Abstract
The wide range of factors associated with the induction of autism is invariably linked with either inflammation or oxidative stress, and sometimes both. The use of acetaminophen in babies and young children may be much more strongly associated with autism than its use during pregnancy, perhaps because of well-known deficiencies in the metabolic breakdown of pharmaceuticals during early development. Thus, one explanation for the increased prevalence of autism is that increased exposure to acetaminophen, exacerbated by inflammation and oxidative stress, is neurotoxic in babies and small children. This view mandates extreme urgency in probing the long-term effects of acetaminophen use in babies and the possibility that many cases of infantile autism may actually be induced by acetaminophen exposure shortly after birth.
Keywords: Autism, inflammation, oxidative stress, acetaminophen, paracetamol, paracetamolo
Introduction
Autism is a complex disorder associated with a wide range of disparate and seemingly unrelated factors such as (a) maternal exposure to various chemical substances, (b) maternal exposure to child abuse, (c) maternal evidence of diabetes or other autoimmune diseases, (d) age of either parent at conception, (e) exposure of the infant to various chemical substances, (f) vitamin D levels of the infant at birth, (g) gender of the infant, and (h) a large number of genetic factors. With this in mind, we believe it is helpful to categorize the factors associated with autism in an effort to identify patterns which may be informative. As described below, these risk factors for autism fall into two primary categories: those associated with inflammation and those associated with oxidative stress.
A number of risk factors for autism can be categorized as risk factors for inflammation or indicators of inflammation (Table 1). Risk factors for inflammation associated with autism include maternal (odds ratio [OR] = 1.6, confidence interval [CI] 95% = 1.1–2.2) and perhaps paternal (OR = 1.4, CI 95% = 1.0–2.0) autoimmune diseases such as diabetes, myasthenia gravis, idiopathic thrombocytopenic purpura, or rheumatic fever,1 maternal obesity (OR = 1.67, CI 95% = 1.1–2.56),2 and febrile episodes during the first two trimesters (OR = 1.6, CI 95% = 1.0–2.5).3 An increase in inflammation among individuals living in Western societies is evidenced by the alarming rise of allergy and autoimmune disease in the United States and other Westernized countries over the past century and can be attributed to five major causes that result directly from social and cultural changes: (a) an “inflammatory diet” high in fat and simple sugars and low in fiber and nutrients, (b) lack of physical exercise, (c) chronic and unrequited psychological stress, (d) vitamin D deficiency, and (e) biome depletion.4–7 The recent rise in autism8,9 could be attributable, at least in part, to this rise in inflammation.10,11
Table 1.
Risk factors associated with autism, in very approximate descending order of risk.
| Factor | Classification |
|---|---|
| High Risk | |
| Acetaminophen use in childrena | Pharmaceutical: oxidative stressor, decreased capacity to handle oxidative stress |
| Excessively high levels of vitamin B12 and folate in maternal blood | Linked to inflammation and possibly to oxidative stress |
| Down’s Syndrome | Oxidative stress |
| Preterm delivery | Risk factor for oxidative stress and indicator of inflammation |
| Cerebral palsy | Indicator of inflammation |
| Environmental toxins: pesticides | Oxidative stressors, inflammatory stimuli |
| Male gender | Risk factor for oxidative stress, susceptibility to oxidative stress |
| Maternal exposure to childhood abuse | Risk factor for inflammation later in life |
| Hepatitis B vaccine, first month of life, pre-1999 | Oxidative stressor, inflammatory stimulus |
| Polymorphic variants (various, involved in methionine and glutathione pathways) | Decreased capacity to handle oxidative stress |
| Mother with diabetes | Indicator of inflammation |
| Mother with lupus | Indicator of inflammation |
| Moderate Risk | |
| Father >50 years old | Risk factor for inflammation |
| Parental autoimmune disorder | Indicator of inflammation |
| Maternal obesity | Risk factor for inflammation |
| Hyperbilirubinemia | Risk factor for oxidative stress |
| Early childhood atopic disorders (dermatitis, respiratory) | Indicator of inflammation |
| Maternal autoimmune disease | Indicator of inflammation |
| Febrile episode >7 days | Symptoms associated with oxidative stress and inflammation |
| Use of acetaminophen during pregnancy | Pharmaceutical, oxidative stressor |
| Infection during pregnancy | Oxidative stressor, inflammatory stimulus |
| Low or Uncertain Risk | |
| Mother smoking during pregnancy | Oxidative stressor |
| Mother >40 years old | Risk factor for inflammation |
| Environmental toxins: air pollution, including vehicular emissions of heavy metals and particulate matter | Oxidative stressors, inflammatory stimuli |
| Aspartame/other sources of methanol | Oxidative stressor |
| Low vitamin D levels at birth | Inflammatory mediator |
| Folate deficiency (anti-folate receptor autoantibodies) | Risk factor for oxidative stress |
| Urbanization/Western society | Risk factor for inflammation |
Citations are in the text.
Determined only in a single, survey-based study.
Aside from inflammation, oxidative stress is the second category in the landscape of autism-associated factors. As pointed out by Chauhan and Chauhan, a wide range of studies “… suggest increased oxidative stress in autism that may contribute to the development of this disease.”12 However, it is unknown whether factors causing oxidative stress have increased in Western societies concomitantly with inflammation. Perhaps the weight of factors causing oxidative stress peaked during the height of the industrial revolution and has since declined from that peak,9 but this is unknown. Indeed, it may be difficult or even impossible to determine whether, in terms of oxidative stress, modern factors such as pharmaceuticals, pollutants from the combustion of fossil fuels, and other factors from industrial processes outweigh more historical factors such as smoke from cooking fires and naturally occurring toxins from food obtained by hunting and gathering. Nevertheless, oxidative stress is now associated with modern diseases. Oxidative stress, like inflammation, is associated with cancer,13 coronary artery disease,14 and a number of psychiatric disorders.15 It is widely thought that inflammation and oxidative stress go hand in hand; as stated by Ghezzi and colleagues, “The mechanism by which oxidative stress induces inflammation and vice versa is unclear but is of great importance.”16 Therefore, either oxidative stress has increased or other changes in modern society (e.g. increased inflammation) have made oxidative stress more dangerous than it was in past generations.
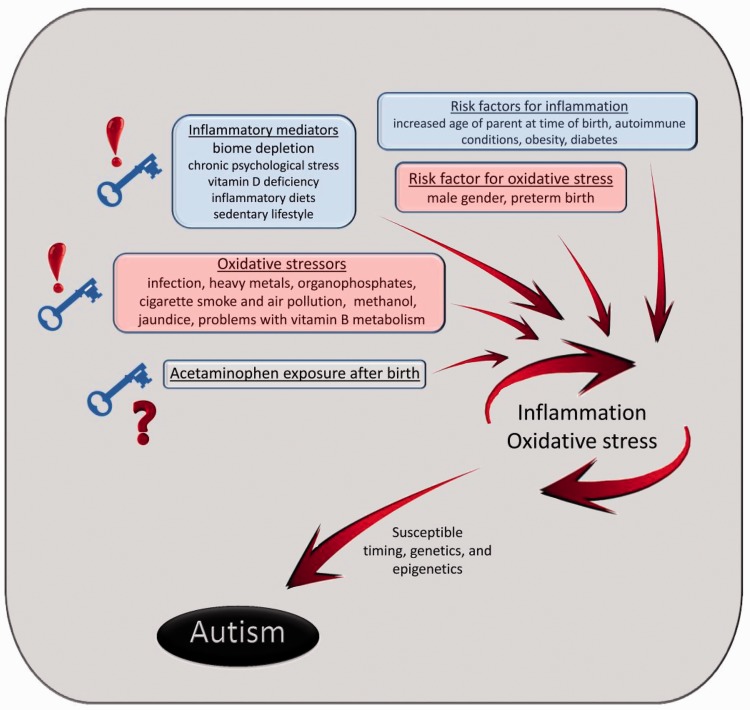
Thus, the list of diverse factors associated with the induction of autism can be viewed as interrelated when examined in the context of two major categories, inflammation and oxidative stress (Figure 1). For the purpose of this discussion, factors involved in the induction of autism will be described as “low risk” (1%–20% increased risk of autism), “moderate risk” (21%–100% increased risk of autism), or “high risk” (any risk of autism above 100% or 2-fold).
Figure 1.
The role of oxidative stress (red), inflammation (blue), and possibly acetaminophen exposure after birth in the induction of autism.
Autism and inflammation
Several risk factors found for autism have very straightforward connections to inflammation (Table 1). Episodes of fever greater than 7 days in the first and second trimester carry a moderate risk factor for autism (hazard ratio [HR] = 1.6, CI 95% = 1.0–2.5).3 In addition, autoimmune diseases in the mother such as lupus, multiple sclerosis, diabetes, and rheumatic fever are associated with moderate-to-high risk for autism induction,17 as is maternal infection during pregnancy.18 Further, atopic dermatitis and respiratory diseases in children under the age of 2 years are also associated with a moderate risk for the development of autism.19
Another moderate risk factor for autism that has a well-known association with inflammation is obesity (OR = 1.67; CI 95% = 1.1–2.56).2 Over one-third of women in the United States of childbearing age are obese and this number is steadily rising.20 Multiple studies link obesity to chronic, low-grade inflammation and the subsequent secretion of pro-inflammatory cytokines and infiltration of immune cells.21 Although it may be tempting to blame the rise in autism on the rise in obesity and autoimmune disease, these risk factors alone, which are generally associated with low-to-moderate increases in autism prevalence, likely do not account for the 30-fold or greater increased prevalence of autism since the 1970s.9,22
The prevalence of autism in children with cerebral palsy is approximately 7-fold greater than in the general population.23 Substantial evidence points toward the view that cerebral palsy, the most common cause of severe motor disability in childhood, is associated with inflammation. For example, many studies have linked fetal exposure to infection to the development of cerebral palsy,24–26 and blood samples from neonates with cerebral palsy contain higher concentrations of many pro-inflammatory cytokines and chemokines compared with controls.27 Further, cerebral palsy is associated with chorioamnionitis,26,28–30 which can stimulate fetal production of inflammatory cytokines including IL-6.31 Thus, cerebral palsy, a high risk factor for the development of autism, is linked to inflammation.
Some risk factors for autism have less obvious associations with inflammation. For example, increasing age of the mother or the father at the time of birth is associated with a low or moderate risk of autism, respectively (OR = 1.15, >40 years for the mother and OR = 1.66, >50 years for the father).32,33 While age itself might not normally be considered inflammatory, there have been direct associations found between increased age of the parents and inflammatory diseases in their offspring. For instance, maternal age ≥30 years at the time of birth is associated with an increased incidence of one or more food allergies in the child compared with controls (75% vs. 55%, p = 0.005).34 Further, the risk of having children diagnosed with multiple sclerosis steadily increases with paternal age at birth from 21 to 55 years (adjusted OR for 21–25-year-old fathers = 1.08 and 51–55-year-old fathers = 2.00).35 Thus, although parental age, a moderate risk factor for autism, is not intuitively associated with inflammation, the association is evident based on published studies.
Maternal exposure to severe emotional stress during her childhood and/or adolescence is another risk factor (OR = 3.7, CI 95% = 2.3–5.8) for autism36 that may not be intuitively associated with inflammation. However, the link between childhood and adolescent adversity and immune dysregulation and inflammation is widely appreciated by the scientific community. Multiple studies demonstrate that a wide range of stressful events taking place in one’s childhood can adversely affect health and inflammatory responses even into the eighth decade of life.37–40 Exposure of women to two or more early childhood stressful events, including physical, emotional, or sexual abuse, is positively correlated with an increased risk of Th1, Th2, and rheumatic autoimmune development later in life compared with women reporting no events in childhood (p < 0.05).41 Markers of inflammation (IL-6 and C-reactive protein) have been found to be elevated in a systemic manner in women who experienced sexual abuse in adolescence compared with women without a history of abuse (p = 0.04 for IL-6 and 0.03 for C-reactive protein).42 Further, a recent study of 28,456 African American women found a positive association between adult-onset asthma and physical or sexual abuse experienced during childhood or adolescence after adjusting for multiple confounders such as parental history of asthma, body mass index, exercise, current smoking habits, and exposure to secondhand smoke.43 Childhood stress also affects a woman’s response to current, daily stressors. For example, elevated levels (2.35-fold) of IL-6 have been found within 24 hours in response to current daily stressful events in sera from women who experienced childhood abuse compared with women who experienced the same number of daily stressors without a history of child abuse.44 Other examples of the impact of early life stress on inflammation abound.37,45–48 Thus, maternal exposure to stress during early life, a high risk factor for autism, is clearly associated with inflammation.
Autism and oxidative stress: Environmental toxins
Exposure to environmental toxins, a source of oxidative stress, has been associated with autism in a number of studies.49 Maternal exposure during gestation to agricultural pesticides such as organophosphates, organochlorines, and pyrethroids has been identified as a moderate-to-high risk factor for autism.50–52 The risk seems to be dependent at least in part on the proximity to the applied chemical and the trimester during which the exposure took place. However, the data are somewhat noisy and, as pointed out by the authors, possibly confounded by unavoidable misclassifications in the estimations of maternal exposure. In contrast to evidence of the effects of exposure in utero, evidence for exposure to pesticides after birth as a risk factor for autism is slim to non-existent. Maternal exposure to traffic-related air pollutants also carries risk factors for autism. For example, exposure to particulate matter during the third trimester is a low-to-moderate risk factor for autism,53–55 while maternal exposure to other traffic-related toxins (e.g. mercury, lead, arsenic, cadmium, manganese, styrene, trichloroethylene, and vinyl chloride) is a moderate-to-high risk factor for autism.56–58 However, these findings were not replicated in a study of four European countries.59 Furthermore, the range of variability in U.S. air pollution (e.g. interquartile ranges of only 4–5 mcg/m3 in fine particulate matter [PM2.5]) over which associations with autism spectrum disorder have been reported53–55 is quite small compared with the more severe air pollution encountered routinely in the developing world (for example, >900 mcg/m3 when indoor cooking stoves are used).60 Additionally, exposure to many environmental toxins such as vehicular and air pollution, polychlorinated biphenyls (PCBs), lead, polycyclic aromatic hydrocarbons (PAHs), and organochlorines and organophosphates has decreased over at least part, if not most, of the time frame in which autism prevalence has continued to climb.9 Further, factors such as exposures to phthalates, atmospheric mercury levels, and total blood mercury have remained relatively stable since the 1990s, although exposure to polybrominated diphenyl ethers (PBDEs) and glyphosates has increased.9 However, to date, few studies have associated exposure to phthalates, PCBs, PBDEs, PAHs, or glyphosates to the development of autism. With this in mind, it seems likely that the relative contribution of any single environmental oxidative stressor to the prevalence of autism is most likely dependent on the location or population in question.
Autism and oxidative stress: Jaundice
Hyperbilirubinemia (the symptoms of which are known as jaundice) is associated with elevated oxidative stress61 and impedes the clearance of acetaminophen,62 a drug suspected of inducing autism (see discussion below). Jaundice is associated with autism,63 although the connection between jaundice and autism had some early critics.64 The idea was that early studies pointing toward a connection between jaundice and autism may have been confounded by some condition or conditions associated with autism but unrelated to jaundice. These conditions may have been responsible for keeping the children in the hospital for longer periods of time, which could have resulted in an increased chance of receiving a diagnosis of jaundice. However, a prospective study design using a large sample size in Taiwan seems to have addressed major concerns with the previous studies, and the connection between jaundice and autism is apparently real.65
Autism and oxidative stress: Sex, Down syndrome, and preterm birth
Perhaps the most widely known risk factor for autism is being male. The increased risk of autism for males was established very early during work with patients having autism, and is approximately 4-fold that of females. Although being male is not widely known to be associated with oxidative stress, male infants are more susceptible to oxidative stress than female infants,66–68 and have been found to have more oxidative stress.69 Further, male children seem to be more susceptible to toxin-induced oxidative stress than females.70 The occurrence of Down syndrome is also a risk factor for autism, with more than 5% of individuals with Down syndrome having autism.71 Down syndrome is also associated with a profound increase in oxidative stress and inflammation,72,73 pointing again toward a connection between oxidative stress and the induction of autism.
Preterm birth, a complex and often idiopathic complication of pregnancy, is a high risk factor for the development of autism. Limperopoulos and colleagues found that 26% of preterm, low birth weight infants had a positive result on the Modified Checklist for Autism in Toddlers (M-CHAT) autism screening tool74 compared with 5.7% of non-preterm infants (OR=4.56).75 This risk is corroborated by several additional studies with odds ratios of 6.3 (CI 95% = 2.2–18.3) for children born preterm in the United Kingdom and Ireland75 and 3.2 (CI 95% = 2.6–4.0) for children who were born preterm in Sweden using a sibling-comparison approach.76 Preterm birth has long been associated with inflammation, with approximately 50% of preterm births being associated with chorioamnionitis.77,78 In addition, oxidative stress has recently been hypothesized as a co-mechanism for the initiation of preterm birth.77,79,80 Further, oxidative stress is associated with preterm birth.81–83 Thus, preterm birth, a risk factor for autism, is associated with both inflammation and oxidative stress.
Autism and oxidative stress: Vitamin B
Excessively high levels of maternal vitamin B12 and vitamin B9 (folate) are additional risk factors linked to autism.84 In a study of the association between vitamin B levels and risk of autism, excessively high prenatal vitamin B12 levels in mothers (HR = 3.01, CI 95% = 1.64–5.52; P value: 0.001) and excessively high prenatal folate levels (HR=2.27, CI 95% = 1.26–4.09; P value: 0.007) were each found to be associated with a significantly increased risk of infant autism. Together, excessively high vitamin B12 and folate levels in maternal blood sera show the highest increased risk of infantile autism (HR: 17.59; P value: <0.001).84
Vitamin B9 and B12 are antioxidants, and thus, intuitively, high levels of these vitamins should be associated with a decreased risk for oxidative stress. However, excessively high levels of vitamin B12 in the blood are common and are often paradoxically indicative of a clinical deficiency of vitamin B12.85 In one case, for example, excessively high levels of vitamin B12 were caused by the presence of anti-vitamin B12 antibodies that led to the formation of functionally inactive IgG-IgM-B12 immune complexes.86 In general, excessively high levels of vitamin B12 and the associated functional deficiency of that antioxidant could lead to difficulties in the ability to cope with oxidative stress, and indeed have been consistently linked to inflammatory diseases such as neoplasms, hematological malignancies, and liver and kidney diseases.85
Not only are exceedingly high levels of folate associated with autism,84 the presence of anti-folate receptor autoantibodies has been shown to be highly prevalent (75.3%) in children with autism.87 Such autoantibodies would create a functional deficiency of folate in the brain regardless of the concentration of folate in the serum. Folate deficiency is highly deleterious and is associated with reduced activity of antioxidant enzymes, as well as overall increased oxidative stress.88,89
Hypothetically, excessive levels of folate in the serum could be an indication of a clinical insufficiency of folate, just as excessively high levels of vitamin B12 are linked to a clinical insufficiency of that vitamin. Regardless of the reasons for excessively high levels of folate, they have been associated with inflammatory bowel disease,90 indicating that the high levels are related in some way to inflammation.
Autism and oxidative stress: Genetic variation
Polymorphic variants related to the metabolism of methionine transmethylation and transsulfuration, which increase susceptibility to endogenous and environmental oxidative stress, are significantly different in children with autism.91–93 Studies show a decreased ability in children with these genetic variants to handle oxidative stress as measured by several metabolic biomarkers including S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), adenosine, homocysteine, cystathionine, cysteine, oxidized and reduced glutathione, endogenous secretory receptor for advanced glycation end-products (RAGE), and the pro-inflammatory ligand S100A9. In addition, polymorphisms in glutathione pathways, which modulate the response to oxidative stress, strongly affect risk for autism. For example, the homozygous GSTM1 deletion genotype imposes a near 2-fold increased risk for autism.94,95 Further, polymorphisms of the glutathione S-transferase P1 gene (GSTP1) in the mother, which could affect the fetus during pregnancy, are high risk factors for the induction of autism (OR = 2.67, CI 95% = 1.39–5.13).96 Thus, several genetic variants that affect pathways involved in oxidative stress are risk factors for autism.
Synergism between oxidative stress and inflammation: Evidence from animal models
Inflammation can be caused by a diverse set of factors associated with Western culture, including chronic psychological stress and biome depletion (Figure 1), which at first glance might seem unrelated to inflammation. However, it is now recognized that these non-chemical inflammatory mediators (e.g. limited resources or social support for the mother, leading to chronic psychological stress) can increase vulnerability of the fetus to chemical stressor exposures (e.g. oxidative stressors such as pollution or toxins).97 This view potentially explains why a single exposure or risk factor in isolation is a modest predictor of autism risk. Given the complex nature of environmental and social exposures, attempts at deciphering the mechanisms that contribute to autism suffer from fatal oversimplification if those models involve only single agents. In contrast, useful and relevant models must include multiple factors. For example, an experimental animal model has been developed that employs the combined effects of an ethologically relevant maternal stressor and an environmentally relevant pollutant, diesel exhaust, both of which have been implicated in autism.55,57,98–102 Using this model, it was demonstrated that maternal exposure to diesel exhaust particles combined with maternal stress, but neither in isolation, produced long-term cognitive deficits and strikingly increased anxiety in male but not female offspring.97
Evidence for a mediator other than inflammation and oxidative stress
The likely role of oxidative stressors and inflammation in the pathogenesis of autism is apparent based on the nature of risk factors associated with autism. This conclusion is corroborated by numerous studies, described above and reviewed elsewhere,10,103–109 that have identified immune activation and inflammation in patients with autism. Further, treatment with antioxidants or anti-inflammatory agents such as sulforaphane110 and helminth therapy,111 respectively, can help some patients with autism. However, it seems unlikely that oxidative stress and inflammation alone account for the dramatic rise in the incidence of autism since 1980. Inflammation in general has increased steadily since the turn of the twentieth century, as indicated by a slow and steady rise in a wide range of allergic disorders, autoimmune conditions, and other inflammation-associated diseases.112 The very rapid rise in autism since the early 1980s might suggest that one or more specific environmental factors are at play. As we and others have pointed out,9,10,113 it is possible that the rapid “increase” in autism is due simply to increased awareness and changing diagnostic criteria, but substantial evidence which contradicts this conclusion is available and a working hypothesis that autism has profoundly increased over the last 40 years holds the most promise for a rapid resolution of the problem.10 That is to say, if autism is indeed an ancient and natural consequence of “being human,” then autism may be much more difficult to prevent than if it is induced by factors present in modern society.10
We have suggested10 that arguments for and against autism as an epidemic (or pandemic) can only be resolved (a) if a specific trigger for autism is found, facilitating the elimination of autism; or (b) if complete normalization of the immune system (leading to a pre-industrial condition of essentially no allergies) succeeds in eliminating autism. Given that complete normalization of the immune system may take years or even generations to accomplish, and given the recent apparent rise in autism described above, the search for very specific and potent triggers for the induction of autism seems worthwhile.
Proposed triggers of the autism epidemic: Aspartame
Aspartame is an artificial sweetener that has been in use since 1981, about the time that the autism epidemic started (Figure 2). Aspartame breaks down in the body and releases methanol, an oxidative stressor and toxin. Its use has been attributed to the rise in autism.114 However, the use of other sweeteners has replaced aspartame in the past 10 years, except in diet soft drinks, and the consumption of diet soft drinks has been in decline. Thus, there is no association over time between autism and aspartame. Further, the presence of methanol in canned vegetables and in cigarette smoke is not new, and thus the relatively low incidence of autism prior to the mid-1900s cannot be accounted for if methanol, by itself, is a major trigger. Further, Pepsi Cola® has at least temporarily removed aspartame from its drinks, which eliminates at least for a time one of the major remaining sources of aspartame consumption in the United States. If indeed aspartame is the major trigger for autism, then the “experiment” has been accomplished already and the rate of autism should be in the process of decreasing. This does not appear to be the case, apparently disproving the hypothesis.
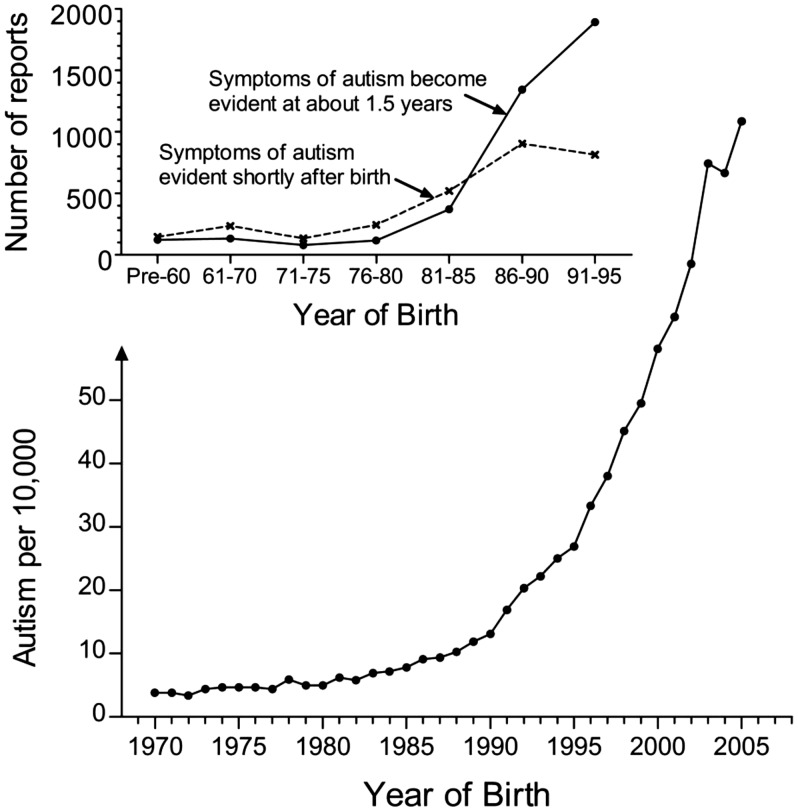
Figure 2.
Apparent changes in the quality and quantity of autism extending over a decade, starting in the early 1980s. In the top diagram, data are from Rimland’s summary168 of the number of surveys (the “E-1 Diagnostic Checklist” and the “E-2 Diagnostic Checklist”) that were collected in a given time period through grass-roots efforts of the Autism Research Institute and the Autism Society of America, the only two national autism organizations in the United States at the time of the data collection. The Y-axis describes the actual number of surveys received, and changes in the number of reports received were attributed by Rimland to increases in the number of children with autism. “Shortly after birth” in this case refers to parents’ reports that symptoms of autism were evident within weeks of birth. In the lower diagram, the prevalence of autism in California as compiled by Nevison9 is shown. Data are a composite of “snapshot” data (information collected at one point in time) from the California Department of Developmental Services (collected in 2002 and covering birth years 1970–1997) and tracking data evaluating 5-year-olds collected under the US Individuals with Disabilities Education Act (covering birth years 1995–2005).9
Proposed triggers of the autism epidemic: Ethyl mercury and vaccines
The view that vaccines and ethyl mercury in particular, a component of the preservative thimerosal used in some vaccines, can induce the development of autism is widespread among non-scientists115,116 and has been discussed widely in the literature.117,118 Proposed mechanisms of induction depend on the difference between methyl and ethyl mercury119 to account for the relatively recent rise of autism compared with the centuries-old use of methyl mercury. However, a study addressing this issue found no association between levels of mercury exposure during vaccination and the incidence of autism 120. Further, a substantial reduction in exposure to ethyl mercury as a result of elimination of thimerosal from vaccines has not reduced the rate of autism. It has been counter-argued that “no level is safe,” suggesting that any amount of ethyl mercury may be dangerous. However, since the induction of autism (by whatever agent) is apparently not near the saturation point (presumably yielding a rate of 100% autism in the population, at which point the rate of autism would be independent of increasing or decreasing levels of inducing agent), then it is expected that a substantial reduction in the amount of inducing agent would lead to at least some reduction in the level of autism. It has been argued that perhaps aluminum adjuvants now replace mercury preservative as the vaccine-associated agent, but then ethyl mercury and aluminum must be different from methyl mercury in some regard, straining the original hypothesis regarding the uniqueness of ethyl mercury. It could be counter-argued that the route of exposure (injection vs. dietary intake) of the metal is important, but in the one widely accepted instance in which a vaccine caused the induction of a neuropsychiatric disorder (narcolepsy with cataplexy), no metal preservatives or metal adjuvants were involved.120 Thus it could be argued that it is the vaccination in general that is critically important, not the metal per se. Yet vaccines are much older in their origins than autism, and it is not intuitive that a vaccine would be worse than an actual life-threatening infection, the origins of which are ancient and pre-date the human race. This view is corroborated by a cohort of parents who did not vaccinate younger siblings of children with autism; failure to vaccinate with one or even all vaccines did not prevent autism.122,123 Most importantly, acetaminophen, the analgesic most commonly administered in conjunction with vaccination and the only analgesic administered to children under the age of 6 months following vaccination, has been identified as a likely inducer of autism.124 Initial studies described below suggest that it is the co-administration of this analgesic with vaccines that may have given many parents the false impression that their child’s autism was induced by a vaccine.
Proposed mediators of the autism epidemic: Acetaminophen
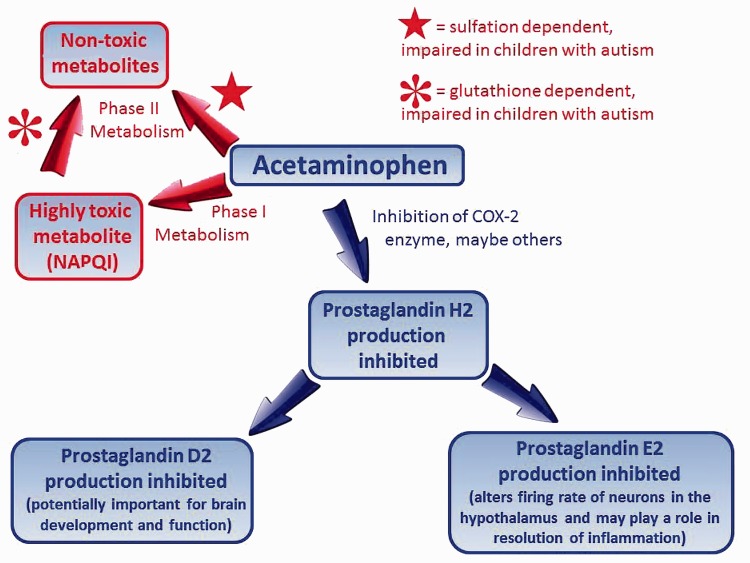
Acetaminophen has been widely used in adults for more than half a century as a pain reliever and anti-pyretic, and is by far the most commonly used medication for pain and fever during pregnancy125 and in the pediatric population.126,127 By the early 1980s, acetaminophen had effectively replaced the analgesic phenacetin, which is metabolized to acetaminophen by the body128,129 and which had been widely used since the late 1800s despite its carcinogenic and toxic nature. The therapeutic action of acetaminophen involves inhibition of prostaglandin synthesis, a surprisingly important biochemical process involved in development and neurological function, as shown in Figure 3. Acetaminophen elimination from the body typically involves biochemical modification in the liver by phase II metabolism, which entails the addition of sulfate (or glucuronide more often in adults130) to the molecule, facilitating its elimination (Figure 3). However, the drug can also be modified via phase I metabolism, producing NAPQI, a highly toxic metabolite which is then processed via phase II metabolism to a non-toxic product by the addition of cysteine in a manner dependent on glutathione (Figure 3). Acetaminophen is known to be safer in children than in adults, presumably because the drug cannot be rapidly converted by the child’s relatively undeveloped liver into toxic metabolites.131 However, this view of acetaminophen’s safety is based strictly on the low rate of acute adverse events such as liver and kidney failure and intestinal bleeding.131,132 Unfortunately, the long-term effects of acetaminophen exposure on neural development have never been evaluated in humans. However, Margaret McCarthy’s lab has shown that drug-mediated inhibition of prostaglandin synthesis (as is accomplished by acetaminophen) in laboratory rats during “a time sensitive window in early postnatal life” not only results in significant long-term modifications to brain development and morphology but also leads to decreased social interactions and reduced sensory function in male but not female animals.133 Further, in vitro studies using human cell lines have shown that acetaminophen can cause “an immediate, reversible, dose-dependent loss of oxygen uptake followed by a slow, irreversible, dose-independent death” and have suggested mechanisms by which acetaminophen may cause toxicity in tissues other than the liver.134
Figure 3.
Action and metabolism of acetaminophen in babies and children. Phase II metabolism involving glucorinadation, like sulfation, leads to detoxification of acetaminophen, but sulfation is the primary mechanism active in infants and children.
Given its suppression of the febrile response, one might expect that the general effects of exposure to acetaminophen would be anti-inflammatory in nature. Unfortunately, this expectation is false. Studies in adult humans demonstrate that even low-dose acetaminophen triggers immune system activation and oxidative stress responses.135 Further, medical professionals evaluating immune responses from a biologists’ perspective have warned that the febrile response is both adaptive (beneficial to survival) and ancient in its origins,136 with behaviors which induce fever-like temperatures evident in cold-blooded animals.137 Thus, biologists argue that inhibition of the febrile response is likely not without costs.137 It has even been postulated that the elimination of fevers may be responsible in part for the induction of autism.138 However, the health costs of eliminating low-grade fevers in general are not known. On the other hand, some data regarding the health costs of using acetaminophen are described in the literature. A multinational study with more than 200,000 children found a dose-dependent association between use of acetaminophen in the first year of life and the occurrence of inflammatory diseases such as asthma, rhinoconjunctivitis, and eczema later in life.139 The causal relationship between use of acetaminophen and the occurrence of asthma has been questioned,140 but the consensus is that a relationship exists141,142 and a population-wide increase in asthma of more than 40% may be caused by the use of acetaminophen.143
Perhaps even more concerning than studies demonstrating acetaminophen-induced social impairment in animal models, acetaminophen-induced injury to cultured cells, and acetaminophen-induced inflammation in adults, are studies pointing toward a connection between acetaminophen and neurological problems in children. A summary of such studies is shown in Table 2. A sibling-controlled study with over 48,000 children in Norway showed that the use of acetaminophen but not ibuprofen by mothers during pregnancy was associated with problems in the psychomotor, behavioral, and temperamental development of children at 3 years of age.144 Further, a study in Bristol, United Kingdom, of more than 7000 children showed that maternal use of acetaminophen during pregnancy was associated with hyperactivity and “emotional symptoms” at age 7.145 In addition, a study at UCLA in collaboration with scientists in Denmark and Taiwan found that children whose mothers used acetaminophen during pregnancy were at higher risk of being diagnosed with hyperkinetic disorder (HR = 1.37, CI 95% = 1.19–1.59). To quote the authors, “Results did not appear to be confounded by maternal inflammation, infection during pregnancy, the mother’s mental health problems, or other [variables they examined].”146 A more recent Danish study came to the same conclusions.147 Further, a study from New Zealand found associations between acetaminophen use during pregnancy and attention-deficit/hyperactivity disorder (ADHD) at 7 and 11 years of age,148 with the authors concluding that their work supports “earlier claims that findings [of increased ADHD with acetaminophen use] are specific to acetaminophen.” The authors further state that “The finding that even low doses of acetaminophen (indicated by the number of weeks of drug exposure) can affect behavior 7 years later is alarming because acetaminophen (paracetamol) is the most commonly used antenatal drug.” Indeed, new studies supporting the view that prenatal acetaminophen use is associated with long-term negative effects on brain function are currently being published on a monthly basis.145,149
Table 2.
Published studies probing the effects of acetaminophen on neuropsychiatric function.
| Study Group | Controls or Variables Considered (other than acetaminophen use) | Symptoms | Odds Ratio |
|---|---|---|---|
| 1. 163 US children, aged 5 years or lessa | Ibuprofen use used as a control. Confounders included maternal and paternal demographics, and child’s health, age, and sex. | Autism | 6.1155 |
| 2. 48,631 Norwegian children, aged 3 years | Ibuprofen use used as a control. Confounders included maternal demographics, health (infections, fevers, pain), use of other medications, alcohol use, tobacco use, and psychological stress. | Problems in psychomotor, behavioral, and temperamental development | 1.51 to 1.69143 |
| 3. 64,322 Danish children, aged up to 15.6 years | Confounders included maternal demographics, health (fever, infection, joint and muscle diseases), use of other medications, alcohol use, tobacco use, psychological conditions, and child’s age and sex. | Autism accompanied by hyperkinetic symptoms | 1.51123 |
| 4. 1491 Danish children, aged 5 years | Confounders included maternal demographics, health (infections, fevers, pain), ibuprofen use, aspirin use, alcohol use, tobacco use, psychological conditions, and child’s sex. | Impaired attention and executive function | 1.5146 |
| 5. 7796 British children, aged 7 years | Maternal postnatal and partner acetaminophen use used as controls. Confounders included maternal demographics, health (infections, fevers, pain), genetic risk factors, alcohol use, tobacco use, psychological stress, and child’s birth weight and gestational age. | Hyperactivity and “emotional symptoms” | 1.29 to 1.46144 |
| 6. 64,322 Danish children, aged 7 years | Confounders included maternal demographics, health (infections, fevers, pain), alcohol use, tobacco use, psychological stress, and child’s birth weight and sex. | Hyperkinetic disorder and ADHDb | 1.13 to 1.37145 |
| 7. 871 New Zealand children, aged 7 and 11 years | Anti-inflammatories, aspirin-based painkillers, antacids, and antibiotic use used as controls. Confounders included maternal demographics, health (fever and inflammation), alcohol use, psychological stress, and medications for psychological conditions. | ADHD | Odds ratio not calculated: approximately 10% difference in ADHD assessment147 |
| 8. 2644 Spanish children, aged 5 years | Confounders included maternal demographics and health (fever, infection, chronic illness), parental mental health, and child’s health, age and sex. | Autism | Odds ratio not calculated: difference in CASTc P value = 0.006161 |
This study was the only study that evaluated acetaminophen exposure during childhood. The rest of the studies evaluated the effects of acetaminophen exposure during pregnancy.
ADHD; Attention-deficit/hyperactivity disorder
CAST; Childhood Autism Spectrum Test
Acetaminophen rapidly enters the cerebrospinal fluid to exert its effects.150 In addition to reducing fever and physical pain, acetaminophen has a profound effect on adult brain function, blunting the response to both negative and positive stimuli, including threatening stimuli,151,152 and reducing behavioral responses to social rejection.153 Further, the drug impairs the ability of adults to identify errors made during the performance of simple tasks.154 In animal models, the use of acetaminophen during development has been shown to cause permanent alterations in cognitive function.155 Despite these effects on neurological function in adults, the widely appreciated connections between acetaminophen use during pregnancy and neurodevelopmental problems, and studies in animal models pointing toward potential problems with acetaminophen for brain development,133,155 acetaminophen is now the most widely used medicine in the pediatric population, readily available for infants in an over-the-counter form labeled as “safe, gentle, and effective,” with no warnings of side effects other than allergic reactions.
A connection between acetaminophen and autism was first identified in 2008 by Schultz et al.,156 who found that acetaminophen use by children was significantly associated with autism in children aged 5 years or less (OR=6.11, CI 95% = 1.42–26.3). Subsequently, several investigators noted that the marked increase in autism, asthma, and ADHD in the early 1980s corresponded with the replacement of aspirin with acetaminophen.157,158 In addition, Schultz noted that the long-term, steady increase in the prevalence of autism was punctuated by short-term decreases coinciding with widely publicized cases of acetaminophen poisoning that temporarily deterred the public from using the drug.159 Further, evidence has surfaced indicating that neural pathways affected by acetaminophen may be “different” in some regards in people with autism.159,160 This observation is potentially a “smoking gun,” suggestive of the role of acetaminophen in the pathogenesis of autism. Interestingly, Bauer and colleagues noted that acetaminophen use with circumcision may be associated with an increased prevalence of autism in some locations.157 A second and more recent study looking at the connection between circumcision and autism, this one by Frisch and Simonsen, found a 2-fold increased risk of autism identified before age 5 in circumcised boys compared with uncircumcised boys.161 But of course, the degree to which acetaminophen use during circumcision is associated with autism will depend not only on the ability of acetaminophen to induce autism but also on other factors such as the relative amount of acetaminophen used following circumcision compared with other uses in the pediatric population.
A Danish National Birth Cohort study recently found that prenatal use of acetaminophen is associated with an increased risk of autism accompanied by hyperkinetic symptoms (HR = 1.51; CI 95% = 1.19–1.92).124 More recently, a Spanish study supported the connection between acetaminophen exposure during pregnancy and autism in the offspring.162 Although the increase from prenatal exposure is statistically significant and indeed concerning, the risk was more than 10-fold less than that originally identified by Schultz when evaluating the use of acetaminophen in children. As discussed below, post-partum (infancy and early childhood) exposure to acetaminophen may result in a much higher risk of developing autism than prenatal exposure.
Schultz, whose 2008 study was the first to identify acetaminophen use in small children as a potential cause of autism, very recently found that older children with autism actually use less (not more) acetaminophen than neurotypical controls.163 Thus, small children who eventually develop autism have more often been exposed to acetaminophen than controls, but older children with autism are less likely to use the drug. Schultz suggested that this observation may be hypothetically explained if parents noticed that their child with autism did not respond to acetaminophen. Studies in animal models have shown that early life exposure to acetaminophen causes a lack of response to the same drug later in life,155 and Schultz postulated that the same may occur in humans.163 Another explanation, not mutually exclusive, is that parents may have learned that acetaminophen is associated with autism, and thus discontinued administration of the drug to their child. The connection between acetaminophen and autism is common knowledge among parents who have children with autism, as it has been promoted by grass-roots organizations such as Reset.Me and SafeMinds.
Plausible mechanisms for the induction of autism by acetaminophen have been formulated,113,158,159 adding further support to the potential role of acetaminophen in the pathogenesis of autism. McCarthy, for example, has provided a plausible explanation for the idea that inhibition of prostaglandin synthesis (by acetaminophen, for example) would have a much different effect on the male brain than on the female brain.164 However, the existence of a plausible mechanism alone may be considered inadequate by itself to support studies regarding the acetaminophen–autism connection, since detailed mechanisms have been proposed for the induction of autism by several factors, including ethyl mercury from vaccine preservatives165 and methanol from diet soft drinks.114 The removal of ethyl mercury from vaccines and the reduction of aspartame consumption have removed these environmental factors from prime suspicion for inducers of autism (see discussion above), despite the publication of hypothetical mechanistic underpinnings. Thus, the availability of a hypothetical mechanism for the induction of autism by a particular agent is not a good indicator for its actual role in the pathogenesis of disease. However, given the weight of the burden of autism on society, it seems reasonable to test all plausible environmental triggers for the induction of autism.
Albeit indirectly, yet another factor points toward a role of acetaminophen in the development of autism: the lack of any published association between cystic fibrosis (CF) and autism stands out as the exception to the rule of association between inflammatory conditions and autism. CF is clearly associated with mucosal inflammation166 but no connection between autism and CF has been published, suggesting that if any association does exist, it is not as conspicuous as that seen with other inflammation-associated conditions (e.g. cerebral palsy, preterm birth, or Down syndrome). Indeed, physicians at two independent clinics, each having treated hundreds of CF patients, recalled no patients with autism whatsoever in discussions with one of the authors (WP). An explanation for this potential exception to the rule of association between inflammation and autism lies in the fact that adults and children with CF tend to metabolize acetaminophen through phase II pathways much more so than do healthy controls.167,168 This feature of metabolism in CF patients may be a “consequence of disease-specific changes in both enzyme activity and/or drug transport within the liver.”168 In this regard, CF patients are very different from patients with autism, who tend to have impaired phase II metabolism (Figure 3). Although speculative at present, the idea that CF may be protective from autism merits further study and may provide insight into the pathogenesis of the latter.
Why evaluate the connection between post-partum acetaminophen exposure and autism?
The possible role of acetaminophen exposure in neonates and young children in the pathogenesis of autism demands further study for a number of reasons. First, as pointed out above, the odds ratio for autism associated with acetaminophen exposure in children is one of the highest ever reported, exceeding the odds ratios consistently reported for exposure in utero. The initial study based on surveys156 was conducted almost 10 years ago and has yet to be confirmed or refuted by more rigorous studies.
Perhaps even more compelling than the reason above, a second line of evidence points toward the need to evaluate the connection between post-partum use of acetaminophen and autism. This evidence is based in part on two important epidemiological observations made by Bernard Rimland, founder of the Autism Research Institute in 1967 and the individual responsible for undermining the horrendous “refrigerator mother” hypothesis that prevailed during the first 20 years of research on autism. As shown in Figure 2, Rimland noted a rapid rise in the rate of autism beginning in the early 1980s.169 Although he did not connect this rise with the use of acetaminophen in neonates and children, the widespread use of acetaminophen in infants, neonates, toddlers, and small children began in the early 1980s as a result of the discovery that aspirin may be associated with Reye syndrome.170 This apparent rise in the prevalence of autism starting in the early 1980s has been corroborated by data collected in the state of California (Figure 2, lower diagram). In addition to an increase in prevalence, Rimland also noted a simultaneous increase in the relative ratio of regressive to infantile autism (Figure 2), as might be expected if a new and extremely potent trigger for the disease was introduced into the population which affected newborns and small children but not necessarily fetuses.
Rimland’s study was not the last to suggest the emergence of a preponderance of regressive autism. In 2010, Ozonoff and colleagues published a prospective evaluation of behavior in children who would eventually be diagnosed with autism.171 Ozonoff collected data starting at 6 months of age and focused on at-risk children, mostly those with siblings that had autism. Surprisingly, more than 85% of the children eventually diagnosed with autism were indistinguishable from neurotypically developing children at 6 months of age, but showed declines in social communication between 6 and 18 months. Thus, shortly prior to 2010, when Ozonoff’s data were collected, most children with autism apparently presented with a regressive phenotype.
Intuitively, a preponderance of the regressive phenotype weakens any assumption that prenatal exposure is centrally or exclusively important. Indeed, there is no “proof” that autism is based on architectural changes laid down before birth. Associations between imaging and neuropathic findings and phenotype have been found,172,173 but no one has systematically assessed the extent to which documented brain architectural differences in individuals diagnosed with autism (a diagnosis that cannot presently be made prenatally or in the first year and a half of life) derive from altered prenatal neurodevelopmental processes as compared with tissue changes acquired over time from disturbances in key phenomena such as excitation/inhibition balance, bioenergetics, immune function, or other metabolic processes. Indeed, it is widely appreciated that pathophysiological changes can be set off not just by early genetic or environmental influences but also in many ways at many times of life.
Another compelling reason to probe the post-partum acetaminophen–autism connection is that, if indeed acetaminophen exposure during early childhood is found to be an important player in the pathogenesis of autism, then reduction of the incidence of autism is readily achievable, with the primary concern being establishing best practices for the treatment of fevers and pain in children under 6 months of age. Given that acetaminophen exposure in pregnant women is associated with an increase in the risk of autism,124 it would be quite surprising and perhaps very informative from a mechanistic perspective if indeed a dramatic reduction in acetaminophen exposure during early childhood did not cause a dramatic reduction in the incidence of autism.
Yet another reason to investigate the role of early childhood exposure to acetaminophen in the pathogenesis of autism is that eliminating acetaminophen exposure early in life is unlikely to cause net harm, regardless of the effect on autism. Acetaminophen has never been shown to save lives in any controlled study and, as described above, is known to be associated with the induction of asthma and the presence of a variety of developmental delays. Going even further, Ohlsson and Shah state in their recent Cochrane report of acetaminophen use during a major thoracic surgery frequently performed on infants (surgical closure of the patent ductus arteriosus):
In view of a recent report in mice of adverse effects on the developing brain from paracetamol [acetaminophen], and another report of an association between prenatal paracetamol and the development of autism or autism spectrum disorder in childhood, long-term follow-up to at least 18 to 24 months postnatal age must be incorporated in any studies of paracetamol in the newborn population. Such trials are required before any recommendations for the use of paracetamol in the newborn population can be made. 174
In other words, Ohlsson and Shah argue that acetaminophen should not be recommended, even for major and necessary surgical procedures, until it is known whether or not it does in fact cause autism.
Another reason to conduct a study of the early childhood acetaminophen–autism connection is the observations of parents. During the 70-year history of investigating the pathogenesis of autism, scientists and pediatricians have made two well-documented and costly errors, both associated with dismissing the observations of parents. The first error was the acceptance for 20 years of the “refrigerator mother” hypothesis.175 The second error was a firm belief, held until 2005, that autism could not be regressive and associated with a decline in previously existing neuropsychiatric function. At present, half of all parents of children with autism suspect vaccines as an underlying cause of their child’s condition.176 One study found that 29% of all mothers, with or without a child with autism, believe that autism can be induced by vaccines.177 Other studies,178 as well as surveys by various polling organizations such as Harris, Thompson Reuters, and the National League of Consumers, have reached similar conclusions. For reasons discussed above, it seems apparent that vaccines are not the underlying cause of the epidemic of autism, but at the same time, if history is any indication, ignoring the perspectives of parents is a grave error. The idea that vaccines induce autism has been widely blamed179 on a single article published by Wakefield,180 but articles published in scientific journals are unlikely to sway public opinion. Even repeated publications regarding the dangers of acetaminophen for neural development, for example, have had little impact on the use of acetaminophen in the pediatric population. Rather, it is social networks that are more likely to be persuasive. Acceptance of social networks is deeply ingrained in the human psyche,181 most likely because of their ancient and critical importance for human survival.182,183 Thus, parents of autistic children are likely to believe that vaccines are responsible for the induction of autism not because of a peer-reviewed article but rather because of their observations and the observations of individuals within their trusted social networks. With this in mind, the story of Steve Schultz is informative. He was convinced that his son’s autism was induced by a vaccine because of what was obvious to him as an observer. Thus, Schultz’s work both accounts for parents’ observations and for the science surrounding the pathogenesis of autism. Whatever the factor inducing the development of autism, it is critical that the scientific community learns from its past errors in ignoring the observations of parents.
It is estimated that the lifetime cost of supporting an individual with autism is currently US$2.4 million for an individual that also has an intellectual disability, or US$1.4 million for an individual with autism but without an intellectual disability.184 It is also estimated that 38% of individuals with autism in the United States also have an intellectual disability.185 This leads to an overall average cost estimation of approximately US$1.8 million to support one child with autism for his or her lifetime. We estimate that the total cost of running a definitive clinical study to test the connection between postnatal acetaminophen exposure and the induction of autism is less than the cost of caring for three to five individuals who have already developed autism.
Conducting an “acetaminophen withdrawal study”: Practical considerations
Given the already established adverse effects of acetaminophen on the developing brain, described above, and the known risk of asthma following acetaminophen exposure, it seems inappropriate to intentionally expose anyone under the age of 5 years to acetaminophen for the purpose of a medical study. Rather, other approaches must be considered. One approach involves large-scale experiments in which acetaminophen exposure is essentially eliminated until the age at which a patient can be considered to be at zero risk for regression into autism. Such an “acetaminophen withdrawal study” would not be trivial. The feasibility of an acetaminophen withdrawal study was previously examined for the purpose of evaluating the connection between acetaminophen and asthma.186 However, that feasibility study involved only 120 infants admitted to hospital after birth, at a time when some acetaminophen exposure may have already happened (e.g. during circumcision). Further, the study allowed some use of acetaminophen in all patients, and only involved 3 months of restricted use in the experimental population versus liberal use in a control population. Complete withdrawal up until the age of 5 years, on the other hand, would require all forms (injectable, oral, and suppository) of acetaminophen to be prospectively identified and reduced to an “essential minimum,” if not eliminated. A few thousand patients may need to be enrolled, depending of course on the magnitude of the anticipated effect. However, fewer patients may be needed if at-risk populations (e.g. preterm delivery, Down syndrome) are selected for the study. The practices associated with circumcision will need to be reconsidered, and the use of acetaminophen during vaccinations, a common but already highly questioned practice,187,188 would need to be eliminated. The possibility of passing acetaminophen to nursing infants and children through breast milk would also need to be eliminated. Extensive education of parents and a wide range of health-care workers (pharmacists and all physicians and nurses associated with obstetrics and pediatrics) regarding the avoidance of acetaminophen would be necessary. Further, comparable control groups with liberal use (current standard) of acetaminophen would need to be monitored simultaneously in order to obtain unequivocal results. Unfortunately, follow-up would require some time, and it would take years before a result is obtained. However, it is hoped that the weight of the current evidence will be considered sufficient for a dramatic reduction in acetaminophen use until incisive studies are completed.
A primary concern with an acetaminophen-elimination experiment would be the treatment of fever and pain. Alternative pharmacological methods of treating pain are available. Although all pharmacological methods have drawbacks, acetaminophen is not considered to be highly effective for pain relief.189 Indeed, as stated by McCullough, “The few clinical studies of (acetaminophen) and ibuprofen in children have struggled to find objective measures of pain capable of reliably distinguishing between active treatment and placebo.”189 Thus it seems unwise to risk potentially permanent neurological injury for apparently ineffective pain relief. Alternative pharmacological means are also available for fever reduction, but these have drawbacks. On the other hand, traditional methods of fever reduction (physical, non-pharmacological) look promising and have few side effects. However, these may be less convenient for clinicians and parents alike, and may prove ineffective in some cases. At the same time, the specter of inducing autism while treating pain or fever with acetaminophen should be weighed when considering the benefits of using acetaminophen, even in dire circumstances.
Given the current value attributed to the therapeutic effects of acetaminophen, it is worthwhile to consider possible ways to utilize the drug in the pediatric population, even if it is shown to be responsible for the epidemic of autism. One possibility is that individuals who are susceptible to acetaminophen-induced autism (e.g. perhaps those whose mothers have excessively high levels of vitamin B) might be identified, and use of the drug in these individuals could be avoided while others could benefit from use of the drug. Another possibility is that co-administration of N-acetyl cysteine (NAC) with acetaminophen could be used to attenuate the toxic effects of acetaminophen in cases where the use of acetaminophen is considered highly desirable. It might be hypothesized that any insufficiencies in phase I or phase II metabolism (Figure 3), which may lead to acetaminophen-induced autism, would be prevented by co-administration of NAC. NAC works both orally and parenterally, which may be important since acetaminophen is available for oral, intravenous, or rectal administration (Table 3). In patients with normal liver and renal function, the half-life of NAC is slightly longer than the half-life of acetaminophen, so a single dose of NAC may be sufficient to cover one dose of acetaminophen. However, the respective half-lives of the two compounds would need to be evaluated in the treatment population, and the idea that NAC may be protective against acetaminophen-induced autism is speculative.
Table 3.
Available US acetaminophen products from birth to early childhood.
| Ingredient | Dosage Form | Strength | Indication | Special Considerations |
|---|---|---|---|---|
| Injectable | ||||
| Acetaminophen | Solution | 1000 mg/100mL | Arthralgia; dental pain; fever; headache; mild/moderate/severe pain; musculoskeletal pain; myalgia; patent ductus arteriosus treatmenta; post-operative/procedural paina | Not studied in infants <28 weeks gestational age |
| Oral | ||||
| Acetaminophen (natural berry, grape, white grape, cherry, and bubble gum flavors) | Solution | 120 mg/5 mL 160 mg/5 mL | Arthralgia; dental pain; fever; headache; mild/moderate/severe pain; musculoskeletal pain; myalgia; post-operative/procedural pain | All dosage forms available OTC |
| Liquid | 160 mg/5 mL 500 mg/15 mL | |||
| Suspension | 160 mg/5 mL 325 mg/10.15 mL 80 mg/2.5 mL 80 mg/0.8 mL | |||
| Drops | 80 mg/0.8 mL | |||
| Chewable tablet | 80 mg 160 mg | |||
| Rapid tablet | 80 mg 160 mg | |||
| Acetaminophen/ Codeineb phosphate | Solution | 120 mg/5 mL | Mild/moderate pain; Arthralgiaa; bone paina; cougha; headachea; myalgiaa | Not recommended: for use in neonates, for post-operative tonsillectomy and/or adenoidectomy pain management; contraindicated in patients who are CYP2D6 ultra-rapid metabolizers |
| Hydrocodone bitartrateb/ acetaminophen | Solution | 108 mg/5 mL 167 mg/5 mL 163 mg/7.5 mL 217 mg/10 mL | Cougha; moderate/severe pain; post-operative/procedural paina | Not recommended in children <2 years of age |
| Oxycodone hydrochlorideb/ acetaminophen | Solution | 325 mg/5 mL | Moderate/severe pain; post-operative/procedural paina | |
| Acetaminophen/ chlorpheniramineb/ dextromethorphanb | Suspension OTC | 7.5 mg/5 mL | Common cold; cough | Not recommended in children <4 years of age |
| Acetaminophen/ dextromethorphanb | Suspension | 160 mg/5 mL | Cough; sore throat | Not recommended in children <4 years of age |
| Acetaminophen/ chlorpheniramineb/ dextromethorphanb/ Phenylephrineb | Effervescent tablet | 250 mg | Common cold; cough; fever; headache; mild pain; nasal congestion; pharyngitis; rhinorrhea; sneezing | Both dosage forms available OTC; not recommended in children <4 years of age |
| Suspension | 160 mg | |||
| Suppository | ||||
| Acetaminophen | Rectal suppository OTC | 80 mg 120 mg 325 mg | Arthralgia; dental pain; fever; headache; mild/moderate/severe pain; musculoskeletal pain; myalgia; post-operative/procedural pain | Not studied in infants <28 weeks gestational age |
“Over-the-counter” is abbreviated OTC.
Off-abel
Combination products contain varying amounts of other active ingredients. The strength listed is for the acetaminophen component only.
Education of medical professionals is imperative for any acetaminophen withdrawal study, given the current medical environment. Acetaminophen use is currently ubiquitous and thought to be the only humane approach to pain and fever reduction for children from the time of birth to 6 months. The drug is available in a very wide range of formats for both prescription and over-the-counter use (Table 3). Almost one-quarter of all infants are given acetaminophen in any given week when in the hospital, making it the number one medication used in infants.190 Given that most medical professionals currently practicing began their careers after 1982, it is not surprising that acetaminophen use is deeply engrained in practice and that alternatives may seem foreign and even primitive. Further, the idea that acetaminophen exposure in early childhood could be dangerous is extremely novel and even disturbing to most medical practitioners (personal observation by co-author WP). Even the best-trained pediatricians are generally unaware that the long-term effects of acetaminophen were never tested in children in controlled trials.191 Further, with the exception of toxic metabolite production (Figure 3) that primarily affects liver function in adults but not babies, most physicians are largely unaware of the varied and complex effects of acetaminophen on the nervous system and on metabolism. Few practitioners are aware of the well-established connection between asthma and acetaminophen use, and fewer still are aware of the increased possibility of cryptorchidism (undescended testis) known to be associated with acetaminophen use.192
The exceedingly common and widespread use of acetaminophen both in the hospital and at home may have led to some degree of complacency in proper dose administration. For example, in a study of outpatient care prescription errors, 15% of children who were prescribed acetaminophen were given a dosage by medical professionals that was higher than recommended.193 Similarly, another study found that 12% of children who were prescribed acetaminophen at a pediatric emergency department were given a prescription for acetaminophen higher than the recommended dosage.194
Interestingly, Li et al.195 found that parents administered acetaminophen in a manner comparable to physicians; dosages administered by parents exceeded recommended amounts about 15% of the time. Li et al.195 also noted that infants were more likely than older children to be given an inaccurate dosage of acetaminophen (RR = 1.40, P < 0.04, CI 95% = 1.06–1.86) by their parents. This latter observation is particularly concerning since it is during infancy that the brain may be most sensitive to damage by acetaminophen from oxidative stress. However, it is unknown to what extent acetaminophen-induced neuropathology can be induced by the recommended dose of the drug, and to what extent excessive doses are responsible for the induction of neurological disorders in the pediatric population.
The bottom line is that hundreds of studies describing the epidemiology of autism and the numerous and varied risk factors for autism have a straightforward explanation: autism could be an acetaminophen-induced brain injury facilitated by oxidative stress and inflammation in newborns and young children. This is certainly an attractive view from an intellectual perspective, as it satisfies Occam’s razor. Most importantly, this model merits urgent testing because (a) it is intuitive and accounts for the observations, (b) the experiment to test the hypothesis is very feasible, and (c) if proven correct, the prevalence of autism in future generations will be dramatically reduced. The urgency of the issue cannot be underestimated, as precious time and resources that could be allocated much more constructively and usefully in this time of serious need are being poured into approaches that offer little to no hope of prevention.
Acknowledgements
The authors are very grateful to Stephen M. Edelson, current director of the Autism Research Institute, for sharing his knowledge of the work with autism that he and Bernard Rimland performed during the 1960s through the 1990s. The study team is also very grateful to Susanne Meza-Keuthen for her generous financial support of this study. The authors wish to thank Michael R. Knowles, Jeffrey P. Baker, Margaret Leigh and Carolyn Durham for very helpful discussions.
Declaration of conflicting interest
The Authors declare that there is no conflict of interest.
Funding
This review received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
No studies using animal models and no research involving human subjects were performed for this study.
References
- 1.Keil A, Daniels JL, Forssen U, et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology 2010; 21: 805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanne JH. Maternal obesity and diabetes are linked to children’s autism and similar disorders. BMJ 2012; 344: e2768–e2768. [DOI] [PubMed] [Google Scholar]
- 3.Atladottir HO, Henriksen TB, Schendel DE, et al. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 2012; 130: e1447–e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker W, Perkins SE, Harker M, et al. A prescription for clinical immunology: the pills are available and ready for testing. Curr Med Res Opin 2012; 28: 1193–1202. [DOI] [PubMed] [Google Scholar]
- 5.Brenner SL, Jones JP, Rutanen-Whaley RH, et al. Evolutionary mismatch and chronic psychological stress. J Evol Med 2015; 3: 235885–235885. [Google Scholar]
- 6.Holick MF., Vitamin D. deficiency. N Engl J Med 2007; 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 7.Bilbo SD, Wray GA, Perkins SE, et al. Reconstitution of the human biome as the most reasonable solution for epidemics of allergic and autoimmune diseases. Med Hypotheses 2011; 77: 494–504. [DOI] [PubMed] [Google Scholar]
- 8.Van Naarden Braun K, Christensen D, Doernberg N, et al. Trends in the prevalence of Autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991–2010. Plos One 2015; 10: e0124120–e0124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nevison CD. A comparison of temporal trends in United States autism prevalence to trends in suspected environmental factors. Environ Health 2014; 13: 73–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilbo SD, Nevison CD, Parker W. A model for the induction of autism in the ecosystem of the human body: the anatomy of a modern pandemic? Microb Ecol Health Dis. 2015. 26: 26253–26253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernell E, Bejerot S, Westerlund J, et al. Autism spectrum disorder and low vitamin D at birth: a sibling control study. Mol Autism 2015; 6: 3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology 2006; 13: 171–181. [DOI] [PubMed] [Google Scholar]
- 13.Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 2006; 160: 1–40. [DOI] [PubMed] [Google Scholar]
- 14.Heitzer T, Schlinzig T, Krohn K, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001; 104: 2673–2678. [DOI] [PubMed] [Google Scholar]
- 15.Ng F, Berk M, Dean O, et al. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 2008; 11: 851–876. [DOI] [PubMed] [Google Scholar]
- 16.Salzano S, Checconi P, Hanschmann EM, et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci U S A 2014; 111: 12157–12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinet E, Pineau CA, Clarke AE, et al. Increased risk of autism spectrum disorders in children born to women with systemic lupus erythematosus: results from a large population-based cohort. Arthritis & rheumatology 2015; 67: 3201–3208. [DOI] [PubMed] [Google Scholar]
- 18.Lee BK, Magnusson C, Gardner RM, et al. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain Behav Immun 2015; 44: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao TC, Lien YT, Wang S, et al. Comorbidity of Atopic Disorders with Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disorder. J Pediatr 2016; 171: 248–255. [DOI] [PubMed] [Google Scholar]
- 20.Krakowiak P, Walker CK, Bremer AA, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012; 129: e1121–e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McArdle MA, Finucane OM, Connaughton RM, et al. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne) 2013; 4: 52–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord 2003; 33: 365–382. [DOI] [PubMed] [Google Scholar]
- 23.Christensen D, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning - Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol 2014; 56: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson KB, Ellenberg JH. Predictors of low and very low birth weight and the relation of these to cerebral palsy. JAMA 1985; 254: 1473–1479. [PubMed] [Google Scholar]
- 25.Cooke RW. Cerebral palsy in very low birthweight infants. Arch Dis Child 1990; 65: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA 1997; 278: 207–211. [PubMed] [Google Scholar]
- 27.Nelson KB, Dambrosia JM, Grether JK, et al. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol 1998; 44: 665–675. [DOI] [PubMed] [Google Scholar]
- 28.Murphy DJ, Sellers S, MacKenzie IZ, et al. Case-control study of antenatal and intrapartum risk factors for cerebral palsy in very preterm singleton babies. Lancet 1995; 346: 1449–1454. [DOI] [PubMed] [Google Scholar]
- 29.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA 2000; 284: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 30.Bracci R, Buonocore G. Chorioamnionitis: a risk factor for fetal and neonatal morbidity. Biol Neonate 2003; 83: 85–96. [DOI] [PubMed] [Google Scholar]
- 31.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol 2007; 50: 652–683. [DOI] [PubMed] [Google Scholar]
- 32.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry 2009; 195: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandin S, Schendel D, Magnusson P, et al. Autism risk associated with parental age and with increasing difference in age between the parents. Mol Psychiatry 2016; 21: 693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dioun AF, Harris SK, Hibberd PL. Is maternal age at delivery related to childhood food allergy? Pediatr Allergy Immunol 2003; 14: 307–311. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery SM, Lambe M, Olsson T, et al. Parental age, family size, and risk of multiple sclerosis. Epidemiology 2004; 15: 717–723. [DOI] [PubMed] [Google Scholar]
- 36.Roberts AL, Lyall K, Rich-Edwards JW, et al. Maternal exposure to childhood abuse is associated with elevated risk of autism. JAMA Psychiatry 2013; 70: 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiecolt-Glaser JK, Gouin JP, Weng NP, et al. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med 2011; 73: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull 2011; 137: 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun 2013; 27: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci 2010; 21: 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dube SR, Fairweather D, Pearson WS, et al. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med 2009; 71: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertone-Johnson ER, Whitcomb BW, Missmer SA, et al. Inflammation and early-life abuse in women. Am J Prev Med 2012; 43: 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coogan PF, Wise LA, O’Connor GT, et al. Abuse during childhood and adolescence and risk of adult-onset asthma in African American women. J Allergy Clin Immunol 2013; 131: 1058–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gouin JP, Glaser R, Malarkey WB, et al. Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med 2012; 44: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor SE, Lehman BJ, Kiefe CI, et al. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry 2006; 60: 819–824. [DOI] [PubMed] [Google Scholar]
- 46.Danese A, Pariante CM, Caspi A, et al. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A 2007; 104: 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slopen N, Lewis TT, Gruenewald TL, et al. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosom Med 2010; 72: 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pace TW, Wingenfeld K, Schmidt I, et al. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun 2012; 26: 13–17. [DOI] [PubMed] [Google Scholar]
- 49.Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry 2014; 4: e360–e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shelton JF, Geraghty EM, Tancredi DJ, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect 2014; 122: 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts EM, English PB, Grether JK, et al. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect 2007; 115: 1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyall K, Croen LA, Sjodin A, et al. Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability. Environ Health Perspect 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raz R, Roberts AL, Lyall K, et al. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect 2015; 123: 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalkbrenner AE, Windham GC, Serre ML, et al. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 2015; 26: 30–42. [DOI] [PubMed] [Google Scholar]
- 55.Becerra TA, Wilhelm M, Olsen J, et al. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect 2013; 121: 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts AL, Lyall K, Hart JE, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect 2013; 121: 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volk HE, Hertz-Picciotto I, Delwiche L, et al. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect 2011; 119: 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volk HE, Kerin T, Lurmann F, et al. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology 2014; 25: 44–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guxens M, Ghassabian A, Gong T, et al. Air pollution exposure during pregnancy and childhood autistic traits in four european population-based cohort studies: the ESCAPE project. Environ Health Perspect 2016; 124: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pokhrel AK, Bates MN, Acharya J, et al. PM2.5 in household kitchens of Bhaktapur, Nepal, using four different cooking fuels. Atmos Environ 2015; 113: 159–168. [Google Scholar]
- 61.Davutoglu M, Guler E, Olgar S, et al. Oxidative stress and antioxidant status in neonatal hyperbilirubinemia. Saudi Med J 2008; 29: 1743–1748. [PubMed] [Google Scholar]
- 62.Palmer GM, Atkins M, Anderson BJ, et al. I.V. acetaminophen pharmacokinetics in neonates after multiple doses. Br J Anaesth 2008; 101: 523–530. [DOI] [PubMed] [Google Scholar]
- 63.Maimburg RD, Bech BH, Vaeth M, et al. Neonatal jaundice, autism, and other disorders of psychological development. Pediatrics 2010; 126: 872–878. [DOI] [PubMed] [Google Scholar]
- 64.Croen LA, Yoshida CK, Odouli R, et al. Neonatal hyperbilirubinemia and risk of autism spectrum disorders. Pediatrics 2005; 115: e135–e138. [DOI] [PubMed] [Google Scholar]
- 65.Chen M-H, Su T-P, Chen Y-S, et al. Is neonatal jaundice associated with autism spectrum disorder, attention deficit hyperactivity disorder, and other psychological development? A nationwide prospective study. Res Autism Spectr Disord 2014; 8: 625–632. [Google Scholar]
- 66.Lavoie JC, Chessex P. Gender and maturation affect glutathione status in human neonatal tissues. Free Radic Biol Med 1997; 23: 648–657. [DOI] [PubMed] [Google Scholar]
- 67.Minghetti L, Greco A, Zanardo V, et al. Early-life sex-dependent vulnerability to oxidative stress: the natural twining model. J Matern Fetal Neonatal Med 2013; 26: 259–262. [DOI] [PubMed] [Google Scholar]
- 68.Bahado-Singh RO, Schenone M, Cordoba M, et al. Male gender significantly increases risk of oxidative stress related congenital anomalies in the non-diabetic population. J Matern Fetal Neonatal Med 2011; 24: 687–691. [DOI] [PubMed] [Google Scholar]
- 69.Bhagde P, Pajai S, Chaudhari AR. Gender disparity in oxidative stress in healthy preterm neonates delivered vaginally. Indian Journal of Basic and Applied Medical Research 2015; 4: 300–306. [Google Scholar]
- 70.Marks AR, Harley K, Bradman A, et al. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect 2010; 118: 1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.DiGuiseppi C, Hepburn S, Davis JM, et al. Screening for Autism spectrum disorders in children with down syndrome: population prevalence and screening test characteristics. J Dev Behav Pediatr 2010; 31: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pagano G, Castello G. Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv Exp Med Biol 2012; 724: 291–299. [DOI] [PubMed] [Google Scholar]
- 73.Wilcock DM, Griffin WS. Down’s syndrome, neuroinflammation, and Alzheimer neuropathogenesis. J Neuroinflammation 2013; 10: 84–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Limperopoulos C, Bassan H, Sullivan NR, et al. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics 2008; 121: 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson S, Hollis C, Kochhar P, et al. Autism spectrum disorders in extremely preterm children. J Pediatr 2010; 156: 525–531 e2. [DOI] [PubMed] [Google Scholar]
- 76.D’Onofrio BM, Class QA, Rickert ME, et al. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry 2013; 70: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Frontiers in immunology 2014; 5: 567–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.O’Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev 2009; 85: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menon R, Bonney E. Oxidative stress and preterm birth. In: Dennery AP, Buonocore G, Saugstad DO. (eds). Perinatal and prenatal disorders, New York, NY: Springer New York, 2014, pp. 95–115. [Google Scholar]
- 80.Ferguson KK, McElrath TF, Chen YH, et al. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. Am J Obstet Gynecol 2015; 212: 208 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee YS, Chou YH. Antioxidant profiles in full term and preterm neonates. Chang Gung medical journal 2005; 28: 846–851. [PubMed] [Google Scholar]
- 82.Jain A, Mehta T, Auld PA, et al. Glutathione metabolism in newborns: evidence for glutathione deficiency in plasma, bronchoalveolar lavage fluid, and lymphocytes in prematures. Pediatr Pulmonol 1995; 20: 160–166. [DOI] [PubMed] [Google Scholar]
- 83.Buonocore G, Perrone S, Longini M, et al. Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr Res 2002; 52: 46–49. [DOI] [PubMed] [Google Scholar]
- 84.Raghavan R, Fallin MD, Wang X. Maternal plasma folate, vitamin B12 levels and multivitamin supplementation during pregnancy and risk of Autism spectrum disorder in the Boston birth cohort. FASEB J 2016; 30 Supplement 151.6. [Google Scholar]
- 85.Andres E, Serraj K, Zhu J, et al. The pathophysiology of elevated vitamin B12 in clinical practice. QJM 2013; 106: 505–515. [DOI] [PubMed] [Google Scholar]
- 86.Bowen RA, Drake SK, Vanjani R, et al. Markedly increased vitamin B12 concentrations attributable to IgG-IgM-vitamin B12 immune complexes. Clin Chem 2006; 52: 2107–2114. [DOI] [PubMed] [Google Scholar]
- 87.Frye RE, Sequeira JM, Quadros EV, et al. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry 2013; 18: 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pravenec M, Kozich V, Krijt J, et al. Folate deficiency is associated with oxidative stress, increased blood pressure, and insulin resistance in spontaneously hypertensive rats. Am J Hypertens 2013; 26: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bahmani F, Karamali M, Shakeri H, et al. The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Clin Endocrinol (Oxf) 2014; 81: 582–587. [DOI] [PubMed] [Google Scholar]
- 90.Heyman MB, Garnett EA, Shaikh N, et al. Folate concentrations in pediatric patients with newly diagnosed inflammatory bowel disease. Am J Clin Nutr 2009; 89: 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boso M, Emanuele E, Minoretti P, et al. Alterations of circulating endogenous secretory RAGE and S100A9 levels indicating dysfunction of the AGE-RAGE axis in autism. Neurosci Lett 2006; 410: 169–173. [DOI] [PubMed] [Google Scholar]
- 92.James SJ, Cutler P, Melnyk S, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 2004; 80: 1611–1617. [DOI] [PubMed] [Google Scholar]
- 93.James SJ, Melnyk S, Jernigan S, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet 2006; 141B: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buyske S, Williams TA, Mars AE, et al. Analysis of case-parent trios at a locus with a deletion allele: association of GSTM1 with autism. BMC Genet 2006; 7: 8–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ming X, Johnson WG, Stenroos ES, et al. Genetic variant of glutathione peroxidase 1 in autism. Brain Dev 2010; 32: 105–109. [DOI] [PubMed] [Google Scholar]
- 96.Williams TA, Mars AE, Buyske SG, et al. Risk of autistic disorder in affected offspring of mothers with a glutathione S-transferase P1 haplotype. Arch Pediatr Adolesc Med 2007; 161: 356–361. [DOI] [PubMed] [Google Scholar]
- 97.Bolton JL, Huff NC, Smith SH, et al. Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect 2013; 121: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Edwards SC, Jedrychowski W, Butscher M, et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect 2010; 118: 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miranda ML, Edwards SE, Chang HH, et al. Proximity to roadways and pregnancy outcomes. J Expo Sci Environ Epidemiol 2013; 23: 32–38. [DOI] [PubMed] [Google Scholar]
- 100.Kinney DK, Munir KM, Crowley DJ, et al. Prenatal stress and risk for autism. Neurosci Biobehav Rev 2008; 32: 1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. J Child Psychol Psychiatry 2005; 46: 963–971. [DOI] [PubMed] [Google Scholar]
- 102.Perera FP, Li Z, Whyatt R, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics 2009; 124: e195–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Becker KG. Autism, asthma, inflammation, and the hygiene hypothesis. Med Hypotheses 2007; 69: 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 2005; 57: 67–81. [DOI] [PubMed] [Google Scholar]
- 105.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res 2011; 69(5 Pt 2): 26R–33 R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Strickland AD. Prevention of cerebral palsy, autism spectrum disorder, and attention deficit-hyperactivity disorder. Med Hypotheses 2014; 82: 522–528. [DOI] [PubMed] [Google Scholar]
- 107.Thomas RH, Meeking MM, Mepham JR, et al. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation 2012; 9: 153–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Macfabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis 2012; 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bilbo SD, Jones JP, Parker W. Is autism a member of a family of diseases resulting from genetic/cultural mismatches? Implications for treatment and prevention. Autism Res Treat 2012; 2012: 910946–910946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh K, Connors SL, Macklin EA, et al. Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci U S A 2014; 111: 15550–15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J, Morey RA, Wilson JK, et al. Practices and outcomes of self-treatment with helminths based on physicians’ observations. J Helminthol 2016, pp. 1–11. [DOI] [PubMed] [Google Scholar]
- 112.Bickler SW, DeMaio A. Western diseases: current concepts and implications for pediatric surgery research and practice. Pediatr Surg Int 2008; 24: 251–255. [DOI] [PubMed] [Google Scholar]
- 113.Shaw W. Evidence that Increased Acetaminophen use in Genetically Vulnerable Children Appears to be a Major Cause of the Epidemics of Autism, Attention Deficit with Hyperactivity, and Asthma. J Restor Med 2013; 2: 1–16. [Google Scholar]
- 114.Walton RG, Monte WC. Dietary methanol and autism. Med Hypotheses 2015; 85: 441–446. [DOI] [PubMed] [Google Scholar]
- 115.Goin-Kochel RP, Mire SS, Dempsey AG. Emergence of autism spectrum disorder in children from simplex families: relations to parental perceptions of etiology. J Autism Dev Disord 2015; 45: 1451–1463. [DOI] [PubMed] [Google Scholar]
- 116.Mercer L, Creighton S, Holden JJ, et al. Parental perspectives on the causes of an autism spectrum disorder in their children. J Genet Couns 2006; 15: 41–50. [DOI] [PubMed] [Google Scholar]
- 117.Gallagher CM, Goodman MS. Hepatitis B vaccination of male neonates and autism diagnosis, NHIS 1997–2002. J Toxicol Environ Health A 2010; 73: 1665–1677. [DOI] [PubMed] [Google Scholar]
- 118.Calandrillo SP. Vanishing vaccinations: why are so many Americans opting out of vaccinating their children? Univ Mich J Law Reform 2004; 37: 353–440. [PubMed] [Google Scholar]
- 119.Dorea JG, Farina M, Rocha JB. Toxicity of ethylmercury (and Thimerosal): a comparison with methylmercury. J Appl Toxicol 2013; 33: 700–711. [DOI] [PubMed] [Google Scholar]
- 120.Ahmed SS, Schur PH, MacDonald NE, et al. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J Autoimmun 2014; 50: 1–11. [DOI] [PubMed] [Google Scholar]
- 121.Hviid AM, Stellfeld JW and Melbye M. Association between thimerosal-containing vaccine and autism. Jama 2003; 290: 1763–1766. [DOI] [PubMed]
- 122.Abu Kuwaik G, Roberts W, Zwaigenbaum L, et al. Immunization uptake in younger siblings of children with autism spectrum disorder. Autism 2014; 18: 148–155. [DOI] [PubMed] [Google Scholar]
- 123.Jain A, Marshall J, Buikema A, et al. Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. JAMA 2015; 313: 1534–1540. [DOI] [PubMed] [Google Scholar]
- 124.Liew Z, Ritz B, Virk J, et al. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: a Danish national birth cohort study. Autism Res 2015; 9: 951–958. [DOI] [PubMed] [Google Scholar]
- 125.Werler MM, Mitchell AA, Hernandez-Diaz S, et al. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol 2005; 193(3 Pt 1): 771–777. [DOI] [PubMed] [Google Scholar]
- 126.Hsieh EM, Hornik CP, Clark RH, et al. Medication use in the neonatal intensive care unit. Am J Perinatol 2014; 31: 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cranswick N, Coghlan D. Paracetamol efficacy and safety in children: the first 40 years. Am j Ther 2000; 7: 135–141. [DOI] [PubMed] [Google Scholar]
- 128.Prescott LF, Sansur M, Levin W, et al. The comparative metabolism of phenacetin and N-acetyl-p-aminophenol in man, with particular reference to effects on the kidney. Clin Pharmacol Ther 1968; 9: 605–614. [DOI] [PubMed] [Google Scholar]
- 129.Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol 1980; 10: 291 S–298 S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther 2014; 19: 262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Penna A, Buchanan N. Paracetamol poisoning in children and hepatotoxicity. Br J Clin Pharmacol 1991; 32: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics 1999; 104: e39–e39. [DOI] [PubMed] [Google Scholar]
- 133.Dean SL, Knutson JF, Krebs-Kraft DL, et al. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur J Neurosci 2012; 35: 1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Prill S, Bavli D, Levy G, et al. Real-time monitoring of oxygen uptake in hepatic bioreactor shows CYP450-independent mitochondrial toxicity of acetaminophen and amiodarone. Arch Toxicol 2016; 90: 1181–1191. [DOI] [PubMed] [Google Scholar]
- 135.Jetten MJ, Gaj S, Ruiz-Aracama A, et al. ’Omics analysis of low dose acetaminophen intake demonstrates novel response pathways in humans. Toxicol Appl Pharmacol 2012; 259: 320–328. [DOI] [PubMed] [Google Scholar]
- 136.Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol 2015; 15: 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Berlim MT, Abeche AM. Evolutionary approach to medicine. South Med J 2001; 94: 26–32. [PubMed] [Google Scholar]
- 138.Torres AR. Is fever suppression involved in the etiology of autism and neurodevelopmental disorders? BMC Pediatrics 2003; 3: 9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Beasley R, Clayton T, Crane J, et al. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from phase three of the ISAAC programme. Lancet 2008; 372: 1039–1048. [DOI] [PubMed] [Google Scholar]
- 140.Sordillo JE, Scirica CV, Rifas-Shiman SL, et al. Prenatal and infant exposure to acetaminophen and ibuprofen and the risk for wheeze and asthma in children. J Allergy Clin Immunol 2015; 135: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McBride JT. The association of acetaminophen and asthma prevalence and severity. Pediatrics 2011; 128: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 142.Magnus MC, Karlstad Ø, Håberg SE, et al. Prenatal and infant paracetamol exposure and development of asthma: the Norwegian mother and child cohort study. Int J Epidemiol 2016; 45: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Beasley RW, Clayton TO, Crane J, et al. Acetaminophen Use and Risk of Asthma, Rhinoconjunctivitis, and Eczema in Adolescents. Am J Respir Crit Care Med 2011; 183: 171–178. [DOI] [PubMed] [Google Scholar]
- 144.Brandlistuen RE, Ystrom E, Nulman I, et al. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol 2013; 42: 1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stergiakouli E, Thapar A, Davey Smith G. Association of acetaminophen use during pregnancy with behavioral problems in childhood: evidence against confounding. JAMA Pediatrics 2016; 170: 964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liew Z, Ritz B, Rebordosa C, et al. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatrics 2014; 168: 313–320. [DOI] [PubMed] [Google Scholar]
- 147.Liew Z, Bach CC, Asarnow RF, et al. Paracetamol use during pregnancy and attention and executive function in offspring at age 5 years. Int J Epidemiol 2016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 148.Thompson JMD, Waldie KE, Wall CR, et al. Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. Plos One 2014; 9: e108210–e108210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liew Z, Ritz B, Virk J, et al. Prenatal use of acetaminophen and child IQ: a Danish cohort study. Epidemiology 2016; 27: 912–918. [DOI] [PubMed] [Google Scholar]
- 150.Kumpulainen E, Kokki H, Halonen T, et al. Paracetamol (acetaminophen) penetrates readily into the cerebrospinal fluid of children after intravenous administration. Pediatrics 2007; 119: 766–771. [DOI] [PubMed] [Google Scholar]
- 151.Randles D, Heine SJ, Santos N. The common pain of surrealism and death: acetaminophen reduces compensatory affirmation following meaning threats. Psychol Sci 2013; 24: 966–973. [DOI] [PubMed] [Google Scholar]
- 152.Durso GR, Luttrell A, Way BM. Over-the-counter relief from pains and pleasures alike: acetaminophen blunts evaluation sensitivity to both negative and positive stimuli. Psychol Sci 2015; 26: 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dewall CN, Macdonald G, Webster GD, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci 2010; 21: 931–937. [DOI] [PubMed] [Google Scholar]
- 154.Randles D, Kam JW, Heine SJ, et al. Acetaminophen attenuates error evaluation in cortex. Soc Cogn Affect Neurosci 2016; 11: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Viberg H, Eriksson P, Gordh T, et al. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicol Sci 2014; 138: 139–147. [DOI] [PubMed] [Google Scholar]
- 156.Schultz ST, Klonoff-Cohen HS, Wingard DL, et al. Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder. The results of a parent survey. Autism 2008; 12: 293–307. [DOI] [PubMed] [Google Scholar]
- 157.Bauer A, Kriebel D. Prenatal and perinatal analgesic exposure and autism: an ecological link. Environ Health 2013; 12: 41–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Becker KG, Schultz ST. Similarities in features of autism and asthma and a possible link to acetaminophen use. Med Hypotheses 2010; 74: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schultz ST. Can autism be triggered by acetaminophen activation of the endocannabinoid system? Acta Neurobiol Exp (Wars) 2010; 70: 227–231. [DOI] [PubMed] [Google Scholar]
- 160.Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron 2013; 78: 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Frisch M, Simonsen J. Ritual circumcision and risk of autism spectrum disorder in 0- to 9-year-old boys: national cohort study in Denmark. J R Soc Med 2015; 108: 266–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Avella-Garcia CB, Julvez J, Fortuny J, et al. Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. Int J Epidemiol 2016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 163.Schultz ST, Gould GG. Acetaminophen use for fever in children associated with autism spectrum disorder. Autism Open Access 2016; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McCarthy MM and Wright CL. Convergence of sex differences and the neuroimmune system in autism spectrum disorder. Biol Psychiatry 2017; 81: 402–410. [DOI] [PMC free article] [PubMed]
- 165.Geier DA, Kern JK, Geier MR. The biological basis of autism spectrum disorders: Understanding causation and treatment by clinical geneticists. Acta Neurobiol Exp (Wars) 2010; 70: 209–226. [DOI] [PubMed] [Google Scholar]
- 166.Cantin AM, Hartl D, Konstan MW, et al. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J Cyst Fibros 2015; 14: 419–430. [DOI] [PubMed] [Google Scholar]
- 167.Hutabarat RM, Unadkat JD, Kushmerick P, et al. Disposition of drugs in cystic fibrosis. III. Acetaminophen. Clin Pharmacol Ther 1991; 50: 695–701. [DOI] [PubMed] [Google Scholar]
- 168.Kearns GL. Hepatic drug metabolism in cystic fibrosis: recent developments and future directions. Ann Pharmacother 1993; 27: 74–79. [DOI] [PubMed] [Google Scholar]
- 169.Rimland B. The autism increase: research needed on the vaccine connection. Autism Res Rev Intl 2000; 14: 3–3. [Google Scholar]
- 170.Rahwan GL, Rahwan RG. Aspirin and Reye’s syndrome: the change in prescribing habits of health professionals. Drug Intell Clin Pharm 1986; 20: 143–145. [DOI] [PubMed] [Google Scholar]
- 171.Ozonoff S, Iosif A-M, Baguio F, et al. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry 2010; 49: 256–266. e1–2. [PMC free article] [PubMed] [Google Scholar]
- 172.Fein D. The neuropsychology of autism, New York: Oxford University Press, 2011. [Google Scholar]
- 173.Hu VW. Frontiers in autism research new horizons for diagnosis and treatment, Hackensack, New Jersey: World Scientific, 2014. [Google Scholar]
- 174.Ohlsson A, Shah PS. Paracetamol (acetaminophen) for patent ductus arteriosus in preterm or low-birth-weight infants. Cochrane Database Syst Rev 2015; 3: CD010061–CD010061. [DOI] [PubMed] [Google Scholar]
- 175.Baker JP. Autism in 1959: Joey the mechanical boy. Pediatrics 2010; 125: 1101–1103. [DOI] [PubMed] [Google Scholar]
- 176.Bazzano A, Zeldin A, Schuster E, et al. Vaccine-related beliefs and practices of parents of children with autism spectrum disorders. Am J Intellect Dev Disabils 2012; 117: 233–242. [DOI] [PubMed] [Google Scholar]
- 177.Freed GL, Clark SJ, Butchart AT, et al. Parental vaccine safety concerns in 2009. Pediatrics 2010; 125: 654–659. [DOI] [PubMed] [Google Scholar]
- 178.Fischbach RL, Harris MJ, Ballan MS, et al. Is there concordance in attitudes and beliefs between parents and scientists about autism spectrum disorder? Autism 2016; 20: 353–363. [DOI] [PubMed] [Google Scholar]
- 179.Roberts W, Harford M. Immunization and children at risk for autism. Paediatr Child Health 2002; 7: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998; 351: 637–641. [DOI] [PubMed] [Google Scholar]
- 181.Cacioppo JT, Cacioppo S. Social relationships and health: the toxic effects of perceived social isolation. Soc Personal Psychol Compass 2014; 8: 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Larson JM. The evolutionary advantage of limited network knowledge. J Theor Biol 2016; 398: 43–51. [DOI] [PubMed] [Google Scholar]
- 183.Apicella CL, Marlowe FW, Fowler JH, et al. Social networks and cooperation in hunter-gatherers. Nature 2012; 481: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Buescher AV, Cidav Z, Knapp M, et al. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr 2014; 168: 721–728. [DOI] [PubMed] [Google Scholar]
- 185.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of Autism spectrum disorders — autism and developmental disabilities monitoring network, 14 Sites, United States, 2008. MMWR Surveillance Summaries 2012; 61: 1–19. [PubMed] [Google Scholar]
- 186.Riley J, Braithwaite I, Shirtcliffe P, et al. Randomized controlled trial of asthma risk with paracetamol use in infancy–a feasibility study. Clin Exp Allergy 2015; 45: 448–456. [DOI] [PubMed] [Google Scholar]
- 187.Prymula R, Siegrist CA, Chlibek R, et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet 2009; 374: 1339–1350. [DOI] [PubMed] [Google Scholar]
- 188.Doedée AM, Boland GJ, Pennings JL, et al. Effects of prophylactic and therapeutic paracetamol treatment during vaccination on hepatitis B antibody levels in adults: two open-label, randomized controlled trials. Plos One 2014; 9: e98175–e98175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McCullough HN. Acetaminophen and ibuprofen in the management of fever and mild to moderate pain in children. Paediatr Child Health 1998; 3: 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vernacchio L, Kelly JP, Kaufman DW, et al. Medication use among children <12 years of age in the United States: results from the Slone Survey. Pediatrics 2009; 124: 446–454. [DOI] [PubMed] [Google Scholar]
- 191.de Fays L, Van Malderen K, De Smet K, et al. Use of paracetamol during pregnancy and child neurological development. Dev Med Child Neurol 2015; 57: 718–724. [DOI] [PubMed] [Google Scholar]
- 192.Jensen MS, Rebordosa C, Thulstrup AM, et al. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology 2010; 21: 779–785. [DOI] [PubMed] [Google Scholar]
- 193.McPhillips HA, Stille CJ, Smith D, et al. Potential medication dosing errors in outpatient pediatrics. J Pediatr 2005; 147: 761–767. [DOI] [PubMed] [Google Scholar]
- 194.Neuspiel DR, Taylor MM. Reducing the risk of harm from medication errors in children. Health Serv Insights 2013; 6: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Li SF, Lacher B, Crain EF. Acetaminophen and ibuprofen dosing by parents. Pediatr Emerg Care 2000; 16: 394–397. [DOI] [PubMed] [Google Scholar]