Abstract
Objective
This meta-analysis aimed to investigate the efficacy and safety of pentoxifylline (PTF) plus angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) for proteinuria and kidney function in chronic kidney disease (CKD).
Methods
CENTRAL, EMBASE, Ovid-MEDLINE, PubMed, and CNKI were searched for relevant, randomized, controlled trials (RCTs). A meta-analysis was performed to review the effect of PTF plus ACEIs/ARBs vs. ACEIs/ARBs alone on proteinuria and kidney function in CKD.
Results
Eleven RCTs including 705 patients were retrieved. PTF plus ACEI/ARB treatment significantly decreased proteinuria in patients with CKD within 6 months (standard mean difference [SMD] −0.52; 95% CI −0.90 to 0.15; I2 = 68%) and significantly attenuated a decrease in estimated glomerular filtration rate (eGFR) in patients with stages 3–5 CKD after 6 months of treatment (standard mean difference [SMD] 0.30; confidence limit [Cl] 95% CI 0.06 to 0.54; I2 = 0%). PTF plus ACEIs/ARBs for 9 to 12 months significantly reduced albuminuria in patients with CKD (SMD−0.30, 95% CI −0.57 to 0.03; I2 = 0%) and alleviated the decline in eGFR in patients with stages 3–5 CKD (SMD 0.51; 95% CI 0.06 to 0.96; I2 = 61%).
Conclusion
The combination of an ACEI or ARB and PTF has a protective effect in reducing proteinuria by ameliorating the decline in eGFR in patients with stages 3–5 CKD.
Keywords: Pentoxifylline, ACEI/ARB, chronic kidney disease, meta-analysis
Introduction
Chronic kidney disease (CKD) is a worldwide problem. The global burden of disease 2013 (GBD 2013) study reported that CKD was the non-communicable cause of death that increased the most in the past 23 years.1 Additionally, CKD was also an increasing cause of years lived with disability.1 A total of 15% to 20% of patients with CKD may be at risk of a progressive loss of kidney function over time, and this develops into end-stage renal disease (ESRD).2 Therapies that effectively slow a decline in kidney function are limited to angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), alone and in combination. However, these agents do not completely delay progression of renal disease.3 Substitution of ACEIs by spironolactone, an aldosterone blocker, provides additional benefits in terms of a reduction in proteinuria in CKD, but there is also a reduction in glomerular filtration rate (GFR).4 Aliskiren, which was the first direct renin inhibitor, may also enhance renin–angiotensin–aldosterone system blockade and can be used in combination with an ACEI or ARB in patients with proteinuria. These concomitant therapies significantly decrease proteinuria, but do not affect a decline in kidney function.5 Additionally, these concomitant therapies must be carefully monitored for hyperkalemia and worsening of kidney function.5 The addition of other agents may enhance the effects of ACEIs and ARBs and further slow progression to ESRD.
The hallmark of CKD is excessive inflammation, which leads to interstitial tissue fibrosis. Patients with CKD show significantly higher tumour necrosis factor (TNF)-α, interleukin (IL)-6, and C-reactive protein levels in serum. Higher TNF-α and IL-6 levels are independently and significantly associated with a lower estimated glomerular filtration rate (eGFR) and higher albuminuria.6 However, a decline in kidney function is associated with IL-6 and monocyte chemoattractant protein (MCP-1) levels in middle-aged and older adults, which suggests that low-grade inflammation and aging reduce renal function.7 Limiting inflammation attenuates interstitial renal fibrosis and a decline in kidney function in an animal model of CKD.8 Therefore, inflammatory molecules and pathways are new potential targets for CKD treatment. Several antagonists of the receptors CCR2 and chemokine receptor (CCR)2/5 of the MCP-1 pathway (e.g., CCX 140-B, TLK-19705, RS102895, PF-04634817, and BMS-813160) have shown positive experimental results and some of them are being evaluated in clinical trials.9,10
Pentoxifylline (PTF) is a non-specific phosphodiesterase inhibitor that is clinically prescribed for patients with peripheral vascular disease. PTF has been investigated as a potential CKD treatment agent. PTF shows anti-inflammatory, anti-proliferative, and anti-fibrotic properties, which attenuate renal disease progression in animal models.11–13 Clinical trials evaluating PTF in patients with CKD used small sample sizes and reported conflicting conclusions. Tian et al.14 and Bolignano et al.15 conducted meta-analyses. Tian et al. assessed the effects of PTF in patients with diabetic kidney disease (DKD) without considering treatment time and CKD stages, and the conclusion was not consistent with a recent study.14 Bolignano et al. analysed the effect of PTF on anaemia rather than kidney function in patients with CKD. Therefore, we conducted a meta-analysis of appropriate, published, randomized, controlled trials (RCTs) to evaluate the efficacy and safety of PTF plus ACEI/ARB in patients with CKD, especially patients with stages 3–5 CKD.
Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).16 The information for the PRISMA checklist is shown in Table 1.
Table 1.
PRISMA 2009 checklist.
| Section/topic | Checklist item |
|---|---|
| TITLE | |
| Title | Identify the report as a systematic review, meta-analysis, or both. |
| ABSTRACT | |
| Structured summary | Provide a structured summary. |
| INTRODUCTION | |
| Rationale | Describe the rationale for the review. |
| Objectives | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). |
| METHODS | |
| Protocol and registration | Indicate if a review protocol exists, if and where it can be accessed and, if available, provide registration information including registration number. |
| Eligibility criteria | Specify study characteristics and report characteristics used as criteria for eligibility, giving rationale. |
| Information sources | Describe all information sources in the search and date last searched. |
| Search | Present full electronic search strategy for at least one database. |
| Study selection | State the process for selecting studies. |
| Data collection process | Describe method of data extraction from reports and any processes for obtaining and confirming data from investigators. |
| Data items | List and define all variables for which data were sought and any assumptions and simplifications made. |
| Risk of bias in individual studies | Describe methods used for assessing risk of bias of individual studies and how this information is to be used in any data synthesis. |
| Summary measures | State the principal summary measures. |
| Synthesis of results | Describe the methods of handling data and combining results of studies, if done, including measures of consistency for each meta-analysis. |
| Risk of bias across studies | Specify any assessment of risk of bias that may affect the cumulative evidence. |
| Additional analyses | Describe methods of additional analyses. |
| RESULTS | |
| Study selection | Give numbers of studies screened, assessed for eligibility, and included in the review. |
| Study characteristics | For each study, present characteristics for which data were extracted and provide the citations. |
| Risk of bias within studies | Present data on risk of bias of each study and, any outcome level assessment. |
| Results of individual studies | For all outcomes considered, present, for each study. |
| Synthesis of results | Present results of each meta-analysis done. |
| Risk of bias across studies | Present results of any assessment of risk of bias across studies. |
| Additional analysis | Give results of additional analyses. |
| DISCUSSION | |
| Summary of evidence | Summarize the main findings. |
| Limitations | Discuss limitations at study and outcome level and at review-level. |
| Conclusions | Provide a general interpretation of the results in the context of other evidence, and implications for future research. |
| FUNDING | |
| Funding | Describe sources of funding for the systematic review and other support. |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097
For more information, visit: www.prisma-statement.org.
Literature search
Ovid-MEDLINE, PubMed, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), and China National Knowledge Infrastructure (CNKI) databases were searched for articles without time restriction up to July 30, 2015. The search strategy is shown in Table 2. Two authors independently performed the search (Zhou-ping Wang and Li Zhang).
Table 2.
Search strategy.
| CENTRAL |
| 1 (chronic kidney disease or chronic renal disease or chronic kidney failure or chronic renal failure): ti, ab, kw. |
| 2 (CKD or CRD or CKF or CRF): ti, ab, kw. |
| 3 (end-stage renal* or end-stage kidney* or endstage renal* or endstage kidney*): ti, ab, kw. |
| 4 (ESRF or ESRF or ESRD or ESKD): ti, ab, kw. |
| 5 (predialysis or pre-dialysis): ti, ab, kw. |
| 6 (diabetic nephropathy): ti, ab, kw. |
| 7 (chronic or diabetic or diabetes) and (kidney* or renal or nephron* or nephritis* or glomerulo*): ti, ab, kw. |
| 8 non-diabetic renal disease. |
| 9 MeSH descriptor kidney failure, chronic explode all trees. |
| 10 MeSH descriptor diabetic nephropathies, this term only. |
| 11 (1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10). |
| 12 MeSH descriptor pentoxifylline, this term only. |
| 13 (oxpentifylline): ti, ab, kw. |
| 14 (torental): ti, ab, kw. |
| 15 (trental): ti, ab, kw. |
| 16 (agapurin): ti, ab, kw. |
| 17 (bl-191): ti, ab, kw. |
| 18 (pentoxifylline): ti, ab, kw. |
| 19 (12 or 13 or 14 or 15 or 16 or 17 or 18). |
| 20 (11 and 19). |
| Ovid-MEDLINE |
| 1 (chronic or diabetic or diabetes) and (kidney$ or renal$ or nephron$ or nephritis$ or glomerulo$) tw. |
| 2 (DKD or CKD or CRD or CKF or CRF) tw. |
| 3 (end-stage renal or end-stage kidney or endstage renal or endstage kidney) tw. |
| 4 (ESRD or ESKD or ESRF or ESKF) tw. |
| 5 (predialysis or pre-dialysis) tw. |
| 6 diabetic nephropathy/. |
| 7 non-diabetic nephropath$, tw. |
| 8 diabetic nephropathy$, tw. |
| 9 or/1–8. |
| 10 pentoxifylline/. |
| 11 oxpentifylline, tw. |
| 12 pentoxifylline, tw. |
| 13 trental, tw. |
| 14 torental, tw. |
| 15 BL-191, tw. |
| 16 agapurin, tw. |
| 17 or/10–16. |
| 18 and 9, 17. |
| EMBASE |
| 1 PENTOXIFYLLINE. |
| 2 pentoxifylline, tw. |
| 3 oxpentifylline, tw. |
| 4 trental, tw. |
| 5 torental, tw. |
| 6 BL-191, tw. |
| 7 agapurin, tw. |
| 8 or/1–7. |
| 9 (chronic or diabetic or diabetes) and (kidney$ or renal$ or nephron$ or nephritis$ or glomerulo$) tw. |
| 10 (DKD or CKD or CRD or CKF or CRF) tw. |
| 11 (end-stage renal or end-stage kidney or endstage renal or endstage kidney) tw. |
| 12 (ESRD or ESKD or ESRF or ESKF) tw. |
| 13 (predialysis or pre-dialysis) tw. |
| 14 diabetic nephropathy/. |
| 15 non-diabetic nephropath$, tw. |
| 16 diabetic nephropathy$, tw. |
| 17 or/9–16. |
| 18 and 8, 17. |
| PubMed |
| (chronic kidney disease or CKD or chronic renal failure or chronic renal insufficiency or CRF or end stage-kidney disease or ESKD or end stage-renal disease or ESRD or pre-dialysis or diabetic kidney disease or diabetic nephropathy or DKD or non-diabetic kidney disease) AND (pentoxifylline or oxipentifylline or trental). |
| CNKI |
| (chronic kidney disease or CKD or chronic renal failure or chronic renal insufficiency or CRF or end stage-kidney disease or ESKD or end stage-renal disease or ESRD or pre-dialysis or diabetic kidney disease or diabetic nephropathy or DKD or non-diabetic kidney disease) AND (pentoxifylline or oxipentifylline or trental). |
Abbreviations: PTF pentoxifylline, CKD chronic kidney disease, DKD diabetic kidney disease, CRD chronic renal disease, CKF chronic kidney failure, CRF chronic renal failure, ESKF end-stage kidney failure, ESRF end-stage renal failure, ESRD end-stage renal disease, ESKD end-stage kidney disease, ti title, ab abstract, kw key words, tw text words.
Study selection
Any RCT that provided information on the efficacy and safety of PTF plus ACEIs/ARBs vs. ACEIs/ARBs alone in patients with CKD was included. The presence of CKD was defined according to the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative guidelines17 using a reduced GFR <90 mL/min/1.73 m2 and/or the persistence of urinary abnormalities, such as albuminuria, proteinuria, or haematuria, for at least 3 months. Studies were included in the meta-analysis if the following criteria were met: RCTs were designed to compare the benefits and harms of PTF plus ACEIs/ARBs with ACEIs/ARBs alone in patients with CKD; and RCTs reported at least one of the outcomes of proteinuria, albuminuria, serum creatinine, creatinine clearance, eGFR, ESRD, and all causes of mortality. Any possible comparator, including placebo or no therapy, was suitable for controlled studies. The study did not restrict the dose or duration of PTF treatment. Studies were included without limitations of follow-up duration. Studies were excluded for any of the following reasons: 1) inclusion of patients with CKD on renal replacement therapy (e.g., haemodialysis or peritoneal dialysis) or children (age <18 years); 2) lack of long-term data on the outcomes of interest (see above); 3) assessment of the efficiency of PTF alone; and 4) reviews, editorials, case reports, and letters.
Data extraction
Two authors (Zhou-ping Wang and Li Zhang) independently screened titles and abstracts, and studies that did not correlate with the topic were discarded. Two authors (Zhou-ping Wang and Li Zhang) independently reviewed the retrieved abstracts and full text of these studies to determine inclusion or exclusion based on the criteria. A third author (Fei-yan Chen) solved possible discrepancies on judgments of studies. Two authors completed data extraction and analyses (Zhou-ping Wang and Li Zhang), which were independently verified by another author (Fei-yan Chen).
Data analysis and synthesis
A pooled meta-analysis was performed if data on the specific outcome were provided by more than one study. Data on outcomes reported by a single study were reported narratively to maximize the information. The standardized mean difference (SMD) was used to assess the effects of treatment on continuous variables. Data that were reported as medians and ranges were converted to mean and standard deviation (SD) using the formula applied by Hozo.18 The mean ≈ median was estimated. The upper and lower ends of ranges were converted to SD using the following formula: estimated SD ≈ range/4 (15 <n <70) or range/6 (n > 70) (range = the upper end of the range − the lower end of the range). The 25th and 75th percentiles and 5th and 95th percentiles were transformed into SDs using the following formulas: SD ≈norm interquartile range (IQR) = (P75−P25) × 0.7413 (IQR: interquartile range, P75: 75th percentile, P25: 25th percentile) and SD ≈ (P95−P5) / (2 × k) (P5: 5th percentile, P95: 95th percentile, k: coefficient of variation, which is 1.645). Heterogeneity was tested using the chi-square test on N-1 degrees of freedom, with an alpha of 0.1 indicating statistical significance and the Cochrane I2. Possible sources of heterogeneity were explored using sensitivity, and if possible, subgroup analyses were performed.19 Fixed-effect analysis was adopted when I2 ≤ 50%. Otherwise, the random-effects model was used. Statistical significance was set at a two-tailed level of 0.05. Adverse effects were described. Statistical analyses were performed using Review Manager (RevMan; Version 5.1).
Quality and risk of bias assessment
The quality of RCTs was assessed independently according to recommendations from the Cochrane Collaboration.20 Quality items that were assessed were the presence of potential selection bias, performance bias, detection bias, attrition bias, reporting bias, and other possible bias.
Results
Search results
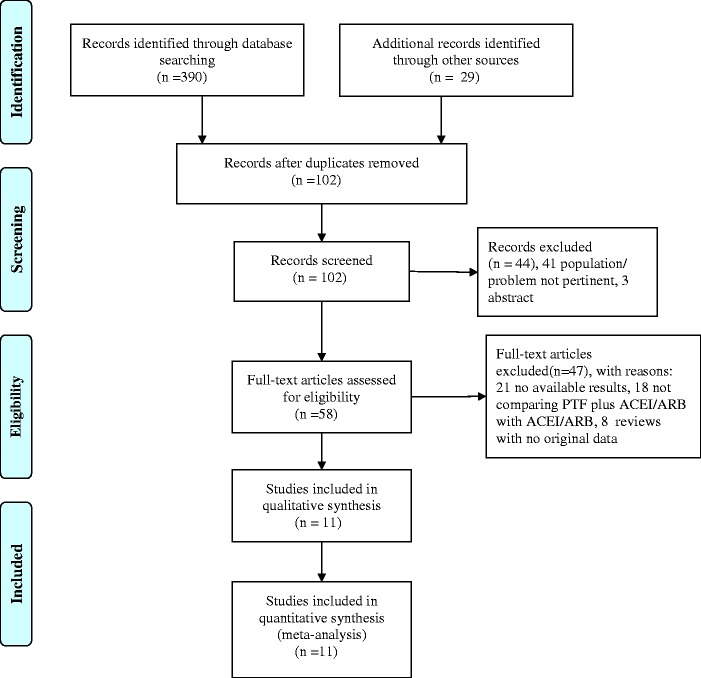
Figure 1 shows a flow diagram of the selection process. A total of 419 potentially relevant references were initially retrieved. A total of 361 citations were excluded following title and abstract screening because of search overlap or inclusion of the wrong population/intervention. Forty-seven of the 58 studies that were selected for full text examination were excluded because of the following factors: no report of outcomes pertinent to the topic (n = 21), no comparison of PTF plus ACEIs/ARBs with ACEIs/ARBs alone (n = 18), and reviews with no original data to be extracted (n = 8). A total of 11 articles were reviewed in detail and included in the meta-analysis. Table 1 shows the main characteristics of these studies.
Figure 1.
Flowchart of the literature search.
Study characteristics
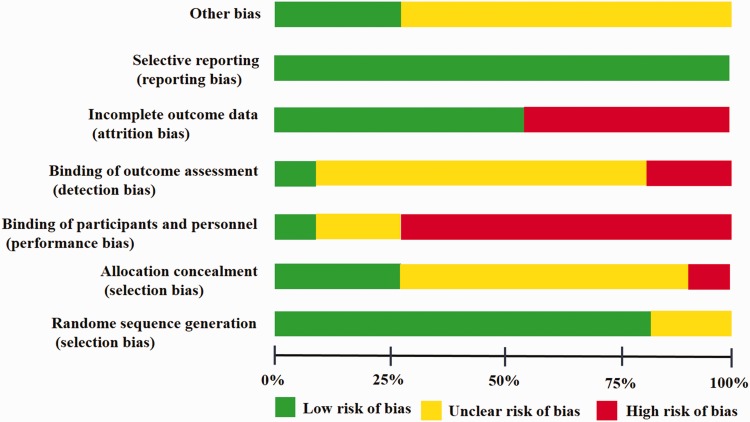
All studies were performed at a single centre. Nine21–29 of the 11 RCTs21–31 used a computer-generated random number table to divide the two groups, and two studies30,31 did not report concrete randomization methods. Three studies24,25,29 were placebo controlled. Only two studies22,29 adopted binding. Figure 2 shows the quality of the trials.
Figure 2.
Risk of bias graph according to recommendations from the Cochrane collaboration.
The final population that was analysed in this review was 705 cases, but the range was variable across studies and spanned from 2224 to 169 patients.21 Eight studies21–23,25,27,28,30,31 enrolled patients with diabetic nephropathy who had routine treatment. Three studies21,26,29 analysed patients with stages 3–5 CKD, and one of the three studies analysed patients with non-diabetic nephropathy.21 The duration of therapy was within 6 months for six trials,22,23,25,27,28,31 and 9 to 12 months for five trials.21,24,26,29,30 Table 3 shows the characteristics of the included RCTs.
Table 3.
Characteristics of the included randomized, controlled trials.
| Study | Design | Population | Size | Intervention | Duration | Outcome |
|---|---|---|---|---|---|---|
| Navarro, 2015 | Single-centre, parallel, open-labelled RCT | Type 2 DM, DN, stages 3–4 CKD | 87 T | PTF (600 mg, twice a day) + ACEIs/ARBs | 24 months | eGFR, UAE, U-TNF-α, adverse effect |
| 82 C | ACEIs + ARBs | |||||
| Ghorbani, 2012 | Single-centre, double-blind, parallel RCT | Type 2 DM, duration 8.9/8.4 years Proteinuria >150 mg/d | 50 T | PTF (400 mg/d)+losartan, 50 mg+ enalapril 15 mg/d | 6 months | Scr, proteinuria, Ccr, Bp, HbA1c |
| 50 C | Losartan, 50 mg+ enalapril 15 mg/d | |||||
| Lv, 2012 | Single-centre, parallel RCT | Type 2 DM, DR, DN, proteinuria >0.5 g/d, Scr < 270 µmol/l | 16 T | PTF 400 mg/d + valsartan 80 mg/d | 21 days | Scr, BUN, Ccr, beta-MG |
| 16 C | Valsartan 80 mg/d | |||||
| Oliaei, 2011 | Single-centre, placebo RCT | Type 2 DM, DN, proteinuria >0.5 g/d, Ccr >60 ml/min (3 months) | 28 T | PTF 400 mg 3 times/d + ACEIs/ARBs | 3 months | Proteinuria, Ccr |
| 28 C | Placebo + ACEIs/ARBs | |||||
| Roozbeh, 2010 | Single-centre, parallel RCT | Type 2 DM, DN, proteinuria >0.5 g/d, Scr normal | 37 T | PTF 400 mg 3 times/d + captopril 75 mg/d | 6 months | Proteinuria, Scr, Bp, Ccr, HBAc1 |
| 37 C | Captopril 75 mg/d | |||||
| Renke, 2010 | Single-centre, placebo-controlled RCT | CKD, NDRD, eGFR >30 ml/min, proteinuria >0.3 g/d | 22 T | PTF 1200 mg once daily + ACEIs/ARBs | 2 months | Proteinuria, Scr, NAG, hCRP |
| 22 C | Placebo + ACEIs/ARBs | |||||
| Perkine, 2009 | Single-centre, double-blind, placebo RCT | CKD stages 3–4, proteinuria >1 g/d (eGFR 20–40/ml/min) or proteinuria >3 g/d (eGFR 20–60 ml/min) | 28 T | PTF 400 mg twice daily + ACEIs/ARBs | 12 months | Scr, eGFR, BUN, Htc |
| 28 C | Placebo + ACEIs/ARBs | 12 months | ||||
| Lin, 2008 | Single-centre, open-labelled RCT | CKD 3–5 (eGFR 10–60 ml/min/1.73 m2, not on dialysis therapy) | 29 T | PTF 400 mg twice/once daily + losartan 100 mg/d | 12 months | Proteinuria, eGFR, urine TNF-α |
| 27 C | Losartan 100 mg/d | |||||
| Navarro, 2005 | Single-centre, parallel RCT | Type 2 DM, DN, proteinuria >0.3 g/d, eGFR >90 ml/min/1.73 m2 | 31 T | PTF 600 mg twice daily + ACEIs/ARBs | 4 months | Scr, albuminuria, Bp, adverse effect |
| 30 C | ACEIs/ARBs | |||||
| Navarro, 2003 | Single-centre, parallel RCT | Type 2 DM, hypertension (treated with ACEIs/ARBs >4 months), DN, proteinuria < 3 g/d, Scr < 1.5 mg/dl | 45 T | PTF 1200 mg/d + ACEIs/ARBs | 4 months | Scr, proteinuria, Bp |
| 45 C | ACEIs/ARBs | |||||
| Harmankaya, 2003 | Single-centre, parallel RCT | Type 2 DM, hypertension stage 3, DKD, UAE 0.03–0.3 g/d | 50 T | PTF 600 mg/day + isinopril 10 mg/day | 9 months | Scr, albuminuria, Bp, adverse effect |
| 50 C | Isinopril 10 mg/day |
PTF pentoxifylline, ARB angiotensin receptor blocker, ACEI angiotensin-converting enzyme inhibitor, DM diabetes mellitus, UAE urinary albumin excretion, Scr serum creatinine, Ccr creatinine clearance, eGFR estimated glomerular filtration rate, Bp blood pressure, CKD, chronic kidney disease, DKD diabetic kidney disease, hCRP human C-reactive protein, Hct haematocrit. TNF tumor necrosis factor, BUN blood urea nitrogen, DN diabetic nephropathy, C control, T trial, RCT randomized controlled trial, HbA1C hemoglobin, A1C NAG N-Acetyl-beta-D-Glucosaminidase.
Quantitative data synthesis
PTF + ACEI/ARB vs. ACEI/ARB treatment for CKD within 6 months
Proteinuria and albuminuria
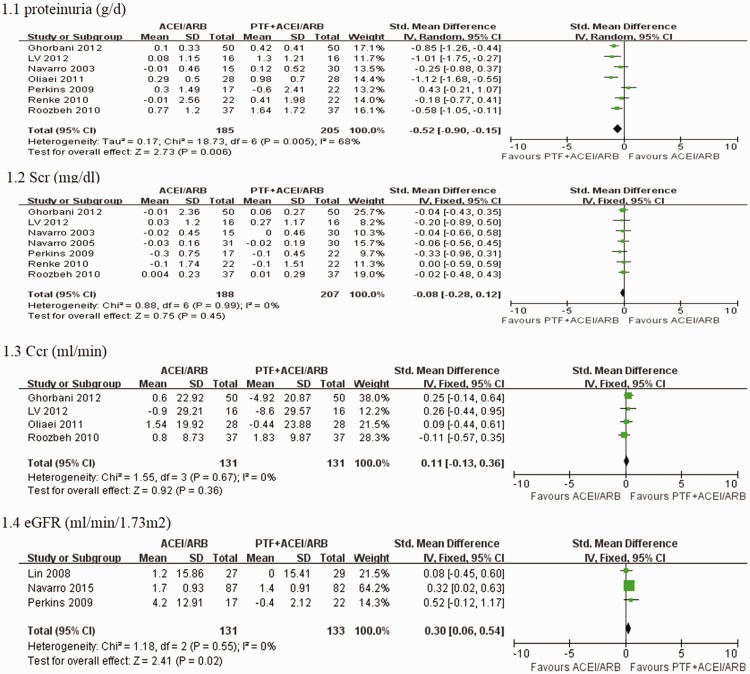
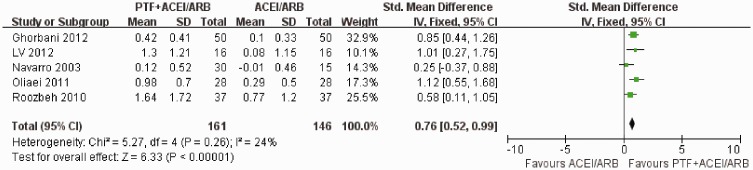
Seven trials reported the primary outcome of proteinuria. Meta-analysis using the random-effects model suggested that PTF plus ACEI/ARB treatment significantly decreased proteinuria in patients with CKD (SMD −0.52; 95% CI −0.90 to −0.15; p = 0.006) (Figure 3). The test for heterogeneity was high (I2 = 68%) (Figure 3). This may have occurred because one study included patients with stages 3–4 CKD with severe proteinuria (>1.0 g/d),32 and the others enrolled patients in the early stages of CKD with mild proteinuria (>0.5 g/d). We performed subgroup analyses, and only included studies of DKD. Meta-analysis using the fixed-effects model suggested that proteinuria was significantly decreased in the PTF plus ACEI/ARB group in patients with DKD (SMD −0.76; 95% CI −0.52 to−0.99; p <0.00001). The test for heterogeneity was low (I2 = 24%) (Figure 4). Two studies reported the effect of PTF and ACEIs/ARBs on albuminuria in patients with DKD.21,23 One of the studies enrolled patients with normal kidney function (eGFR > 90) who were followed up for 4 months.23 The other study enrolled patients with stages 3–4 CKD who were followed up for 6 months.21 In the study of patients with normal kidney function, urinary albuminuria excretion (UAE) was significantly decreased in the treatment group (900 mg/d [466 to 1542 mg/d] at baseline to 791 mg/d [309 to 1400 mg/d]) by the end of the study.23 No significant changes in UAE were observed in the control group.23 UAE in the study of patients with stages 3–4 CKD showed a mean percentage increase of 1.4% (95% CI −0.8% to 3.8%) in the control group versus a mean percentage reduction of 10.6% (95% CI 8.2% to 13%) in the PTF group after treatment for 6 months.21
Figure 3.
Effects of PTF + ACEI/ARB vs. ACEI/ARB treatment within 6 months on proteinuria, serum creatinine levels, creatinine clearance, and eGFR in patients with CKD.
Figure 4.
Effects of PTF + ACEI/ARB vs. ACEI/ARB treatment within 6 months on proteinuria in patients with CKD.
Kidney function
Seven trials, six of which enrolled patients with early-stage CKD, reported serum creatinine levels. Pooled analysis demonstrated no significant effect of PTF on changes in serum creatinine levels (SMD −0.08; 95% CI −0.28 to 0.12; p = 0.45) (Figure 3). The test for heterogeneity was low (I2 = 0%) (Figure 3). Four trials22,25,28,31 that enrolled patients with early CKD reported creatinine clearance, and PTF plus ACEI/ARB treatment did not significantly change creatinine clearance compared with ACEI/ARB treatment alone (SMD 0.11; 95% CI −1.3 to 0.36; p = 0.36; I2 = 0%) (Figure 3). Three trials21,26,29 enrolled patients in stages 3–5 CKD and reported changes in eGFR. PTF plus ACEI/ARB treatment significantly decreased the decline in eGFR compared with ACEI/ARB treatment (SMD 0.30; 95% CI 0.06 to 0.54; p = 0.02; I2 = 0%) (Figure 3).
PTF + ACEI/ARB vs. ACEI/ARB treatment for 9 to 12 months in patients with CKD
Albuminuria and proteinuria
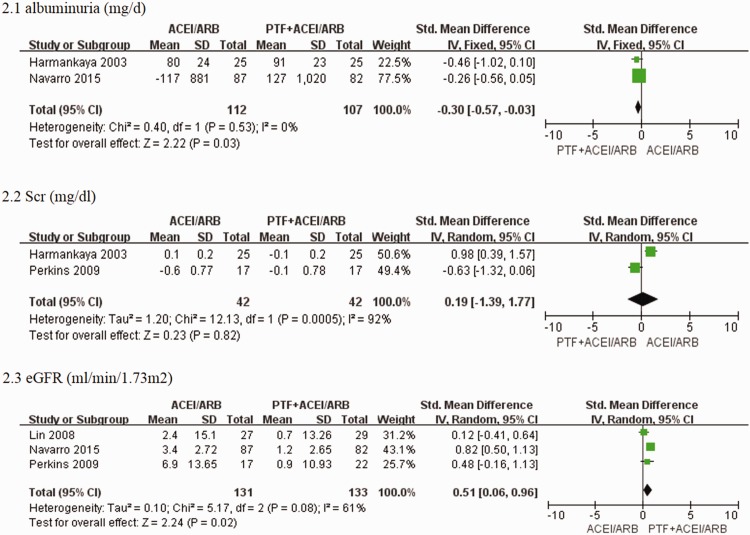
Two trials21,30 assessed the effect of PTF plus ACEIs/ARBs on albuminuria. The combination of PTF and ACEIs/ARBs significantly reduced albuminuria (SMD −0.30, 95% CI −0.57 to 0.03; p = 0.03; I2 = 0%) (Figure 5). Only one study29 assessed the effect of PTF plus AECIs/ARBs on proteinuria in patients with stages 3–4 CKD with severe proteinuria (>1 g/d). This study showed that proteinuria was not different between the PTF and placebo groups at 1 year.
Figure 5.
Effects of PTF plus ACEI/ARB vs. ACEI/ARB treatment for 9 to 12 months on albuminuria, serum creatinine levels, and eGFR in patients with CKD.
Kidney function
Only two studies29,30 assessed the effect of PTF plus AECIs/ARBs on serum creatinine levels. Meta-analysis with high heterogeneity showed that serum creatinine levels were not different between the PTF plus ACEI/ARB and ACEI/ARB groups at 1 year (SMD 0.19; 95% CI −1.39 to 1.77; p = 0.82; I2 = 92%) (Figure 5). The high heterogeneity may have been caused by the different types of patients enrolled in the trials. Harmankaya et al.30 recruited patients with stage 3 diabetic kidney disease and normal kidney function, and Perkins et al.29 recruited patients with stages 3–4 CKD. Three trials21,26,29 enrolled patients in stages 3–5 CKD and reported changes in eGFR. PTF plus ACEI/ARB treatment significantly reduced the decline in eGFR compared with ACEI/ARB treatment alone (SMD 0.51; 95% CI 0.06 to 0.96; p = 0.02; I2 = 61%) (Figure 5). Navarro et al.21 evaluated the efficacy of PTF plus ACEI/ARB treatment for 2 years on eGFR in patients with stages 3–5 CKD. The eGFR decreased by a mean ± SEM of 2.1 ± 0.4 ml/min per 1.73 m2 in patients treated with PTF versus 6.5 ± 0.4 ml/min per 1.73 m2 in the control group. This resulted in a significant mean difference of 4.3 ml/min per 1.73 m2 (95% CI 3.1 to 5.5 ml/min per 1.73 m2; p <0.001) in favour of PTF.
Adverse effects
Six studies21,23,25,26,29,30 that included 218 participants reported adverse events, and the proportion was 39/218 (17.9%). The most frequent adverse effects in patients (n = 30) were gastrointestinal symptoms (e.g., abdominal discomfort, flatus, dyspepsia, nausea, and vomiting). Seven patients suffered from dizziness, one patient complained of mild tremors, and one patient reported suture bleeding. Seven patients withdrew from the trials as a result of side effects of PTF.
Discussion
This meta-analysis included a total of 11 studies with 705 patients and evaluated the efficacy and safety of oral PTF plus ACEIs/ARBs on CKD. The addition of PTF significantly reduced urinary protein excretion compared with ACEI/ARB alone. Pooled analyses did not show any significant effects of PTF plus ACEIs/ARBs on changes in serum creatinine levels or creatinine clearance in patients with CKD, most of whom were in the early stages of CKD. However, PTF plus ACEI/ARB treatment significantly attenuated the decline in eGFR in patients with stages 3–5 CKD. The lack of benefit for serum creatinine and creatinine clearance may be related to the short observation time and relative insensitivity of serum creatinine/creatinine clearance to GFR. This is because of secretion of creatinine by renal tubules in the early stage of CKD. Several clinical trials with a longer follow-up showed that PTF significantly slowed the reduction in eGFR in patients with stages 3–4 CKD.21,33 More large RCTs are required to provide concrete evidence of the add-on effect of PTF for different stages of CKD. Adverse transient digestive symptoms and dizziness were the most common adverse reactions in patients with CKD. The safety of PTF was verified in patients with peripheral vascular disease for longer than 30 years.
None of the RCTs evaluated the effect of PTF + ACEIs/ARBs on ESRD (requiring dialysis), cardiovascular mortality and overall death. Chen et al.34 conducted a retrospective study of 661 patients with an eGFR <45 mL/minute/1.73 m2. A total of 419 patients used PTF and ACEIs or ARBs, and 242 patients used ACEIs or ARBs only.34 The baseline characteristics of the patients were not different. The median follow-up period was 2.25 years.34 Participants using PTF had a better renal outcome compared with patients without PTF use.34 However, PTF treatment did not affect overall mortality and cardiovascular death.34 Another large retrospective study35 analysed 7366 PTF users and 7366 propensity score-matched non-users. PTF reduced the risks of ESRD, but not mortality. Kuo et al.36 conducted a prospective cohort study to evaluate the effect of PTF plus ACEIs vs ACEIs/ARBs alone in patients with CKD stage 5 who had not yet received dialysis. They reported that after propensity score-matching, use of PTF was associated with a lower-term dialysis or death in ACEI/ARB users or ARB users.36
CKD caused by primary glomerular diseases or secondary kidney diseases (e.g., diabetes mellitus) is an inflammatory state. Evidence suggests that proinflammatory cytokines play a pathogenic role in increasing glomerular permeability and renal interstitial injury.37 The antiproteinuric and renoprotective effects of PTF in CKD may arise from its ability to inhibit production of pro-inflammatory cytokines, such as MCP-1, IL-1, IL-6, and TNF-α.33,38 A change in urinary TNF-α levels was correlated directly with changes in UAE and inversely correlated with changes in eGFR in the PREDIAN trial and other clinical studies.21,26 Inflammation also plays a pivotal role in the genesis and worsening of anaemic states secondary to CKD. The effect of PTF on erythropoiesis-stimulating agent-hyporesponsive anaemia in patients with CKD was assessed in several studies.32,39,40 Unfortunately, there was no concrete conclusion in these studies. A large, randomized, placebo-controlled trial (HERO) demonstrated that PTF did not significantly modify ESA hyporesponsiveness, but increased haemoglobin concentrations.39
Our review has some strengths and limitations that should be mentioned. Strengths of our meta-analysis include the inclusion of RCTs, division of the treatment period, and performance of subgroup analyses. A previous analysis by Tian14 only included patients with diabetic nephropathy, did not consider the effect of treatment time and CKD stages, and failed to collect data on eGFR. The trials in our analysis enrolled patients with CKD and included 28.6%–61.5% patients with DKD. However, 37.3–68.6% of patients with diabetes mellitus who had undergone renal biopsy were diagnosed with non-diabetic renal disease,41,42 and patients who were diagnosed with diabetic nephropathy without renal biopsy had a risk of misdiagnosis. Therefore, all of the trials that assessed CKD or only diabetic nephropathy without renal biopsy according to the definition of CKD were analysed in our meta-analysis. There are some potential limitations of our study. First, some included trials did not adopt blinding and placebos, which might have favoured the treatment group. Second, the included studies primarily focussed on albuminuria, proteinuria, serum creatinine, and creatinine clearance. These factors acted as surrogate endpoints, not hard outcomes, such as the incidence of ESRD, cardiovascular events, and mortality. Lastly, subgroup analyses could not be conducted to compare the effect of doses and baseline proteinuria levels on each outcome of PTF treatment because of the limited number of studies.
Conclusions
The combination of an ACEI or ARB and PTF may lead to a greater reduction in proteinuria in patients with CKD and attenuate the decline in eGFR in patients with stages 3–5 CKD. Further large, multicentre, high-quality studies of long duration are advocated to confirm whether the combination of ACEIs/ARBs and PTF treatment reduces hard endpoints, such as ESRD and death.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This analysis was supported by a grant from the National Natural Science Foundation of China (81100233).
References
- 1.Carney EF. Epidemiology: global burden of disease study 2013 reports that disability caused by CKD is increasing worldwide. Nat Rev Nephrol 2015; 11: 446–446. [DOI] [PubMed] [Google Scholar]
- 2.Indridason OS, Thorsteinsdóttir I, Pálsson R. Advances in detection, evaluation and management of chronic kidney disease. Laeknabladid 2007; 93: 201–207. [in Icelandic, English Abstract]. [PubMed] [Google Scholar]
- 3.Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351: 1952–1961. [DOI] [PubMed] [Google Scholar]
- 4.Quiroga B, Arroyo D, de Arriba G. Present and future in the treatment of diabetic kidney disease. J Diabetes Res 2015; 2015: 801348–801348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lizakowski S, Tylicki L, Rutkowski B. Direct renin inhibition–a promising strategy for renal protection? Med Sci Monit 2013; 19: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BT, Ahmed FA, Hamm LL, et al. Association of C-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC nephrol 2015; 16: 77–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costello-White R, Ryff CD, Coe CL. Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age (Dordr) 2015; 37: 9808–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchal PO, Kavvadas P, Abed A, et al. Reduced NOV/CCN3 expression limits inflammation and interstitial renal fibrosis after obstructive nephropathy in mice. PloS One 2015; 10: e0137876–e0137876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naidoo S, Meyers AM. Drugs and the kidney. S Afr Med J 2015; 105: 2683–2683. [DOI] [PubMed] [Google Scholar]
- 10.Machowska A, Carrero JJ, Lindholm B, et al. Therapeutics targeting persistent inflammation in chronic kidney disease. Transl Res 2016; 167: 204–213. [DOI] [PubMed] [Google Scholar]
- 11.Lin SL, Chen YM, Chien CT, et al. Pentoxifylline attenuated the renal disease progression in rats with remnant kidney. J Am Soc Nephrol 2002; 13: 2916–2929. [DOI] [PubMed] [Google Scholar]
- 12.Yagmurlu A, Boleken ME, Ertoy D, et al. Preventive effect of pentoxifylline on renal scarring in rat model of pyelonephritis. Urology 2003; 61: 1037–1041. [DOI] [PubMed] [Google Scholar]
- 13.Soni HM, Patel PP, Patel S, et al. Effects of combination of aliskiren and pentoxyfylline on renal function in the rat remnant kidney model of chronic renal failure. Indian J Pharmacol 2015; 47: 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian ML, Shen Y, Sun ZL, et al. Efficacy and safety of combining pentoxifylline with angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in diabetic nephropathy: a meta-analysis. Int Urol Nephrol 2015; 47: 815–822. [DOI] [PubMed] [Google Scholar]
- 15.Bolignano D, D’Arrigo G, Pisano A, et al. Pentoxifylline for anemia in chronic kidney disease: a systematic review and meta-analysis. PloS One 2015; 10: e0134104–e0134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 17.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 18.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J Am Soc Nephrol 2015; 26: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghorbani A, Omidvar B, Beladi-Mousavi SS, et al. The effect of pentoxifylline on reduction of proteinuria among patients with type 2 diabetes under blockade of angiotensin system: a double blind and randomized clinical trial. Nefrologia 2012; 32: 790–796. [DOI] [PubMed] [Google Scholar]
- 23.Navarro JF, Mora C, Muros M, et al. Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: a short-term, randomized, controlled trial. J Am Soc Nephrol 2005; 16: 2119–2126. [DOI] [PubMed] [Google Scholar]
- 24.Renke M, Tylicki L, Rutkowski P, et al. Effect of pentoxifylline on proteinuria, markers of tubular injury and oxidative stress in non-diabetic patients with chronic kidney disease - placebo controlled, randomized, cross-over study. Acta Biochim Pol 2010; 57: 119–123. [PubMed] [Google Scholar]
- 25.Oliaei F, Hushmand S, Khafri S, et al. Efficacy of pentoxifylline for reduction of proteinuria in type II diabetic patients. Caspian J Intern Med 2011; 2: 309–313. [PMC free article] [PubMed] [Google Scholar]
- 26.Lin SL, Chen YM, Chiang WC, et al. Effect of pentoxifylline in addition to losartan on proteinuria and GFR in CKD: a 12-month randomized trial. Am J Kidney Dis 2008; 52: 464–474. [DOI] [PubMed] [Google Scholar]
- 27.Navarro JF, Mora C, Muros M, et al. Effects of pentoxifylline administration on urinary N-acetyl-beta-glucosaminidase excretion in type 2 diabetic patients: a short-term, prospective, randomized study. Am J Kidney Dis 2003; 42: 264–270. [DOI] [PubMed] [Google Scholar]
- 28.Roozbeh J, Banihashemi MA, Ghezlou M, et al. Captopril and combination therapy of captopril and pentoxifylline in reducing proteinuria in diabetic nephropathy. Ren Fail 2010; 32: 172–178. [DOI] [PubMed] [Google Scholar]
- 29.Perkins RM, Aboudara MC, Uy AL, et al. Effect of pentoxifylline on GFR decline in CKD: a pilot, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis 2009; 53: 606–616. [DOI] [PubMed] [Google Scholar]
- 30.Harmankaya O, Seber S, Yilmaz M. Combination of pentoxifylline with angiotensin converting enzyme inhibitors produces an additional reduction in microalbuminuria in hypertensive type 2 diabetic patients. Ren Fail 2003; 25: 465–470. [DOI] [PubMed] [Google Scholar]
- 31.LV JL, LV LQ, Shao Y, et al. Valsartan combined with pentoxifylline in treatment of diabetic retinopathy and diabetic nephropathy. Rec Adv Ophthalmol 2012; 32: 945–948.
- 32.Ferrari P, Mallon D, Trinder D, et al. Pentoxifylline improves haemoglobin and interleukin-6 levels in chronic kidney disease. Nephrology (Carlton) 2010; 15: 344–349. [DOI] [PubMed] [Google Scholar]
- 33.Goicoechea M, García de Vinuesa S, Quiroga B, et al. Effects of pentoxifylline on inflammatory parameters in chronic kidney disease patients: a randomized trial. J Nephrol 2012; 25: 969–975. [DOI] [PubMed] [Google Scholar]
- 34.Chen PM, Lai TS, Chen PY, et al. Renoprotective effect of combining pentoxifylline with angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker in advanced chronic kidney disease. J Formos Med Assoc 2014; 113: 219–226. [DOI] [PubMed] [Google Scholar]
- 35.Wu PC, Wu CJ, Lin CJ, et al. Pentoxifylline decreases dialysis risk in patients with advanced chronic kidney disease. Clin Pharmacol Ther 2015; 98: 442–449. [DOI] [PubMed] [Google Scholar]
- 36.Kuo KL, Hung SC, Liu JS, et al. Add-on protective effect of pentoxifylline in advanced chronic kidney disease treated with renin-angiotensin-aldosterone system blockade - a nationwide database analysis. Sci Rep 2015; 5: 17150–17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambers Heerspink HJ, Gansevoort RT. Albuminuria is an appropriate therapeutic target in patients with CKD: the pro view. Clin J Am Soc Nephrol 2015; 10: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro JF, Milena FJ, Mora C, et al. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol 2006; 26: 562–570. [DOI] [PubMed] [Google Scholar]
- 39.Johnson DW, Pascoe EM, Badve SV, et al. A randomized, placebo-controlled trial of pentoxifylline on erythropoiesis-stimulating agent hyporesponsiveness in anemic patients with CKD: the handling erythropoietin resistance with oxpentifylline (HERO) trial. Am J Kidney Dis 2015; 65: 49–57. [DOI] [PubMed] [Google Scholar]
- 40.Mohammadpour AH, Nazemian F, Khaiat MH, et al. Evaluation of the effect of pentoxifylline on erythropoietin-resistant anemia in hemodialysis patients. Saudi J Kidney Dis Transpl 2014; 25: 73–78. [DOI] [PubMed] [Google Scholar]
- 41.Chong YB, Keng TC, Tan LP, et al. Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: a single centre review. Ren Fail 2012; 34: 323–328. [DOI] [PubMed] [Google Scholar]
- 42.Pallayova M, Mohammed A, Langman G, et al. Predicting non-diabetic renal disease in type 2 diabetic adults: the value of glycated hemoglobin. J Diabetes Complications 2015; 29: 718–723. [DOI] [PubMed] [Google Scholar]