Abstract
Objective
To explore the aetiology of congenital insensitivity to pain with anhidrosis (CIPA) in two Chinese siblings with typical CIPA symptoms including insensitivity to pain, inability to sweat, and self-mutilating behaviours.
Methods
Clinical examination and genetic testing were conducted of all available family members, and the findings were used to create a pedigree. Mutation screening using PCR amplification and DNA Sanger sequencing of the entire neurotrophic tyrosine kinase receptor type 1 gene (NTRK1) including intron–exon boundaries was used to identify mutations associated with CIPA.
Results
A novel nonsense mutation (c.7C > T, p. Arg3Ter) and a known splice-site mutation (c.851-33 T > A) were detected in NTRK1 and shown to be associated with CIPA.
Conclusion
Our findings expand the known mutation spectrum of NTRK1 and provide insights into the aetiology of CIPA.
Keywords: Congenital insensitivity to pain with anhidrosis, hereditary sensory and autonomic neuropathy type IV, mutation, NTRK1
Introduction
Congenital insensitivity to pain with anhidrosis (CIPA, MIM 256800) is a rare, autosomal, recessively inherited disease initially reported by Swanson in 1963,1 which is also referred to as hereditary sensory and autonomic neuropathy type IV. The clinical characteristics of CIPA include insensitivity to pain, anhidrosis (the inability to sweat), and mental retardation. Pain insensitivity often leads to repeated injuries including self-mutilating behaviours, bruising, multiple bone fractures, and dislocations. Furthermore, anhidrosis disturbs the homeostasis of body temperature, resulting in recurrent episodic fever.2,3
Mice deficient in the tropomyosin receptor kinase A gene (TrkA) show phenotypic features of CIPA, except for anhidrosis. The human TrkA homologue, neurotrophic tyrosine kinase receptor type 1 (NTRK1, MIM 191315), is located on chromosome 1q21-q22, comprises 17 exons and 16 introns, and encodes a high-affinity tyrosine kinase receptor for nerve growth factor (NGF).4,5 Upon binding to NGF, NTRK1 is autophosphorylated, and activates an intracellular signal transduction pathway, thus mediating the survival, growth, differentiation, and synaptic plasticity of neurons.6,7 Homozygous or compound heterozygous NTRK1 mutations identified in CIPA patients strongly suggest that it is the causative gene of CIPA. To date, 84 different NTRK1 mutations have been reported in patients with CIPA from different ethnic groups (Human Gene Mutation Database, http://www.hgmd.cf.ac.uk/ac/index.php), including missense, nonsense, small insertions or deletions, splice-site mutations, and gross deletions.8–10
In this study, we describe two Chinese siblings with typical CIPA phenotypes. Mutation screening of NTRK1 revealed that a novel nonsense mutation and a known splice-site mutation were responsible for the disorder in these individuals.
Patients and methods
Patients
This study was approved by the institutional ethics committee of Suzhou Hospital affiliated to Nanjing Medical University. Written informed consent was obtained for publication from all individuals enrolled in this study. Parental consent was obtained from the children aged under 18 years. The two patients diagnosed with CIPA are siblings. All individuals of the patients’ family were subjected to a comprehensive physical examination and full medical history evaluation. A pedigree of their family was created after the clinical examination and genetic testing of all available family members.
Mutational analysis
Peripheral blood was collected from all family members after informed consent was received. Chromosomal karyotyping was performed for both affected children. Genomic DNA was extracted from blood samples using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. All 17 exons and intron–exon boundaries of NRTK1 were amplified by PCR using LA Taq with GC buffer (TaKaRa, Shiga, Japan). After an initial denaturation at 95℃ for 3 min, 35 cycles of amplification were performed, with denaturation at 95℃ for 30 s, annealing at 60℃ for 30 s, and extension at 72℃ for 45 s; a final extension was then carried out at 72℃ for 7 min. Amplified DNA fragments were purified and sequenced in both directions using an ABI 3130 Genetic Analyzer (Applied Biosystems). The resulting sequences were analysed and compared with the reference sequence of NTRK1 (NM_002529.3) in the NCBI database.
Results
Clinical data
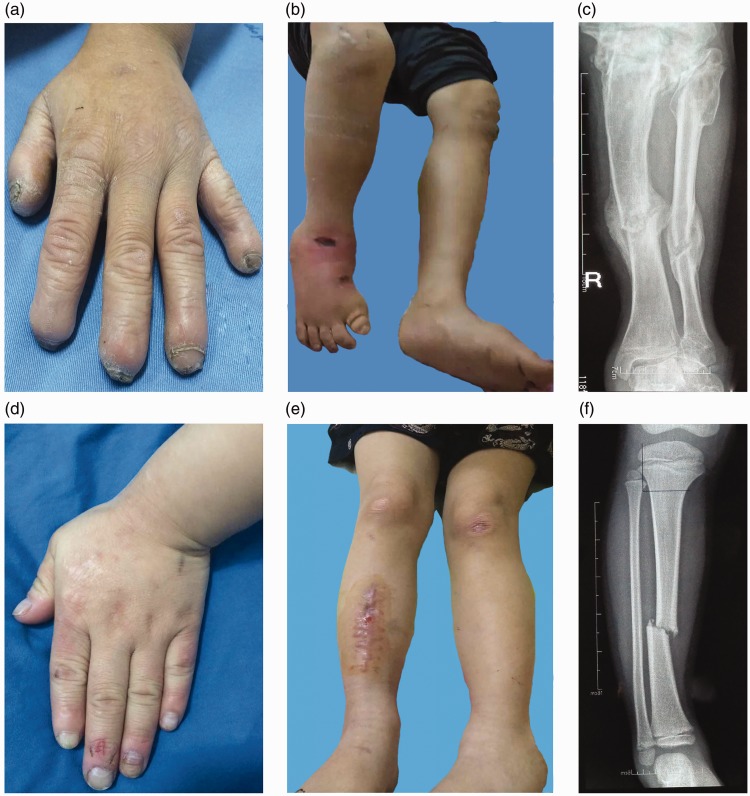
The two patients are siblings who were born after normal pregnancy and delivery from non-consanguineous parents. Patient 1 was a 13-year-old boy, and patient 2 was his younger brother, aged 7 years. Clinical examination revealed that they both exhibited typical CIPA features such as insensitivity to pain, the inability to sweat, and self-mutilating behaviours, but that their parents, grandparents, and half-brother were unaffected. After birth, both patients experienced unexplained episodes of high fever that could only be relieved by physical cooling. They bit their fingers and tongues after tooth eruption, and the skin of their palms and toes was hyperkeratotic. Their medical history revealed frequent bone fractures, poor wound healing, osteomyelitis with sinus formation, and many surgical interventions (Figure 1). Because of old malunited fractures of the right tibia, fibula, and both femurs and a deformity of the right ankle, patient 1 was unable to walk and had to use a wheelchair after the age of 10 years (Figure 1 a–c). Patient 2 also suffered a comminuted fracture of the right tibia and chronic osteomyelitis with sinus formation; however, his clinical presentation of CIPA was less severe than that of his affected brother until they presented at our hospital (Figure 1 d–f).
Figure 1.
Clinical features of patient 1 (a–c) and patient 2 (d–f). (a) Hyperkeratosis, missing tips, and deformed nails from self-mutilating behaviour. (b) Ankle deformity and ulcer of the right foot. (c) Previous radiograph of old fractures of the right tibia and fibula. (d) Hand showing signs of self-mutilation. (e) Scars on the right leg. (f) Previous radiograph of a fracture of the right tibia.
Genetic analysis
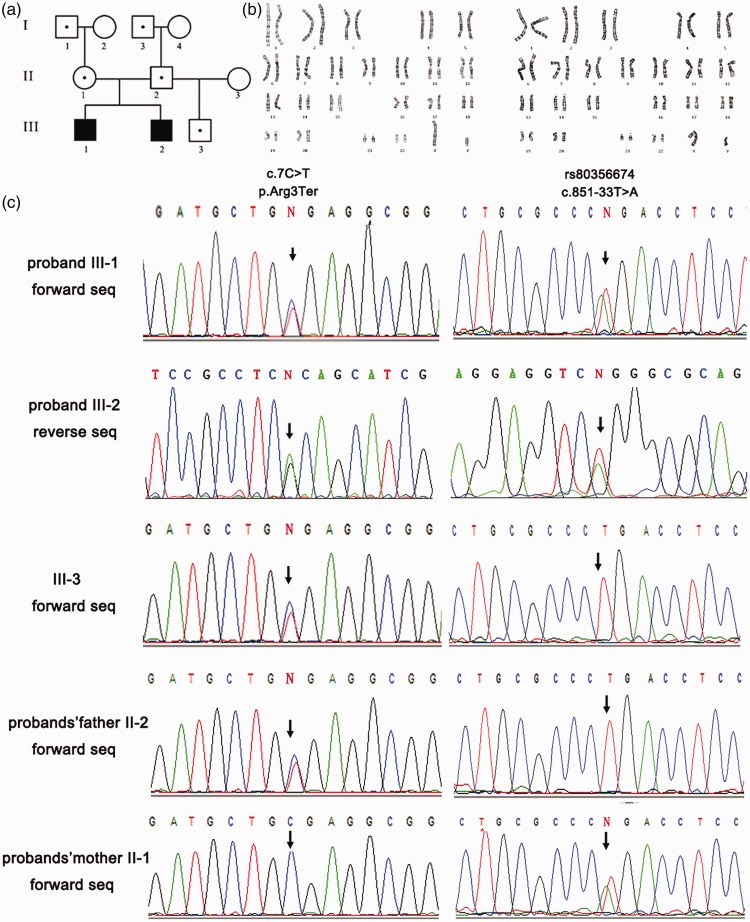
A pedigree of this family is shown in Figure 2a. All 10 individuals were available for study. Both of the affected siblings showed normal chromosomal karyotypes (Figure 2b).
Figure 2.
Genetic analysis of the family. (a) Pedigree of the family. (b) Chromosomal karyotypes of the two probands. (c) Sequencing chromatographs of NTRK1 revealing mutations in the probands, their half-brother, and their parents. Mutations are indicated by black arrows.
Direct Sanger sequencing revealed the existence of two compound heterozygous NTRK1 mutations in the two probands, which were inherited from their parents (Figure 2a, c). Both parents, two grandfathers, and a half-brother of the affected siblings are carriers of one heterozygous mutation, as shown in the three-generation pedigree of the family (Figure 2a, c). The paternal mutation c.7C > T, found within NTRK1 exon 1, is a novel nonsense mutation resulting in the premature termination of mRNA translation. This mutation is not found in dbSNP (http://www.ncbi.nlm.nih.gov/SNP/) or in the 1000 Genomes Project database (http://www.1000genomes.org/). The maternal mutation c.851-33 T > A is a splice-site mutation in intron 7 which causes aberrant splicing; it has previously been shown to be a pathogenic mutation in Japanese, Korean, and Chinese CIPA patients 11–15 (Figure 2c).
Discussion
The human NTRK1 protein is composed of 790 or 796 amino acid residues divided into an extracellular domain important for NGF binding, a single transmembrane region, and an intracellular domain consisting of a juxtamembrane region, a tyrosine kinase domain required for signal transduction, and a short carboxyl-terminal tail.16 To date, a total of 84 NTRK1 mutations associated with CIPA have been identified, causing aberrant protein production and loss of function of NTRK1.
In this study, two compound heterozygous mutations, c.7C > T and c.851-33 T > A, were identified in NTRK1 of the two probands, which were inherited from their father and mother, respectively. The novel nonsense mutation (c.7C > T, Arg3Ter) creates a stop codon at amino acid position Arg 3, leading to translational termination at the initial stage of protein synthesis or nonsense-mediated mRNA decay. To our knowledge, this is the ‘earliest’ stop–gain mutation identified in NTRK1 in Chinese CIPA patients. The splice-site mutation (c.851-33 T > A), also known as IVS7-33 T > A, has previously been reported in Japanese, Korean, and Chinese patients with CIPA. The transition from T to A at the fourth position of the branch site in intron 7 severely reduces the splicing efficiency and results in the insertion of a 137-bp segment in the transcript.14 c.851-33 T > A has been considered one of the founder mutations in Korean CIPA patients, and is the second most common mutation in Japanese CIPA patients.
Our finding supports the notion that this splice-site mutation plays a founder effect in the Asian population, suggesting that it could be a NTRK1 mutation hotspot in Asian CIPA patients.11–15 Because no cure is currently available for CIPA patients, prenatal diagnosis is essential to prevent the birth of an affected child. The probands’ father and their stepmother sought genetic counselling and prenatal diagnosis for a subsequent pregnancy. The fetus was shown to be heterozygous for the paternal mutation, which was confirmed postnatally (Figure 2c).
In conclusion, we identified a novel nonsense mutation and a known splice-site mutation of NTRK1 in two Chinese siblings with CIPA in this study. These findings not only expand the spectrum of NTRK1 mutations but also suggest that Chinese patients with CIPA share some of the same genetic background as other Asian ethnicities.
Acknowledgements
We thank the families for participating in this research project.
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This work is supported by the National Natural Science Foundation of China (30871355), Suzhou Key Medical Center (Szzx201505), Suzhou science and technology support program (SS201647), Suzhou Industry Technology Innovation Project (SYS201568).
References
- 1.Swanson AG. Congenital insensitivity to pain with anhydrosis. A unique syndrome in two male siblings. Arch Neurol 1963; 8: 299–306. [DOI] [PubMed] [Google Scholar]
- 2.Indo Y. Genetics of congenital insensitivity to pain with anhidrosis (CIPA) or hereditary sensory and autonomic neuropathy type IV. Clinical, biological and molecular aspects of mutations in TRKA(NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Clin Auton Res 2002; 12(Suppl 1): I20–32. [DOI] [PubMed] [Google Scholar]
- 3.Toscano E, della Casa R, Mardy S, et al. Multisystem involvement in congenital insensitivity to pain with anhidrosis (CIPA), a nerve growth factor receptor(Trk A)- related disorder. Neuropediatrics 2000; 31: 39–41. [DOI] [PubMed] [Google Scholar]
- 4.Smeyne RJ, Klein R, Schnapp A, et al. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 1994; 368: 246–249. [DOI] [PubMed] [Google Scholar]
- 5.Indo Y, Tsuruta M, Hayashida Y, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet 1996; 13: 485–488. [DOI] [PubMed] [Google Scholar]
- 6.Indo Y. Nerve growth factor and the physiology of pain: lessons from congenital insensitivity to pain with anhidrosis. Clin Genet 2012; 82: 341–350. [DOI] [PubMed] [Google Scholar]
- 7.Mardy S, Miura Y, Endo F, et al. Congenital insensitivity to pain with anhidrosis: novel mutations in the TRKA (NTRK1) gene encoding a high-affinity receptor for nerve growth factor. Am J Hum Genet 1999; 64: 1570–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huehne K, Zweier C, Raab K, et al. Novel missense, insertion and deletion mutations in the neurotrophic tyrosine kinase receptor type 1 gene (NTRK1) associated with congenital insensitivity to pain with anhidrosis. Neuromuscul Disord 2008; 18: 159–166. [DOI] [PubMed] [Google Scholar]
- 9.Mardy S, Miura Y, Endo F, et al. Congenital insensitivity to pain with anhidrosis (CIPA): effect of TRKA (NTRK1) missense mutations on autophosphorylation of the receptor tyrosine kinase for nerve growth factor. Hum Mol Genet 2001; 10: 179–188. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q, Guo S, Duan G, et al. Novel and novel de novo mutations in NTRK1 associated with congenital insensitivity to pain with anhidrosis: a case report. Medicine (Baltimore) 2015; 94: e871–e871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo YC, Liao KK, Soong BW, et al. Congenital insensitivity to pain with anhidrosis in Taiwan: a morphometric and genetic study. Eur Neurol 2004; 51: 206–214. [DOI] [PubMed] [Google Scholar]
- 12.Jung CL, Ki CS, Kim BJ, et al. Atypical hereditary sensory and autonomic neuropathy type IV with neither mental retardation nor pain insensitivity. J Child Neurol 2013; 28: 1668–1672. [DOI] [PubMed] [Google Scholar]
- 13.Lee ST, Lee J, Lee M, et al. Clinical and genetic analysis of Korean patients with congenital insensitivity to pain with anhidrosis. Muscle Nerve 2009; 40: 855–859. [DOI] [PubMed] [Google Scholar]
- 14.Miura Y, Mardy S, Awaya Y, et al. Mutation and polymorphism analysis of the TRKA (NTRK1) gene encoding a high-affinity receptor for nerve growth factor in congenital insensitivity to pain with anhidrosis (CIPA) families. Hum Genet 2000; 106: 116–124. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Liang JY, Sun ZH, et al. Novel nonsense and frameshift NTRK1 gene mutations in Chinese patients with congenital insensitivity to pain with anhidrosis. Genet Mol Res 2012; 11: 2156–2162. [DOI] [PubMed] [Google Scholar]
- 16.Indo Y, Mardy S, Tsuruta M, et al. Structure and organization of the human TRKA gene encoding a high affinity receptor for nerve growth factor. Jpn J Hum Genet 1997; 42: 343–351. [DOI] [PubMed] [Google Scholar]