Abstract
Objective
The ideal agents for conscious sedation during ambulatory inguinal hernia repair are still unclear. We aimed to compare the analgesic, sedative, haemodynamic, and side effects of dexmedetomidine with those of propofol in combination with fentanyl for conscious sedation in patients undergoing inguinal hernia repair.
Methods
Eighty patients undergoing unilateral inguinal hernia repair were prospectively randomized to receive either dexmedetomidine (n = 40) or propofol (n = 40). Dexmedetomidine and propofol dosages were adjusted to maintain the targeted level of sedation.
Results
After administration of sedative drugs, patients who received dexmedetomidine had a significantly lower heart rate. The intraoperative requirement of fentanyl was significantly lower in patients who received dexmedetomidine compared with patients who received propofol. Administration of dexmedetomidine was associated with a reduced postoperative pain score, longer time for onset of sedation, and a slightly longer recovery time. No serious adverse events occurred in either group. The patients’ overall satisfaction score was comparable between the two groups.
Conclusion
Dexmedetomidine is an effective adjuvant when co-administered with fentanyl for conscious sedation in patients who undergo inguinal hernia repair. Administration of dexmedetomidine decreases the requirement of fentanyl and the pain score, but slightly prolongs the time to sedation and recovery.
Keywords: Dexmedetomidine, inguinal hernia repair, conscious sedation, recovery
Introduction
Inguinal hernia repair is one of the most commonly performed surgical procedures in ambulatory surgery. There are several anaesthetic techniques that can be used for inguinal herniorrhaphy, such as general anaesthesia, spinal anaesthesia, and local infiltration anaesthesia. For ambulatory surgery, the ideal method of anaesthesia is to provide safe and effective anaesthesia with minimal side effects and rapid recovery.1 Wound infiltration with a local anaesthetic is recommended as the preferred anaesthetic technique for patients undergoing inguinal hernia repair.2 Local anaesthesia has some proven advantages, including less postoperative pain, faster mobilization, and earlier fulfilment of discharge criteria than other anaesthetic techniques. However, local anaesthesia alone may be uncomfortable and the procedure may be distressful for patients.3 Therefore, conscious sedation is required to relieve patients’ psychological and physiological stress and to increase patients’ comfort.
The most suitable agents for conscious sedation during ambulatory surgical procedures are still unclear. An ideal anaesthetic drug that is used for conscious sedation should relieve a patient’s stress, achieve rapid recovery, and have few side effects. Although propofol is a widely used sedative hypnotic, this drug possesses some inherent limitations. Propofol may cause respiratory depression, which can be amplified in the presence of opioids.4 Moreover, intravenous propofol may cause haemodynamic instability in older or feeble patients.
Dexmedetomidine is a highly selective α-2 agonist that has many clinical benefits, such as sedation, analgesia, preventing detrimental stress responses, and minimal respiratory depression.5 An advantage of dexmedetomidine when used for conscious sedation is that it can induce a unique pattern of sleep, which resembles physiological sleep, while enabling easy arousal.6 Several clinical studies have shown dexmedetomidine to be a useful sedative drug for conscious sedation during ambulatory surgeries.5,7
The present study aimed to compare the analgesic, sedative, haemodynamic, and side effects of dexmedetomidine with those of propofol in combination with fentanyl for conscious sedation in patients undergoing inguinal hernia repair.
Methods
The present study was a prospective, randomized, controlled trial. All patients, postoperative assessors, and statisticians were not provided information on the allocation of groups. Only the anaesthesiologists were aware of the group assignment of their patients. This study was approved by the Medical Ethics Committee of Zhejiang Hospital and was registered at the Chinese clinical trial registry (trial registration number: ChiCTR-IOR-15007086). Written informed consent was obtained from the patients
Eighty adult patients aged between 18 and 70 years old with American Society of Anesthesiologists physical statuses I and II, who underwent unilateral inguinal hernia repair from August 2014 to August 2015, were enrolled in this study. Patients with complicated hernia (bilateral hernia, giant hernia, recurrent hernia), chronic pain, a history of opioid addiction, body mass index greater than 40 kg/m2, and allergies to local anaesthetics were excluded. Patients were randomly allocated to receive either intravenous dexmedetomidine (Dex group, n = 40) or propofol (Pro group, n = 40) during the operation. Randomization was based on a computer-generated random number table.
Patients were taken to the operation room without any premedication. Standard intraoperative monitoring was performed for electrocardiography, non-invasive blood pressure, heart rate (HR), respiratory rate (RR), arterial oxygen saturation (SpO2), and capnography (the carbon dioxide sample line of the capnograph was placed close to the nostrils). Oxygen supplementation was achieved through a nasal cannula. The sedation level was assessed and recorded every 10 min throughout the surgical procedure by using the Ramsay sedation scale (RSS).8
Patients in the Dex group were infused with dexmedetomidine at a loading dose of 0.5 µg/kg over 10 min, followed by a maintenance infusion of 0.5 µg/kg/h until the end of surgery. Patients in the Pro group were infused with propofol at a loading dose of 2 mg/kg over 10 min, followed by a maintenance infusion of 1.5 mg/kg/h until the end of surgery. To achieve the target sedation level (RSS = 3) in both groups, infusion doses of dexmedetomidine or propofol were increased by 20% if sedation was inadequate (RSS > 3) and decreased by 20% if patients were over-sedated (RSS < 3).
All patients received 0.5 µg/kg fentanyl intravenously 5 min before surgical incision. After achieving the predefined targeted level of sedation (RSS = 3), local anaesthesia with 10 mL of 1% lidocaine was introduced in the superficial and deep layers of the surgical incision. A volume of 20 mL of 0.5% ropivacaine was then infiltrated to block the nerves of the groin region, as described by Amid et al.9 If the patient complained of pain or the body moved because of pain during the surgical procedure, a 0.2–0.5-µg/kg fentanyl bolus was administered as a rescue drug in both groups. The total amount of intraoperative fentanyl required was recorded.
Perioperative adverse events, such as apnoea, oxygen desaturation, hypertension, hypotension, tachycardia, bradycardia, nausea, vomiting or any other event, were monitored and recorded. Apnoea was monitored by using capnography and defined as cessation of breathing for >20 s.10 Oxygen desaturation was defined as an SpO2 value of <92%. Hypotension and bradycardia were defined as a decrease from baseline value by 20%. Hypertension and tachycardia were defined as an increase from baseline value by 20%. We managed adverse respiratory events with a jaw thrust, mask ventilation, or by increasing oxygen flow. Adverse haemodynamic events were managed with ephedrine, nitroglycerin, atropine, or esmolol.
All of the patients were monitored in the post-anaesthetic care unit until their discharge The Aldrete score reached 10 (full recovery from sedation).11 The times to full sedation and full recovery were recorded for all patients. Postoperative pain scores at rest and on movement were evaluated by numeric rating scale (0 = no pain, 10 = worst pain imaginable). Patients’ overall satisfaction was assessed using a 7-point Likert-like verbal rating scale immediately following discharge from the post-anaesthetic care unit.12 The primary outcome of this study was the requirement of fentanyl. The secondary outcomes were time to targeted sedation, recovery time, perioperative adverse events, postoperative pain scores, and patients’ overall satisfaction.
Statistical analysis
Descriptive data are shown as mean and standard deviation or number. The chi-square and Student’s t-tests were used to compare significant differences in categorical and continuous variables, respectively, between the two study groups. For comparison of continuous variables, factorial ANOVA with repeated measures was applied. Post hoc comparison between the groups at each time point and among the repeated measures in each group was performed by the Tukey HSD test, if appropriate. Statistical analysis was performed using SPSS v19.0 (SPSS Inc., Chicago, IL, USA). A P value less than 0.05 was considered significant.
The sample size was estimated based on pilot data of our preliminary study. The primary outcome of the preliminary study showed that the requirement of fentanyl in the Dex group was 52.3 ± 11.6 µg and that in the Pro group was 60.4 ± 13.2 µg. The sample size calculation was based on detection of a difference in the requirement of fentanyl between the two groups with α = 0.05 and power = 80%.13 This analysis showed that each group would require 37 patients. For possible dropouts, we decided to include 40 patients in each group.
Results
During the study period, 103 patients with unilateral inguinal hernia were screened for eligibility. Of the patients who were screened for enrolment, 23 were excluded because of the exclusion criteria. A total of 80 patients were enrolled in our study with 40 patients in the Dex group and 40 in the Pro group. There were no significant differences in age, sex, body mass index, comorbidities, and duration of surgery between the two groups (Tables 1 and 2).
Table 1.
Demographic characteristics of the patients.
| Dex group (n = 40) | Pro group (n = 40) | P | |
|---|---|---|---|
| Age (years) | 68.5 ± 13.6 | 66.9 ± 14.1 | 0.607 |
| Sex (male/female) | 38/2 | 38/2 | 1.000 |
| BMI (kg/m2) | 24.3 ± 4.2 | 25.1 ± 3.9 | 0.380 |
| ASA (I/II) | 3/37 | 5/35 | 0.456 |
| Comorbidities | |||
| Hypertension | 11 | 12 | 0.805 |
| COPD | 5 | 6 | 0.745 |
| Diabetes | 4 | 4 | 1.000 |
Values are presented as mean ± SD or number of patients.
BMI: body mass index; ASA: American Society of Anesthesiologists; COPD: chronic obstructive pulmonary disease.
Table 2.
Intraoperative and postoperative characteristics.
| Dex group (n = 40) | Pro group (n = 40) | P | |
|---|---|---|---|
| Duration of surgery (min) | 79.8 ± 20.3 | 83.7 ± 22.0 | 0.413 |
| Time to targeted sedation (min) | 25.5 ± 6.4 | 12.3 ± 4.2 | 0.001 |
| Recovery time (min) | 8.9 ± 2.7 | 5.6 ± 2.1 | 0.001 |
| Fentanyl requirement (µg) | 50.8 ± 10.3 | 82.0 ± 12.6 | 0.001 |
| Patients’ satisfaction | 6.1 ± 0.4 | 6.0 ± 0.5 | 0.326 |
| Adverse events | |||
| Nausea | 6 | 4 | 0.499 |
| Vomiting | 0 | 0 | 1.000 |
| Apnoea | 0 | 5 | 0.021 |
| Desaturation | 0 | 0 | 1.000 |
Values are presented as mean ± SD or number of patients.
In the Pro group, four (10%) patients needed to decrease the infusion rate because of over-sedation. However, none of the patients needed to adjust the infusion rate in the Dex group. All patients in both groups achieved the targeted level of sedation. However, there was a significant difference in the time required to achieve the same level of sedation. Targeted sedation was achieved within 12 min with propofol, but took nearly 25 min with dexmedetomidine (Table 2). During recovery, patients who had received dexmedetomidine during surgery had a slightly longer recovery time compared with patients who received propofol.
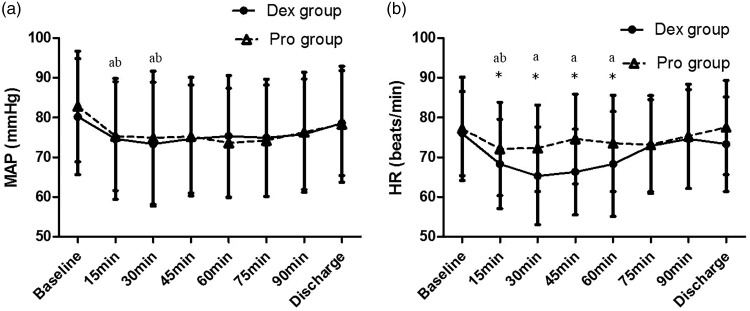
Baseline HR and MAP were similar between the groups. After administration of sedative drugs, HR and mean arterial pressure (MAP) were significantly decreased from baseline in both groups (P < 0.05). The reduction in HR was significantly greater in patients who received dexmedetomidine than in patients who received propofol (Figure 1). No patients in either group required intervention because of adverse haemodynamic events.
Figure 1.
Changes in haemodynamic variables in the two treatment groups.
MAP: mean arterial blood pressure; HR: heart rate. *Significant difference between the groups (P < 0.05). aSignificant difference compared with baseline in the Dex group (P < 0.05). bSignificant difference compared with baseline in the Pro group (P < 0.05).
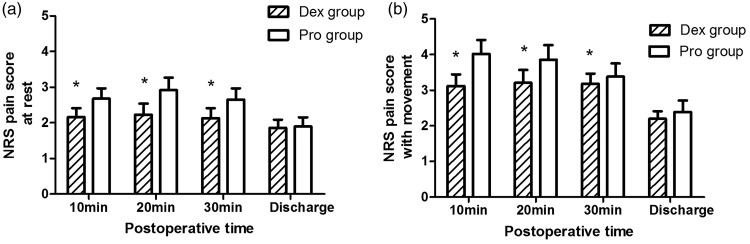
The requirement of fentanyl was significantly lower in the Dex group than in the Pro group (Table 2). No significant difference was observed in the incidence of postoperative nausea (P = 0.499) between the two groups. No patient in either group vomited postoperatively. The postoperative pain score was higher in the Pro group than in the Dex group at 10, 20, and 30 min postoperatively (Figure 2). At discharge, the postoperative pain score was comparable between the two groups. The patients’ overall satisfaction was comparable between both groups.
Figure 2.
Pain score during recovery.
Numeric rating scale (0 = no pain, 10 = worst pain imaginable). *Significant difference between the groups (P < 0.05).
Five apnoea events occurred in the Pro group (Table 2). No cases of oxygen desaturation were observed during the entire study period in either group. No serious adverse events occurred in this study and all patients were discharged as scheduled without any complications.
Discussion
In the present study, we found that the use of dexmedetomidine for conscious sedation in patients who underwent ambulatory inguinal hernia repair was associated with a reduced requirement for opioids, a longer time for onset of sedation, a slightly longer recovery time, and fewer adverse events compared with propofol at similar sedation levels.
For ambulatory surgery, an adequate level of sedation is required to alleviate patients’ anxiety and increase their comfort. In our study, all of the patients achieved targeted sedation levels. Although the level of sedation that was achieved by all patients was sufficient for the procedure to be completed, more fentanyl was required in the Pro group than in the Dex group to reduce or eliminate surgical pain. The analgesic effect of dexmedetomidine might have resulted in a reduction in requirement for fentanyl in the Dex group. Intravenous dexmedetomidine as an anaesthetic adjuvant decreases opioid and anaesthetic requirements in various clinical applications.14 Regardless of lower fentanyl consumption, patients in the Dex group had a lower postoperative pain score than those in the Pro group. In our study, infusion of dexmedetomidine was terminated at the end of the surgical procedure. Because the half-life of dexmedetomidine is 3 h, the analgesic effects of dexmedetomidine were likely to have persisted in the recovery period.15
Bradycardia and hypotension are among the most commonly reported adverse events in patients who received dexmedetomidine.14 The reduction in HR and MAP that were observed in the Dex group could be explained by the central and peripheral sympatholytic effects that are caused by dexmedetomidine. However, no serious haemodynamic events (hypotension or bradycardia) occurred in this study. The relatively low dose of dexmedetomidine used in our study may explain the absence of adverse haemodynamic events. Additionally, the low initial loading dose followed by continuous infusion of dexmedetomidine provided adequate, well-controlled sedation.16 High doses of sedative drugs are likely to cause complications, such as hypertension, hypotension, bradycardia, or sinus arrest.
Opioids are the cornerstone of pain management. However, their use is associated with a variety of adverse effects, such as respiratory depression, nausea, and vomiting.17 Postoperative nausea and vomiting may cause severe discomfort and delayed discharge among ambulatory patients. In our study, although the requirement of fentanyl was significantly higher in patients who received propofol than in those who received dexmedetomidine, the incidence of nausea and vomiting was comparable between the two groups. A possible explanation for this finding is that propofol may be an effective antiemetic when used as sedative.18 In addition, our study showed that propofol in combination with fentanyl for conscious sedation may have caused potential respiratory depression, while dexmedetomidine was associated with minimal respiratory depression.
Our study has several limitations. An important limitation is that the present study was not completely blinded. The anaesthesiologists were aware of the group assignment of their patients. Another limitation is that we did not collect follow-up data, such as the pain score, and the rate of nausea and vomiting after discharge.
In conclusion, dexmedetomidine is an effective adjuvant when co-administered with fentanyl for conscious sedation in patients who undergo inguinal hernia repair. Administration of dexmedetomidine decreases the requirement of fentanyl and the postoperative pain score, but slightly prolongs the time to sedation and recovery.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Key Project of Chinese Ministry of Health and Medical Scientific Research Foundation of Zhejiang Province (No. WKJ-ZJ-1708).
References
- 1.White PF, Eng M. Fast-track anesthetic techniques for ambulatory surgery. Curr Opin Anaesthesiol 2007; 20: 545–557. [DOI] [PubMed] [Google Scholar]
- 2.Simons MP, Aufenacker T, Bay-Nielsen M, et al. European hernia society guidelines on the treatment of inguinal hernia in adult patients. Hernia 2009; 13: 343–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaikh AR, Rao AM, Muneer A. Inguinal mesh hernioplasty under local anaesthesia. J Pak Med Assoc 2012; 62: 566–569. [PubMed] [Google Scholar]
- 4.Uzümcügil F, Canbay O, Celebi N, et al. Comparison of dexmedetomidine-propofol vs. fentanyl-propofol for laryngeal mask insertion. Eur J Anaesthesiol 2008; 25: 675–680. [DOI] [PubMed] [Google Scholar]
- 5.Farag E, Argalious M, Abd-Elsayed A, et al. The use of dexmedetomidine in anesthesia and intensive care: a review. Curr Pharm Des 2012; 18: 6257–6265. [DOI] [PubMed] [Google Scholar]
- 6.Mason KP, O’Mahony E, Zurakowski D, et al. Effects of dexmedetomidine sedation on the EEG in children. Paediatr Anaesth 2009; 19: 1175–1183. [DOI] [PubMed] [Google Scholar]
- 7.Fan TW, Ti LK, Islam I. Comparison of dexmedetomidine and midazolam for conscious sedation in dental surgery monitored by bispectral index. Br J Oral Maxillofac Surg 2013; 51: 428–433. [DOI] [PubMed] [Google Scholar]
- 8.Sen J, Sen B. A comparative study on monitored anesthesia care. Anesth Essays Res 2014; 8: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amid PK, Shulman AG, Lichtenstein IL. Local anesthesia for inguinal hernia repair step-by-step procedure. Ann Surg 1994; 220: 735–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beitz A, Riphaus A, Meining A, et al. Capnographic monitoring reduces the incidence of arterial oxygen desaturation and hypoxemia during propofol sedation for colonoscopy: a randomized, controlled study (ColoCap Study). Am J Gastroenterol 2012; 107: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 11.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg 1970; 49: 924–934. [PubMed] [Google Scholar]
- 12.Alhashemi JA. Dexmedetomidine vs midazolam for monitored anaesthesia care during cataract surgery. Br J Anaesth 2006; 96: 722–726. [DOI] [PubMed] [Google Scholar]
- 13.Archibald CP, Lee HP. Sample size estimation for clinicians. Ann Acad Med Singapore 1995; 24: 328–332. [PubMed] [Google Scholar]
- 14.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol 2008; 21: 457–461. [DOI] [PubMed] [Google Scholar]
- 15.Venn RM, Karol MD, Grounds RM. Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive care. Br J Anaesth 2002; 88: 669–675. [DOI] [PubMed] [Google Scholar]
- 16.Manne GR, Upadhyay MR, Swadia V. Effects of low dose dexmedetomidine infusion on haemodynamic stress response, sedation and post-operative analgesia requirement in patients undergoing laparoscopic cholecystectomy. Indian J Anaesth 2014; 58: 726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am 2015; 95: 301–318. [DOI] [PubMed] [Google Scholar]
- 18.Soppitt AJ, Glass PS, Howell S, et al. The use of propofol for its antiemetic effect: a survey of clinical practice in the United States. J Clin Anesth 2000; 12: 265–269. [DOI] [PubMed] [Google Scholar]