated with midazolam was significantly lower than that of controls without midazolam pretreatment (42/151 versus 123/151 patients; RR 0.34, 95% CI 0.26, 0.44; P < 0.00001; Figure 2).
Figure 2.
Forest plot showing fixed-effects model analyses of pooled overall myoclonus incidence data from randomized controlled trials of etomidate-induced general anaesthesia with or without use of midazolam. Incidence rate of myoclonus following etomidate injection was significantly lower in patients pretreated with midazolam than in controls. CI, confidence interval; M-H, Cochran–Mantel–Haenszel χ2-test.
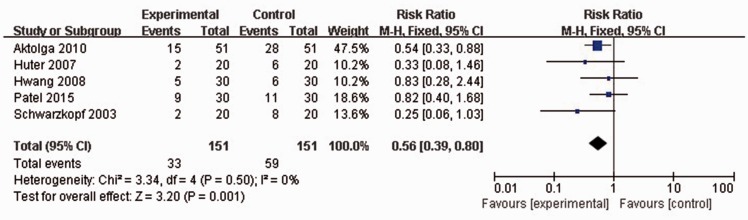
All included studies reported the rates of mild etomidate-induced myoclonus. No statistically significant heterogeneity was found among any of the studies (P = 0.5, I2 = 0%), thus a fixed effects model was used for meta-analysis. Mild myoclonus incidence rate following etomidate injection was significantly lower in patients pretreated with midazolam than in controls (33/151 versus 59/151 patients; RR 0.56, 95% CI 0.39, 0.80; P = 0.001; Figure 3).
Figure 3.
Forest plot showing fixed-effects model analyses of pooled mild myoclonus incidence data from randomized controlled trials of etomidate-induced general anaesthesia with or without use of midazolam. Incidence rate of mild myoclonus following etomidate injection was significantly lower in patients pretreated with midazolam than in controls. Mild myoclonus, only mild fasciculation involving face and/or distal upper and/or lower extremities; CI, confidence interval; M-H, Cochran–Mantel–Haenszel χ2-test.
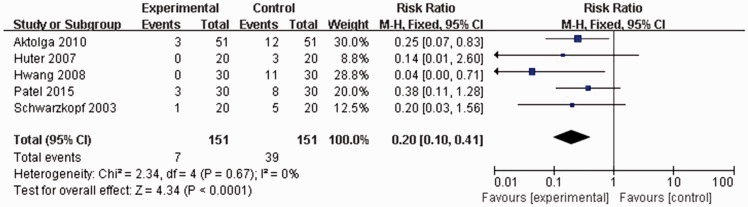
All included studies reported the rates of moderate etomidate-induced myoclonus. No statistically significant heterogeneity was found among any of the studies (P = 0.67, I2 = 0%), thus data were analysed using a fixed effects model. Moderate myoclonus incidence rate following etomidate injection was significantly lower in patients pretreated with midazolam than in controls (7/151 versus 39/151 patients; RR 0.20, 95% CI 0.10, 0.41; P < 0.0001; Figure 4).
Figure 4.
Forest plot showing fixed-effects model analyses of pooled moderate myoclonus incidence data from randomized controlled trials of etomidate-induced general anaesthesia with or without use of midazolam. Incidence rate of moderate myoclonus following etomidate injection was significantly lower in patients pretreated with midazolam than in controls. Moderate myoclonus, marked movements of the face and/or limbs; CI, confidence interval; M-H, Cochran–Mantel–Haenszel χ2-test.
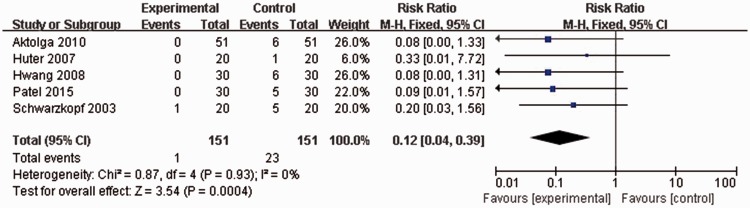
All included studies reported the rates of severe etomidate-induced myoclonus. Again, no statistically significant heterogeneity was found among any of the studies (P = 0.93, I2 = 0%), thus a fixed effects model was used for meta-analysis. Incidence rate severe myoclonus following etomidate injection was significantly lower in patients pretreated with midazolam than in controls (1/151 versus 23/151 patients; RR 0.12, 95% CI 0.04, 0.39; P = 0.0004; Figure 5).
Figure 5.
Forest plot showing fixed-effects model analyses of pooled severe myoclonus incidence data from randomized controlled trials of etomidate-induced general anaesthesia with or without use of midazolam. Incidence rate of severe myoclonus following etomidate injection was significantly lower in patients pretreated with midazolam than in controls. Severe myoclonus, involving limbs and trunk; CI, confidence interval; M-H, Cochran–Mantel–Haenszel χ2-test.
Funnel plot analysis (data not shown) indicated that the results were asymmetrical. The fixed effects model, risk difference, and SMD, respectively were converted into a random effects model, odds ratio, and mean difference. Similar results were achieved (data not shown), indicating that the results of the meta-analysis were stable.
Discussion
The results of the current meta-analysis showed that midazolam is effective in reducing the incidence of etomidate-induced myoclonus, and mitigating the degree of etomidate-induced myoclonus.
Although the mechanism of etomidate-induced myoclonus remains unclear, there are several suggested hypotheses. One study4 reported that etomidate-induced myoclonus was related to subcortical central depression, whereas another study14 suggested that myoclonus may occur following etomidate injection due to the fact that the suppressive neuronal circuit was inhibited earlier than the excitatory neuronal circuit. Another potential mechanism15 may be that etomidate acts on the Ɣ-aminobutyric acid (GABA)A receptor, thereby inhibiting the central nervous reticular activating system. Inhibition of neurotransmission through GABAA receptor activation may result in skeletal muscle control of relevant signal transmission, allowing the occurrence of autonomic nervous conduction.
Midazolam is a water-soluble benzodiazepine. Its pharmacological characteristics includes fast-action, short half-life, rapid and complete absorption by muscle tissue and mucous membranes, and bioavailability >90%.16 Under physiological pH, midazolam can quickly enter into the central nervous system through the blood brain barrier, thus rapidly playing its pharmacological role.16 One potential reason for the prevention of etomidate-induced myoclonus by midazolam may be that midazolam interferes with neurohumor GABA reuptake, leading to accumulation of GABA. Small doses of midazolam have been found to readily suppress the effect of stimulation of the medullary reticular formation on electrochemical responses, leading to relaxation of central muscle, thus inhibiting or mitigating myoclonus.13
The results of the present study are limited by a number of factors. The included studies were limited by quality, setting and design, and few were exactly matched with the inclusion criteria. The sample sizes were relatively small, and allocation concealment schemes were not reported, which may have given rise to selection or measurement bias. The midazolam dose, and time between midazolam administration and anaesthesia induction, varied between the studies. Direct comparative studies of midazolam and different treatment drugs and methods are scarce, thus, there is no strong evidence for which agent is the better choice in reducing myoclonus following etomidate-induced anaesthesia.
Treatment drugs and methods for preventing etomidate-induced myoclonus should be short-acting, and should not have significant effects on respiration or haemodynamics. Midazolam may meet these conditions, however, further randomized controlled trials with larger high-quality samples are needed to confirm the present findings.
In summary, the present study shows that pretreatment with midazolam can effectively reduce the occurrence of myoclonus.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Moss E, Powell D, Gibson RM, et al. Effect of etomidate on intracranial pressure and cerebral perfusion pressure. Br J Anaesth 1979; 51: 347–352. [DOI] [PubMed] [Google Scholar]
- 2.Weiss-Bloom LJ, Reich DL. Haemodynamic responses to tracheal intubation following etomidate and fentanyl for anaesthetic induction. Can J Anaesth 1992; 39: 780–785. [DOI] [PubMed] [Google Scholar]
- 3.Kaushal RP, Vatal A, Pathak R. Effect of etomidate and propofol induction on hemodynamic and endocrine response in patients undergoing coronary artery bypass grafting/mitral valve and aortic valve replacement surgery on cardiopulmonary bypass. Ann Card Anaesth 2015; 18: 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doenicke AW, Roizen MF, Kugler J, et al. Reducing myoclonus after etomidate. Anesthesiology 1999; 90: 113–119. [DOI] [PubMed] [Google Scholar]
- 5.Berry JM, Merin RG. Etomidate myoclonus and the open globe. Anesth Analg 1989; 69: 256–259. [PubMed] [Google Scholar]
- 6.Ebrahim ZY, Deboer GE, Luders H, et al. Effect of etomidate on the electroencephalogram of patients with epilepsy. Anesth Analg 1986; 65: 1004–1006. [PubMed] [Google Scholar]
- 7.Shulman MS, Edelmann R. Use of etomidate for elective cardioversion. Anesthesiology 1988; 68: 656–656. [DOI] [PubMed] [Google Scholar]
- 8.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 9.Patel MH, Rajesh C, Ramani MN, et al. A comparison of dexmedetomidine and midazolam for the prevention of myoclonic movements and pain following etomidate injection. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2015; 6: 161–168. [Google Scholar]
- 10.Aktolga S, Gunes Y, Gunduz M, et al. A comparison of midazolam and dexmedetomidine for the prevention of myoclonic movements and pain following etomidate injection. J Anaesth Clin Pharmacol 2010; 26: 162–166. [Google Scholar]
- 11.Hwang JY, Kim JH, Oh AY, et al. A comparison of midazolam with remifentanil for the prevention of myoclonic movements following etomidate injection. J Int Med Res 2008; 36: 17–22. [DOI] [PubMed] [Google Scholar]
- 12.Huter L, Schreiber T, Gugel M, et al. Low-dose intravenous midazolam reduces etomidate-induced myoclonus: a prospective, randomized study in patients undergoing elective cardioversion. Anesth Analg 2007; 105: 1298–1302. [DOI] [PubMed] [Google Scholar]
- 13.Schwarzkopf KR, Hueter L, Simon M, et al. Midazolam pretreatment reduces etomidate-induced myoclonic movements. Anaesth Intensive Care 2003; 31: 18–20. [DOI] [PubMed] [Google Scholar]
- 14.Kugler J, Doenicke A and Laub M. The EEG after etomidate. In Anaesthesiology and Resuscitation Volume 106; Doenicke A (ed) Etomidate: an intravenous hypnotic agent first report on clinical and experimental experience. Berlin Heidelberg New York: Springer-Verlag, 1977, pp.31–48.
- 15.Gancher S, Laxer KD, Krieger W. Activation of epileptogenic activity by etomidate. Anesthesiology 1984; 61: 616–618. [DOI] [PubMed] [Google Scholar]
- 16.Sadegh AB. Comparison of intranasal administration of xylazine, diazepam, and midazolam in budgerigars (Melopsittacus undulatus): clinical evaluation. J Zoo Wildl Med 2013; 44: 241–244. [DOI] [PubMed] [Google Scholar]