Abstract
Objective
To discuss the relevance of heparanase and syndecan-1 and regulation of the heparanase-syndecan1 axis in the invasiveness of gallbladder carcinoma cells.
Methods
1. Generation of a gallbladder cancer cell line overexpressing a heparanase (GBD-SD) transgene. 2. Western blot analysis of syndecan-1 levels of GBD-SD and control gallbladder carcinoma (GBC-SD) cells. 3. RT-PCR analysis of syndecan-1 mRNA levels of GBD-SD and GBC-SD. 4. Evaluation of invasion and migration of GBD-SD and GBC-SD cells.
Results
1. Heparanase expression in GBD-SD cells was significantly increased. 2. The syndecan-1 mRNA level of GBD-SD cells was significantly lower compared with that of GBC-SD cells. 3. The syndecan-1 DNA copy number in GBD-SD cells was significantly lower compared with that of GBC-SD. 4. The invasiveness and migration of GBD-SD cells were significantly higher compared with GBC-SD cells.
Conclusions
1. The expression of heparanase negatively correlated with that of syndecan-1 in a gallbladder carcinoma cell line. 2. The expression of heparanase and syndecan-1 in gallbladder carcinomas negatively correlated, similar to other tumours. 3. The heparanase/syndecan1 axis in gallbladder carcinoma plays an important role in the invasion and metastasis, thus providing a new therapeutic target. 4. Further research is required to identify the detailed mechanisms.
Keywords: Gallbladder carcinoma, heparanase, metastasis, syndecan-1, RT-PCR; western blot
Abbreviations
HS: Heparan sulphate
VEGF: Vascular endothelial cell growth factor
RT-PCR: Reverse transcriptase-polymerase chain reaction
ECM: Extracellular matrix
SDS-PAGE: Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
Introduction
With a highly malignant phenotype and poor prognosis, gallbladder carcinoma has increased in incidence in recent years and is a serious hazard to human health. Heparanase is a vital enzyme and a therapeutic target. After the year 2000, research on heparanase has drawn worldwide attention, and heparanase is increasingly implicated as a key contributor to the invasiveness and metastasis of carcinomas.1
Heparanase is an endo-β-D-glucuronidase that specifically degrades heparan sulfate (HS), a component of the extracellular matrix (ECM). Heparanase catalyses the cleavage of the β (1,4)-glycosidic bond between glucuronic acid and a glucosamine residue1 to release numerous HS-linked molecules such as growth factors, cytokines, and enzymes, which are involved in inflammation, wound healing, and tumour invasion. Numerous studies2 indicate that heparanase mRNA is overexpressed in human tumours. A prometastatic and proangiogenic role for heparanase is established, high levels of heparanase correlate with lymph node and distant metastasis and shorter survival of patients with cancer.3 Heparanase may serve as a promising therapeutic target because of its unique activity.
The syndecan family includes four transmembrane heparin sulfate proteoglycans, which are mainly located on the cell surface.4,5 The four syndecans comprise a core protein surrounded by different numbers of glycosaminoglycan (GAG) chains. Syndecans function through these GAG chains, and the core protein’s domains affect syndecan function.6,7 Among the members of the syndecan family, syndecan-1 is the most important and resides on the surface of human epithelial cells, mesenchymal cells, and immature and mature lymphoid cells.4 Syndecan-1 contributes to diverse biological processes associated with cell differentiation and growth,8 adhesion,6 spreading, migration, infiltration, angiogenesis,7,9 and cytoskeletal organization.10,11
Elevation of heparanase levels in myeloma cells reduces the amount of syndecan-1 in the nucleus. Syndecan-1 is linked to heparan sulfate chains, and exogenous heparan sulfate inhibits the activity of histone acetyltransferases in vitro. Therefore, the decrease in the levels of nuclear syndecan-1 may be caused by high levels of heparanase. The relationship between the localization of heparanase and syndecan-1 has emerged as an important regulatory element in myelomas, intestinal carcinomas, and other cancers. Heparanase reduces syndecan-1 protein levels in myelomas, intestinal carcinomas, colorectal carcinomas, oesophageal carcinomas, renal carcinomas, and breast cancer cells; and decreased levels of syndecan-1 in the tumour microenvironment drives tumour angiogenesis, metastasis, and growth.9,13–17 However, whether this occurs in gallbladder carcinoma cells is unclear, and few published studies address this question.
Therefore, we hypothesized that heparanase may play a role in the down-regulation of syndecan-1 protein in gallbladder carcinoma cells. The aim of this study was to investigate whether heparanase decreases syndecan-1 protein levels in a gallbladder carcinoma cell line and if the regulation of the heparanase-syndecan-1 axis affects the invasiveness of gallbladder carcinoma cells.
Materials and methods
Materials
The human gallbladder carcinoma cell line GBC-SD was from the Cell Bank National Academy of Science of China (Shanghai, China). Molecular size markers, dNTPs, and PCR primers were from Shanghai Shenggong Company (Shanghai, China). DMEM medium, RPMI-1640 medium, FBS, Trizol solution, and Lipofectamine 2000 were from Invitrogen Co. (Carlsbad, CA, USA). Reverse transcription kits, restriction endonuclease BglII and SalI, T4 DNA ligase, the BCA protein quantitation and real-time qPCR kits were from MBI Fermentas China Co., Ltd. (Shenzhen, China). Plasmid pcDNA3.1 was from Beinuo Biotech Co. Ltd. (Shanghai, China). PCR purification and plasmid extraction kits were from Axygen Scientific Inc. (Carlsbad, CA, USA). A rabbit-anti-heparanase polyclonal antibody and horseradish peroxidase- (HRP-) conjugated anti-rabbit IgG were from Boshide Biotech Co. Ltd. (Wuhan, China). Matrigel was from BD Bioscience (San Jose, CA, USA).
Plasmid construction and characterization
To construct heparanase overexpression vectors, the genomic sequence of hepasranse gene was retrieved from GenBank and the cDNA encoding hepasranse gene silencing was designed. Synthetic single-stranded oligonucleotides were designed according to the heparanase sequence (Gene ID ENSG00000225609.1) and converted into double-stranded form by denaturation and subsequent annealing. Specific sequence was inserted into the vector pGPU6/GFP/Neo. The products were then used to transform competent Escherichia coli, which were cultivated on a plate containing spectinomycin overnight at 37C. Plasmid DNAs were extracted and sequenced. The samples with correct sequences were named DNA-overexpressedheparanase.
Transfection and analyses of expression levels
Recombinant plasmids were used to transfect gallbladder carcinoma cells in the presence of Lipofectamine 2000 according to the manufacturer’s protocol. Transfection efficiency was determined using an inverted fluorescence microscope 24 h after transfection. The experiments were performed three times. The transfected cell line was maintained in DMEM medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen) and designated the GBD-SD cell line. GBD-SD cells were incubated in a humidified air containing 5% CO2 at 37℃.
RT-PCR analysis of heparanase mRNA levels of GBC-SD and GBD-SD cells
RT-PCR Analysis of Heparanase mRNA levels
Primers for heparanase, syndecan-1, and β-actin were designed from sequences acquired from the GenBank database using Primer Express software (PE Applied Biosystems). The sequences of heparanase primers were as follows: forward, 5′-AGCCTCGAAGAAAGACGG-3′; reverse, 5′-GTAGTGATGCCATGTAACTGAATC-3′. The sequences of β-actin primers were as follows: forward, 5′-GGAAATCGTGCGTGACATTAAG-3′; reverse, 5′-GGAAATCGTGCGTGACATTAAG-3′. After total RNA was extracted using Trizol Reagent, RT-PCR was performed as described in the manufacturer’s instruction. β-Actin mRNA served as an internal reference. PCR products were separated using 1% agarose gel electrophoresis. The bands were scanned, and heparanase mRNA levels were determined relative to those of β-actin.
Western blot analysis of heparanase expression
Proteins were extracted from GBD-SD and GBC-SD cells, and equal amounts of heparanase were fractionated using SDS-PAGE. Antibodies against heparanase (Abcam, CA, USA) and β-actin (Santa Cruz Biotechnology, Inc.; Santa Cruz, CA, USA) were diluted 1:500. The blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Specific bands were detected using the enhanced chemiluminescent (ECL) western blot detection system (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Coomassie-blue staining of fractionated GBC-SD cell lysates was performed as a loading control.
Comparison of syndecan-1 levels of GBC-SD and GBD-SD cell lines
RT-PCR Analysis of syndecan-1 mRNA levels
The sequences of the syndecan-1 primers were as follows: forward, 5′-GGAAAGAGGTGCTGGGAGGG-3′; reverse, 5′-TTGGTGGGCTTCTGGTAGGC-3′. The sequences of the β-Actin primers were the same as those shown in Section 2.4.1. After total RNA was extracted using Trizol Reagent, RT-PCR was performed as described in the manufacturer’s instruction. β-Actin served as an internal reference. PCR products were separated using 1% agarose gel electrophoresis. The bands were scanned, and syndecan-1 mRNA levels were determined relative to those of β-actin.
Western blot analysis of syndecan-1 levels
Proteins were extracted from GBD-SD and GBC-SD cells. Equal amounts of total syndecan-1 were fractionated using SDS-PAGE. Antibodies against syndecan-1 (Abcam, CA, USA) and β-Actin (Santa Cruz, CA, USA), were diluted 1:500. The blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Specific bands were detected using the Enhanced Chemiluminescent (ECL) western blot detection system (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Coomassie-blue staining of fractionated GBC-SD cell lysates was performed as a loading control.
Cell invasion and migration assays
Transwell invasion and migration assay. The experiments were performed as previously described18 using 5 × 104 GBC-SD and 5 × 104 GBD-SD cells in serum-free RPMI-1640 that were added to the upper chambers of each well of a 24-well plate with inserts (8-mm pores; Millipore, Billerica, MA, USA) coated with Matrigel. For the migration assay, the upper chambers were not coated with Matrigel. RPMI-1640 containing 10% FBS was placed in the lower chambers as a chemoattractant. After 24 h incubation, cells on the upper surface of the membrane were wiped off, and cells that invaded through the Matrigel membrane were fixed with paraformaldehyde and stained with crystal violet. The number of invasive cells was then counted (five randomly chosen fields for each membrane) using an inverted microscope (200x). Each assay was performed in triplicate.
Statistical Analysis
The data are expressed as the mean ± standard deviation (SD). Comparisons among multiple groups were performed using one-way analysis of variance (ANOVA) followed by the t-test. P < 0.05 indicates statistical significance.
Results
Identification of recombinant vectors
The sequencing results showed that DNA vectors were consistent with the designed sequences. Deletions, insertions, or mutations were not detected.
Transfection efficiency
Bright fluorescence in cells transfected with the recombinant plasmids was observed 48 h after transfection. The average transfection efficiency of recombinant plasmids was 81.6%. Gallbladder cells with recombinant plasmids GBD-SD were isolated (Figure 1).
Figure 1.
Fluorescence microscopy (A) and light microscopy (B) of cells transfected with the DNA-overexpressed-heparanase plasmid.
Effect of recombinant plasmids on heparanase mRNA levels in gallbladder carcinoma cells
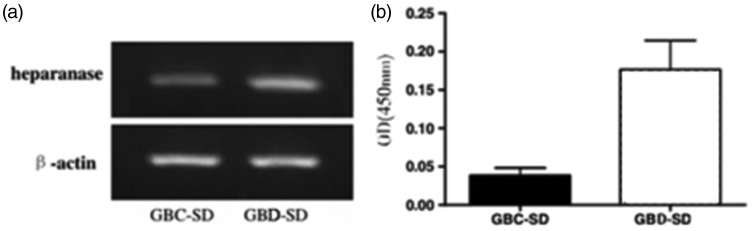
The levels of heparanase mRNA and protein in transfected cells were significantly higher compared with those of control groups (P < 0.05). These cells were selected for the following experiments (Figure 2).
Figure 2.
The expression of heparanase in GBD-SD cells and GBC-SD cells.
(a) Western blot analysis of heparanase expression.
(b) Real time RT-PCR analysis of heparanase mRNA expression. The amounts of heparanase mRNA, normalized to those of β-actin in GBD-SD cells were significantly higher compared with those in GBC-SD cells. P < 0.05.
Effect of plasmid transfection on syndecan-1 protein levels in gallbladder carcinoma cells
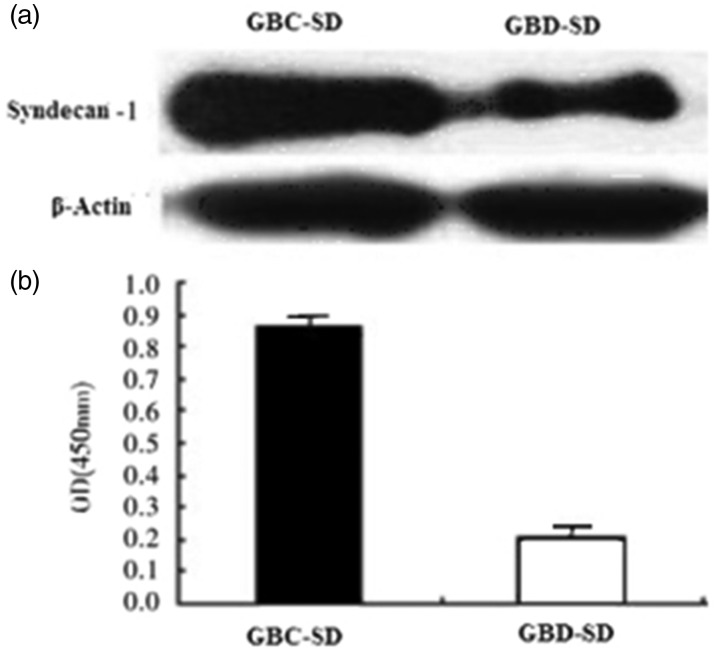
The levels of syndecan-1 in GBD-SD cells were significantly lower compared with that in GBC-SD cells (P < 0.05). The levels of syndecan-1 in GBD-SD cells were significantly lower compared with those in GBC-SD cells (P < 0.05) (Figure 3).
Figure 3.
The expression of syndecan-1 in GBD-SD cells and GBC-SD cells.
(a) Western blot analysis of syndecan-1 expression.
(b) Real time RT-PCR analysis of syndecan-1 expression. The amounts of syndecan-1 mRNA, normalized to those of β-actin in GBD-SD cells, were significantly higher compared with those in GBC-SD cells., P < 0.05.
Effect of plasmid transfection on the invasiveness and migration of gallbladder carcinoma cells
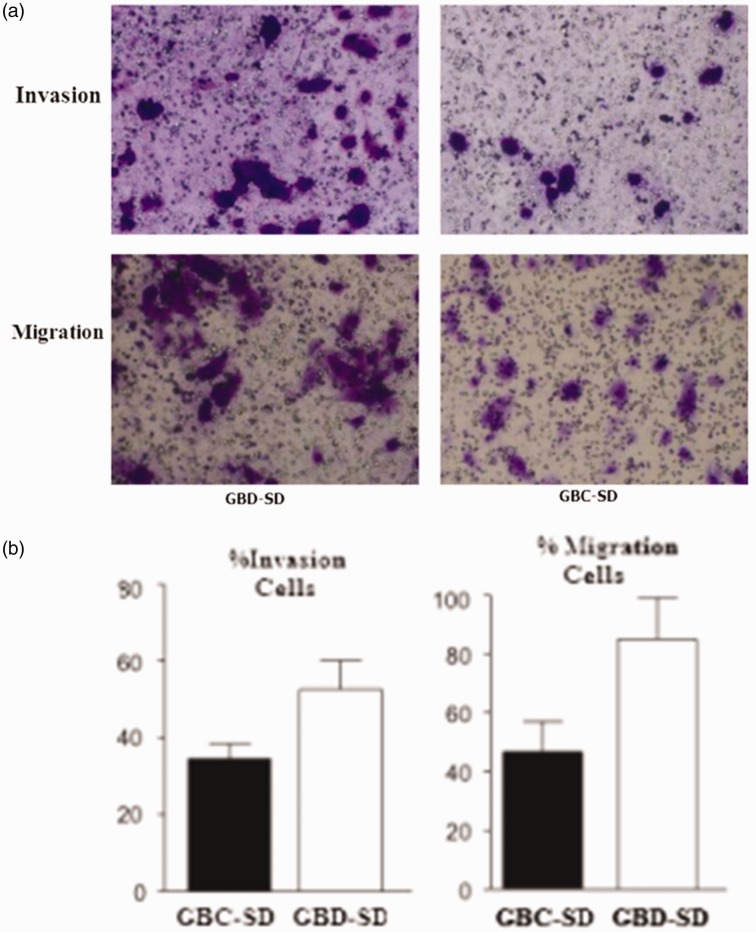
The number of invading GBC-SD cells was significantly less compared with that of GBD-SD cells (P < 0.05). The number of migrated GBC-SD cells was significantly less than compared with that of GBD-SD cells (P < 0.05) (Figure 4).
Figure 4.
Effect of heparanase overexpression on the invasiveness and migration of GBD-SD and GBC-SD cells.
(a) Invasion and migration experiments.
(b) Numbers of invading and migrating gallbladder carcinoma cells. The numbers of invading and migrating GBD-SD cells were higher compared with those of GBC-SD cells. P < 0.05.
Discussion
Heparanase has been widely studied.19 Here we show that the levels of syndecan-1 protein negatively correlated with those of heparanase. Evidence indicates that heparanase plays a vital function in tumour invasion and metastasis.20–30 To demonstrate the ability of heparanase to induce malignant transformation, vector DNA-overexpressed-heparanase was generated using recombinant DNA technology. Cells transfected with the recombinant plasmids significantly increased heparanase levels in gallbladder carcinoma cells, and DNA-overexpressed-heparanase strongly promoted the malignant phenotype of the transfected cells. DNA-overexpressed-heparanase reduced the levels of syndecan-1. Therefore, up-regulation of heparanase expression led to down-regulation of syndecan-1, consistent with the results of other studies.31,32,9
Recent studies suggest that heparanase plays a role in promoting adhesion and coagulation activity associated with tumour metastasis and inflammation.33,34 Furthermore, HS synthesis by heparanase may affect inflammation through inducing the release of chemokines and cytokines anchored within cell surfaces and the ECM, facilitating the migration of leukocytes to the sites of inflammation and activating immune cells.35 The effects of HS on acute inflammation in tissues may differ according to the levels of HS.36
Syndecan-1, a proteoglycan, plays an important role in the growth and metastasis of tumours and affects the invasiveness of tumour cells in different environments. Previous studies show that syndecan-1 promotes the growth of myelomas, angiogenesis, and metastasis.9,12,37 Although the mechanism of heparanase-mediated regulation is of interest, the main conclusion of the present study is that as heparanase levels rise (as in most tumours), the levels of syndecan-11 decrease. This reveals a vital and novel mechanism through which heparanase promotes tumour aggressiveness.
There is increasing evidence that heparanase up-regulates the expression of genes that mediate cancer metastasis and angiogenesis, glucose metabolism, the immune response, inflammation, and atherosclerosis,38–45 suggesting that heparanase belongs to an emerging class of proteins that play a significant role in regulating transcription. For example, in 2006, Nadir et al.46 found that heparanase induces tissue-factor expression in vascular endothelial and cancer cells. In 2010, Yang et al.47 found that heparanase enhances systemic osteolysis in multiple myelomas by up-regulating the expression and secretion of Receptor Activator for Nuclear Factor-κ B Ligand (RANKL). In 2011, Ramani et al.48 found that heparanase plays a dual role in driving hepatocyte growth factor (HGF) signalling by enhancing HGF expression and activity. In 2012, Cohen-Kaplan 49 found that heparanase induces signal transducer and activator of transcription (STAT) phosphorylation. Moreover, Masola reported in 2014 that heparanase is a key player in renal fibrosis by regulating transforming growth factor-β (TGF-β) expression. These findings suggest that heparanase exerts extranuclear functions, and plays a significant role in regulating transcription.
Our findings that heparanase may down-regulate syndecan-1 expression in tumours indicate that this may represent a significant mechanism through which heparanase promotes tumour invasion. Although heparanase and heparan sulfate proteoglycans have multiple effects within the tumour cells, it is likely that the most important effect of the heparanase/syndecan-1 axis in tumours and other diseases may be related to its role in vascularization. HS and VEGF are important regulators of tumour vascularization. Therefore, our present findings, along with the recent discovery18 that heparanase increases the levels of VEGF, indicate that heparanase and syndecan-1 are major components that condition the tumour microenvironment and drive angiogenesis.
The current finding that heparanase inhibits syndecan-1 expression contributes a novel insight into how heparanase activates tumour invasion and metastasis. Heparanase inhibitors may therefore serve as become promising drugs for treating cancer.
Acknowledgments
The authors thank Dr. Shaobo Zhou for excellent technical assistance in experiments on behalf of the Central Laboratory, Zhuhai People’s Hospital. This study was supported by the Natural Science Foundation of Anhui province, China (Grant no. Bykf12A07).
Authors’ contributions
Shaobo Zhou conceived and designed the study. Hao Jin drafted the manuscript and participated in the RT-PCR study. Song Yang constructed the recombinant vectors and performed transfections. Haiming Cao participated in the western blot analysis. All authors read and approved the final paper.
Ethical approval
This research did not conduct studies of human participants or animals.
Disclosure
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This study was funded by Zhuhai City People’s Hospital.
References
- 1.Chang XZ, Zhan MW, Jin MY. Isolation of a human gallbladder cancer cell clone with high invasive phenotype in vitro and metastatic potential in orthotopic model and inhibition of its invasiveness by heparanase antisense oligodeoxynucleotides. Clin Exp Metastasis 2007; 24: 25–38. [DOI] [PubMed] [Google Scholar]
- 2.Gomes AM, Stelling MP, Pavão MS. Heparan sulfate and heparanase as modulators of breast cancer progression. Biomed Res Int 2013; 2013: 852093–852093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramani VC, Purushothaman A, Stewart MD, et al. The heparanase/syndecan-1 axis in cancer: mechanisms and therapies. FEBS J 2013; 280: 2294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masola V, Secchi MF, Gambaro G, et al. Heparanase as a target in cancer therapy. Curr Cancer Drug Targets 2014; 14: 286–93. [DOI] [PubMed] [Google Scholar]
- 5.Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol 1989; 108: 1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernfield M, Gotte M, Park PW, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 1999; 68: 729–777. [DOI] [PubMed] [Google Scholar]
- 7.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates αvβ3 integrin activily in human mammary carcinoma cells. J Cell Bio 2004; 167: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beauvais DM, Ell BJ, McWhorter AR, et al. Syndecan-1 regulates αvβ3 and αvβ5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med 2009; 206: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Litwack ED, Stanley MJ, et al. Heparan sulfate proteoglycans as adhesive and antiinvasive molecules. Syndecans and glypican have distinct functions. J Bio Chem 1998; 273: 22825–22832. [DOI] [PubMed] [Google Scholar]
- 10.Beauvais DM, Rapraeger AC. Syndecan-1-mediated cell spreading requires signaling by αvβ3 integrins in human breast carcinoma cells. Exp Cell Res 2003; 286: 219–232. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Park H, Chung H. Syndecan-2 regulates the migratory potential of melanoma cells. J Bio Chem 2009; 284: 27167–27175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purushothaman A, Uyama T, Kobayashietal F. Heparanase enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood 2010; 115: 2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee H, Kim Y, Choi Y, et al. Syndecan-2 cytoplasmic domain regulates colon cancer cell migration via interaction with syntenin-1. Biochem Bioph Res Co 2011; 409: 148–153. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Macleod V, Miao HQ, et al. Heparanase Enhances Syndecan-1 Shedding: a novel mechanism for stimulation of tumor growth and metastasis. J Bio Chem 2007; 282: 13326–13333. [DOI] [PubMed] [Google Scholar]
- 15.Mikami S, Ohashi K, Usui Y. Loss of Syndecan-1 and increased expression of heparanase in invasive esophageal carcinomas. Jpn J Cancer Res 2001; 92: 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peretti T, Waisberg J, Mader AM, et al. Heparanase-2, syndecan-1, and extracellular matrix remodeling in colorectal carcinoma. Eur J Gastroenterol Hepatol 2008; 20: 256–265. [DOI] [PubMed] [Google Scholar]
- 17.Giordano RJ. Heparanase-2 and syndecan-1 in colon cancer: the ugly ducklings or the beautiful swans? Eur J Gastroenterol Hepatol 2008; 20: 716–718. [DOI] [PubMed] [Google Scholar]
- 18.Masola V, Gambaro G, Tibaldi E, et al. Heparanase and Syndecan-1 interplay orchestrates fibroblast growth factor-2-induced epithelial-mesenchymal transition in renal tubular cells. J Biol Chem 2012; 287: 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang GB, Zhou XY, Wang XQ. Relationship between serum heparanase and microscopic venous invasion in patients with hepatocellular carcinoma. Am J Clin Pathol 2010; 134: 242–248. [DOI] [PubMed] [Google Scholar]
- 20.Xiong Z, Lu MH, Fan YH, et al. Downregulation of heparanase by RNA interference inhibits invasion and tumorigenesis of hepatocellular cancer cells in vitro and in vivo. Int. J. Oncol 2012; 40: 1601–1609. [DOI] [PubMed] [Google Scholar]
- 21.Tang D, Zhang Q, Zhao S, et al. The expression and clinical significance of microRNA-1258 and heparanase in human breast cancer. Clin Biochem 2013; 46: 926–932. [DOI] [PubMed] [Google Scholar]
- 22.Zheng L, Pu J, Jiang G, et al. Abnormal expression of early growth response 1 in gastric cancer: association with tumor invasion, metastasis and heparanase transcription. Pathol Int 2010; 60: 268–277. [DOI] [PubMed] [Google Scholar]
- 23.Vlodavsky I, Beckhove P, Lerner I, et al. Significance of heparanase in cancer and inflammation. Cancer Microenviron 2012; 5: 115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mogler C, Herold-Mende C, Dyckhoff G, et al. Heparanase expression in head and neck squamous cell carcinomas is associated with reduced proliferation and improved survival. Histopathology 2011; 58: 944–52. [DOI] [PubMed] [Google Scholar]
- 25.Zeng C, Ke ZF, Luo WR, et al. Heparanase overexpression participates in tumor growth of cervical cancer in vitro and in vivo. Med Oncol 2013; 30: 403–403. [DOI] [PubMed] [Google Scholar]
- 26.Liu P, Gou M, Yi T, et al. The enhanced antitumor effects of biodegradable cationic heparin-polyethyleneimine nanogels delivering HSulf-1 gene combined with cisplatin on ovarian cancer. Int J Oncol 2012; 41: 1504–1512. [DOI] [PubMed] [Google Scholar]
- 27.Lui NS, van Zante A, Rosen SD, et al. SULF2 expression by immunohistochemistry and overall survival in oesophageal cancer: a cohort study. BMJ Open 2012; 2: e001624–e001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruan J, Trotter TN, Nan L, et al. Heparanase inhibits osteoblastogenesis and shifts bone marrow progenitor cell fate in myeloma bone disease. Bone 2013; 57: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barash U, Zohar Y, Wildbaum G, et al. Heparanase enhances myeloma progression via CXCL10 downregulation. Leukemia 2014; 121: 1038–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Yang JM, Wang HJ, et al. Synthesized multiple antigenic polypeptide vaccine based on B-cell epitopes of human heparanase could elicit a potent antimetastatic effect on human hepatocellular carcinoma in vivo. PLoS One 2013; 8: e52940–e52940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waisberg J, Theodoro TR, Matos LL, et al. Immunohistochemical expression of heparanase isoforms and syndecan-1 proteins in colorectal adenomas. Eur J Histochem 2016; 60: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roucourt B, Meeussen S, Bao J, et al. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Research 2015; 25: 412–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast 2013; 22(Suppl 2): 66–72. [DOI] [PubMed] [Google Scholar]
- 34.Axelsson J, Xu D, Kang BN, et al. Inactivation of heparan sulfate 2-O-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice. Blood 2012; 120: 1742–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akbarshahi H, Axelsson JB, Said K, et al. TLR4 dependent heparan sulphate-induced pancreatic inflammatory response is IRF3-mediated. J. Transl. Med 2011; 9: 219–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo CH, Koo CY, Bay BH, et al. Comparison of the effects of differentially sulphated bovine kidney- and porcine intestine-derived heparin sulphate on breast carcinoma cellular behaviour. Int. J. Oncol 2007; 31: 1415–1423. [PubMed] [Google Scholar]
- 37.Heidari-Hamedani G, Vivès RR, Seffouhetal A, et al. Syndecan-1 alters heparan sulfate composition and signaling pathways in malignant mesothelioma. Cell Signal 2015; 27: 2054–2067. [DOI] [PubMed] [Google Scholar]
- 38.Ilan N, Elkin M, Vlodavsky I. Regulation, function, and clinical significance of heparanase in cancer metastasis and angiogenesis. Int. J. Biochem. Cell Biol 2006; 38: 2018–2039. [DOI] [PubMed] [Google Scholar]
- 39.Levy-Adam F, Ilan N, Vlodavsky I. Tumorigenic and adhesive propertities of heparanase. Semin. Cancer Biol 2010; 20: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barash U, Cohen-Kaplan V, Doweck I, et al. Proteoglycans in health and diseases: New concepts for heparanase function in tumor progression and metastasis. FEBS J 2010; 277: 3890–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zetser A, Bashenko Y, Edovitsky E, et al. Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and SRC activation. Cancer Res 2006; 66: 1455–1463. [DOI] [PubMed] [Google Scholar]
- 42.Purushothaman A, Hurst DR, Pisano C, et al. Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT) activity to promote expression of genes that drive an aggressive tumor phenotype. J Biol Chem 2011; 286: 30377–30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahtouk K, Hose D, Raynaud P, et al. Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood 2007; 109: 4914–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okawa T, Naomoto Y, Nobuhisa T, et al. Heparanase is involved in angiogenesis in esophageal cancer through induction of cyclooxygenase-2. Clin Cancer Res 2005; 11: 7995–8005. [DOI] [PubMed] [Google Scholar]
- 45.Cohen-Kaplan V, Naroditsky I, Zetser A, et al. Heparanase induces VEGF-C and facilitates tumor lymph angiogenesis. Int J Cancer 2008; 123: 2566–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nadir Y, Brenner B, Zetser A, et al. Heparanase induces tissue factor expression in vascular endothelial and cancer cells. J Thromb Haemost 2006; 4: 2443–51. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y, Ren Y, Ramani VC, et al. Heparanase enhances local and systemic osteolysis in multiple myeloma by up-regulating the expression and secretion of RANKL. Cancer Res 2010; 70: 8329–8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramani VC, Yang Y, Ren Y, et al. Heparanase plays a dual role in driving hepatocyte growth factor (HGF) signaling by enhancing HGF expression and activity. J. Biol. Chem 2011; 286: 6490–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen-Kaplan V, Jrbashyan J, Yanir Y, et al. Heparanase induces signal transducer and activator of transcription (STAT) protein phosphorylation: preclinical and clinical significance in head and neck cancer. J Biol Chem 2012; 287: 6668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masola V, Zaza G, Secchi MF, et al. Heparanase is a key player in renal fibrosis by regulating TGF-β expression and activity. Biochim Biophys Acta 2014; 1843: 2122–2128. [DOI] [PubMed] [Google Scholar]