Abstract
Objective
To evaluate the effect of the phenolic compound naringenin on thermal burn-induced inflammatory responses and oxidative stress in rats.
Methods
First degree thermal burn injuries were induced in shaved rats by 10 s immersion of the back surface in water at 90℃. Naringenin treatment (25, 50 and 100 mg/kg/day) was initiated 24 h following burn injury, and continued for 7 days. On treatment day 7, serum tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, nitric oxide (NO), prostaglandin (PG)E2, caspase-3, leukotriene (LT)-B4 and nuclear factor (NF)-κB levels were quantified. Skin sample glutathione (GSH) and thiobarbituric acid reactive substances (TBARS) levels, and catalase, superoxide dismutase (SOD), glutathione-S-transferase (GST) and glutathione peroxidase (GPx) activities, were also measured.
Results
Serum inflammatory biomarkers were significantly increased in thermal-burn injured rats versus uninjured controls. Naringenin significantly inhibited the increased proinflammatory markers at day 7 of treatment. Increased TBARS levels and decreased GSH levels in wounded skin were significantly restored by naringenin treatment at day 7. SOD, catalase, GPx and GST activities were markedly inhibited in wounded skin tissues, and were significantly increased in naringenin-treated rats.
Conclusion
Naringenin treatment showed anti-inflammatory and antioxidant effects in rats with thermal burn-induced injury.
Keywords: Naringenin, thermal burn, oxidative stress, inflammation
Introduction
Thermal burns are known to be responsible for many pathophysiological changes in the skin and other organs,1 and burn trauma results in several complications, such as increased infection rate, long hospital stay and increased mortality rate.2 The use of burn-trauma animal models has helped identify multiple inflammatory mediators that contribute to burn-associated systemic inflammatory response syndrome.3
Inflammatory cytokines are involved in the hypermetabolic host response to thermal injury. Various types of trauma are associated with different types of physiologic stress, resulting in a wide range of post-injury cytokine responses at varying time-points.4 Information concerning the early development of acute-phase response to thermal-injury stress, however, remains sparse.5 Various models of inflammation have shown that inflammatory cytokines work in sequence,6 and potential sources of cytokines in thermal injury include neutrophils, phagocytic cells, lymph nodes and hepatocytes.7 Dynamic alterations in circulating inflammatory cytokines implicates their pathological role in early thermal injury responses.8
Phenolic compounds and flavonoids are documented to possess anti-inflammatory and antioxidant activities, and the flavonoid naringenin is proposed to have pharmacologically significant effects against chronic diseases including diabetes, hypertension, inflammation and allergy.9 Naringenin has been demonstrated to attenuate the release of inflammatory biomarkers by inactivating nuclear factor (NF)-κB and suppressing mitogen-activated protein kinases.10 Further investigations are necessary to validate the potentially beneficial role of naringenin in burn-associated wounds and inflammation. Thus, the aim of the present study was to explore the mode of action of naringenin against experimentally-induced thermal-burn wounds in rats.
Materials and methods
Experimental animals
This study, conducted at the Department of Dermatology and Venerology, College of Medicine, Al Imam Mohammad Ibn Saud Islamic University, Riyadh, Saudi Arabia, included male Wistar albino rats (aged approximately 11–12 weeks; weight, 220–240 g) supplied by the Experimental Animal Care Centre, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. Rats were housed under controlled conditions (temperature, 22 ± 1℃; humidity, 50–55%; and 12-h dark/12-h light cycle) and were provided free access to Purina rat chow (Grain Silos & Flour Mills Organization, Riyadh, Saudi Arabia) and drinking water. The study was conducted in accordance with the 1996 National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23), and was approved by the Research Ethics Committee, College of Pharmacy, King Saud University.
Thermal burn wound induction
Thermal injury, comprising 20% of total body surface area, was induced following a method described previously.11 Briefly, normal healthy Wistar rats were anaesthetized with 94 mg/kg Ketamine (AGRAR holland, EC Soest, The Netherlands) and 14 mg/kg Xylazine (Laboratorios Calier, S.A., Spain) mixture (i.p.) and the back area was shaved, followed by demarcation of the proposed burn area. Each anaesthetized rat was then fixed flat on its back on a perforated Plexiglas™ plate (Shanghai Kingsign International Trade Co. Ltd, Shanghai, China) and the delimited skin surface was immersed through a hole of 3 cm diameter into a 90℃ water bath for 10 s, to induce first degree burns with superficial partial skin injury. To stop the burn process after 10 s, the rat was immediately plunged into fresh water (20 ± 22℃) and dried with a towel before awakening. A burn area measuring about 6 cm2 corresponded to approximately 20% of the total body surface area. Immediately on awakening, the burned animals were administered 1 ml/kg saline Ringer solution (i.p.), then each animal was housed in a separate cage. A moist saline dressing was used to cover the wounded area to prevent the grooming or licking response.
Experimental design
The body weight of each rat was recorded immediately prior to wound induction and on day 7 of post-burn treatment. Naringenin (Sigma-Aldrich, Gillingham, Dorset, UK) was suspended in 0.25% carboxymethyl cellulose. Wounded rats were subdivided into four groups (six rats per group), and a group of six healthy rats were used as controls as follows: group 1, healthy controls; group 2, sham control wounded rats (treated with 0.25% carboxymethyl cellulose vehicle); group 3, wounded rats treated with 25 mg/kg/day naringenin (oral); group 4, wounded rats treated with 50 mg/kg/day naringenin (oral); and group 5, wounded rats treated with 100 mg/kg/day naringenin (oral). Naringenin treatments were initiated 24 h following thermal-wound induction and continued daily for 7 days. Wound images were obtained using a digital camera mounted onto a Leica EZ4 HD stereomicroscope (Leica Microsystems, Heerbrugg, Switzerland). At 2 h following the last treatment on day 7, wound area was measured using Vernier callipers (Fastenal, Winona, MN, USA), blood samples were collected through cardiac puncture into sterilized plain tubes under light anaesthesia using inhaled diethyl ether (Sigma Aldrich, Munich, Germany), and finally animals were decapitated prior to dissection of the wound skin. Skin samples were sectioned into two parts: one part was preserved in 10% formaldehyde and the other was stored at –80℃. Blood samples were placed for approximately 1 h at ambient temperature to allow clotting and centrifuged at 1 800 g for 15 min at room temperature, then serum samples were separated and preserved at –20℃ prior to analysis.
Serum inflammatory biomarker levels, comprising tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β, nitric oxide (NO), prostaglandin (PG)E2, caspase-3, leukotriene (LT)B4 and NF-κB were evaluated using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. All inflammatory biomarkers and oxidative stress parameters were analysed using colorimetric-based ELISA except NF-κB estimations, which were performed using fluorescence-based ELISA. TNF-α, IL-6, IL-1β, PGE2 and LTB4 values are presented as pg/ml, and NO, caspase-3 and NF-κB are presented as mmol/l, % of control group and m fluorescence/µl, respectively. Oxidative stress parameters, including thiobarbituric acid reactive substances (TBARS), glutathione (GSH), superoxide dismutase (SOD), catalase, glutathione-S-transferase (GST) and glutathione peroxidase (GPx), were measured in the previously frozen skin tissue samples using commercially available kits (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions.
Histological analyses
Cross sections of the dermal tissues fixed in 10% formaldehyde solution were embedded into standard paraffin wax blocks. Samples were then cut into 5-µm thick sections using a microtome, and the sections were stained with haematoxylin and eosin. Mounted, stained sections were observed for cellular changes by a histopathologist in a blinded fashion. General histopathological examination was performed without classifying any changes in predefined histological features.
Statistical analyses
Data are presented as mean ± SE. Differences between the groups were analysed using one-way analysis of variance. Within-group differences were analysed using Student–Newman–Keuls multiple comparison test. All statistical analyses were performed using GraphPad Prism software, version 5 (GraphPad Software, La Jolla, CA, USA). A P value < 0.05 was considered to be statistically significant.
Results
There were no statistically significant differences in age and baseline weight between the study groups, and no statistically significant differences in body weight between the thermal-burn injury groups and uninjured controls on day 7 of treatment. Mean wound surface area was compared between day 0 and day 7 of treatment, and showed that in rats treated with naringenin (25, 50 or 100 mg/kg/day), wound surface area was significantly reduced at day 7 of treatment compared with day 0 (P < 0.05; Table 1). On day 7 of treatment, there was a statistically significant difference in reduction of mean wound surface area between rats treated with a relatively high naringenin dose (100 mg/kg/day) and sham-treated controls (P < 0.05; Table 1). Changes in mean wound surface area were not significantly different between rats treated with 25 or 50 mg/kg/day naringenin and sham-treated controls.
Table 1.
Effect of naringenin treatment over 7 days following burn induction on body weight and wound surface area in male Wistar rats with thermally-induced burn injury.
| Study group (n = 6/group) | Body weight, g |
Wound surface area, cm2 |
||||
|---|---|---|---|---|---|---|
| Baseline | Treatment day 7 | Baseline | Treatment day 7 | % Change | Statistical significance (day 7 versus day 0)a | |
| Uninjured control | 238.45 ± 4.56 | 268.57 ± 7.51 | – | – | – | |
| Sham treated | 236.76 ± 3.24 | 258.52 ± 8.15 | 6.75 ± 0.31 | 5.87 ± 0.37 | –13 % | NS |
| Naringenin, 25 mg/kg/day | 236.45 ± 4.15 | 267.52 ± 5.75 | 6.76 ± 0.32 | 5.47 ± 0.34 | –19 % | P = 0.020 |
| Naringenin, 50 mg/kg/day | 237.19 ± 2.87 | 269.87 ± 4.56 | 6.86 ± 0.24 | 4.92 ± 0.21 | –28 % | P = 0.001 |
| Naringenin, 100 mg/kg/day | 235.86 ± 3.34 | 271.29 ± 7.48 | 6.79 ± 0.34 | 4.36 ± 0.32* | –36 % | P = 0.0004 |
Data presented as mean ± SE.
Within-group differences analysed by Student–Newman–Keuls multiple comparison test.
P < 0.05, versus sham-treated group (one-way analysis of variance).
NS, no statistically significant difference at treatment day 7 versus day 0.
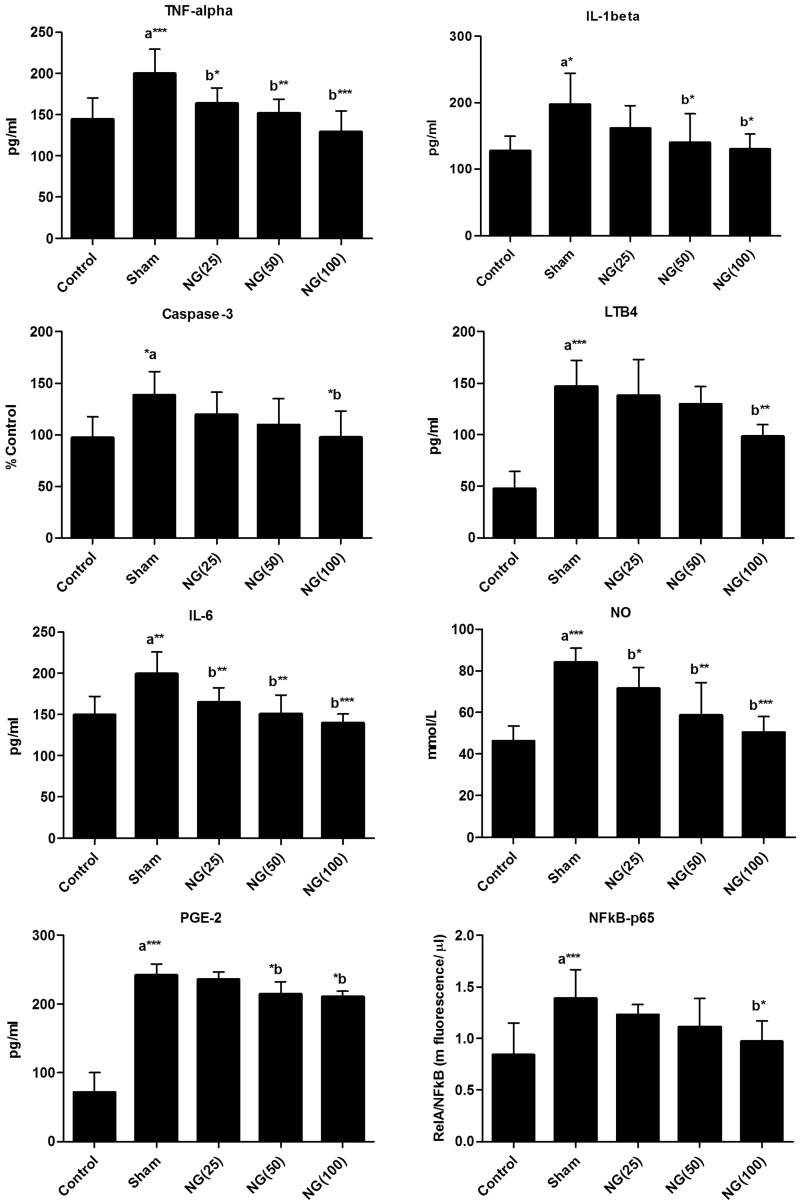
Serum TNF-α, IL-6, IL-1β, NO, caspase-3, LTB4, PGE2 and NF-κB levels were all significantly increased in the sham-treated burn injury group compared with uninjured controls at day 7 of treatment following burn injury (P < 0.05; Figure 1). Serum TNF-α, IL-6 and NO levels were significantly lower at day 7 of 25, 50 or 100 mg/kg/day naringenin treatment following thermal-burn injury compared with sham-treated controls (P < 0.05). Serum IL-1β and PGE2 levels were significantly lower in the 50 or 100 mg/kg/day naringenin treated groups versus the sham-treated controls (P < 0.05; Figure 1). Serum caspase-3, LTB4, and NF-κB levels were only significantly lower than the sham-treated controls at day 7 in rats treated with 100 mg/kg/day naringenin (P < 0.05; Figure 1).
Figure 1.
Effect of oral naringenin treatment over 7 days following burn induction on serum tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, nitric oxide (NO), caspase-3, leukotriene (LT)B4, prostaglandin (PG)E2 and nuclear factor (NF)-κB in male Wistar rats with thermally-induced burn injury. Treatment day 7 data presented as mean ± SE (n = 6); aUninjured control versus sham-treated group; bversus sham-treated group. Statistically significant differences at *P < 0.05, **P < 0.01 and ***P < 0.001 (one-way analysis of variance). NG(25), 25 mg/kg/day naringenin; NG(50), 50 mg/kg/day naringenin; NG(100), 100 mg/kg/day naringenin
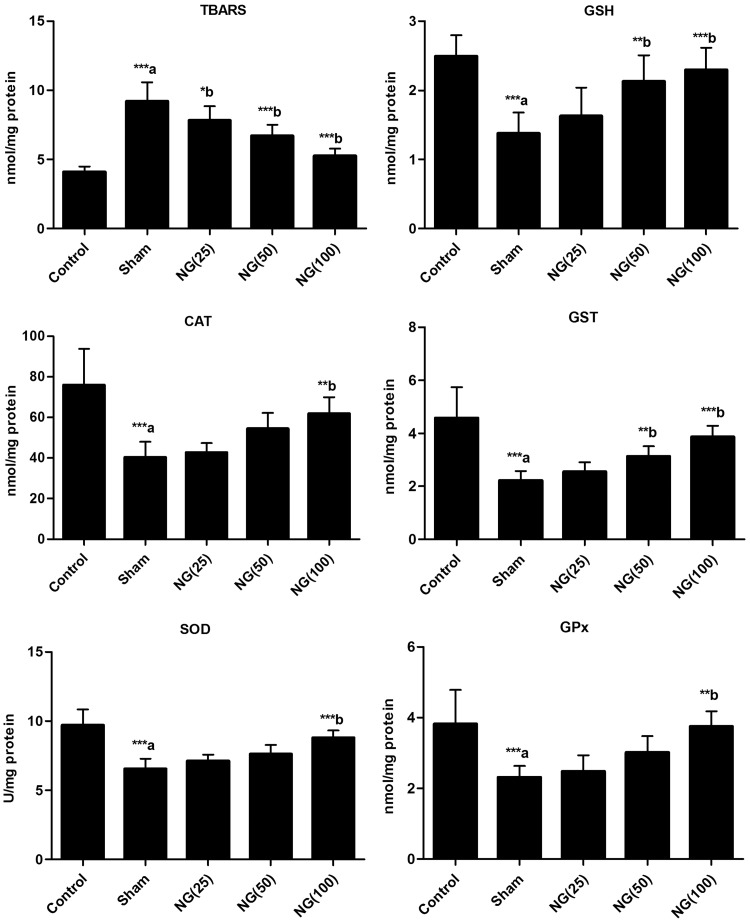
Skin tissue levels of TBARS were significantly higher, and GSH levels were significantly lower, in sham-treated burn injury rats versus uninjured controls (P < 0.001; Figure 2). Seven-day treatment with 25, 50 or 100 mg/kg/day naringenin was associated with significantly lower skin tissue TBARS levels versus sham-treated controls (P < 0.05). GSH levels were significantly increased in rats treated with 50 or 100 mg/kg/day naringenin compared with the sham-treated group (P < 0.01). Skin tissue SOD, catalase, GST and GPx activity were significantly inhibited in sham-treated burn-injury group compared with uninjured controls (P < 0.001). Treatment with 100 mg/kg/day naringenin for 7 days following burn injury significantly increased SOD, catalase, GST and GPx activities versus sham-treated controls (P < 0.01; Figure 2), and GST was also significantly increased versus sham treatment in rats treated with 50 mg/kg/day naringenin (P < 0.01).
Figure 2.
Effect of oral naringenin treatment over 7 days following burn induction on wounded skin thiobarbituric acid reactive substances (TBARS) and glutathione (GSH) levels, and on superoxide dismutase (SOD), catalase, glutathione-S-transferase (GST) and glutathione peroxidase (GPx) activity in male Wistar rats with thermally-induced burn injury. Treatment day 7 data presented as mean ± SE (n = 6); aUninjured control versus sham-treated group; bversus sham-treated group. Statistically significant differences at *P < 0.05, **P < 0.01 and ***P < 0.001 (one-way analysis of variance). NG(25), 25 mg/kg/day naringenin; NG(50), 50 mg/kg/day naringenin; NG(100), 100 mg/kg/day naringenin
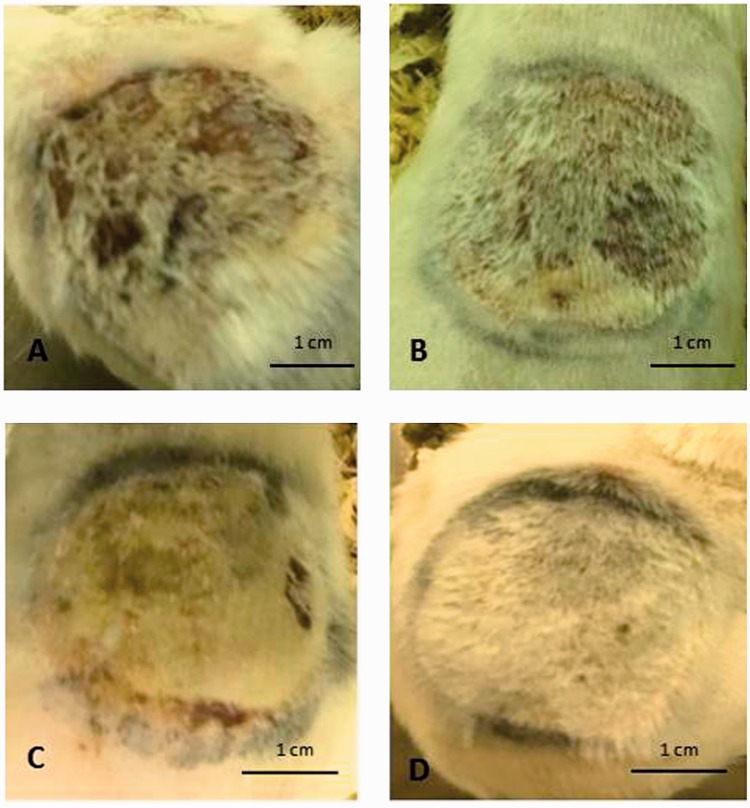
Digital images of experimentally-induced thermal wounds showed: (A) the intensity of the wound in sham-treated rats at day 7 following wound induction; (B) increased healing process at day 7 of 25 mg/kg/day naringenin treatment compared with sham-treated rats; (C) improved healing at day 7 of 50 mg/kg/day naringenin treatment compared with sham-treated rats; and (D) almost normal skin with full hair at day 7 of 100 mg/kg/day naringenin treatment (Figure 3).
Figure 3.
Representative digital images showing effect of oral naringenin treatment over 7 days in male Wistar rats with thermally-induced burn injury: (A) day 7 of sham treatment; (B) day 7 of 25 mg/kg/day naringenin treatment showing improved healing versus sham-treated group; (C) day 7 of 50 mg/kg/day naringenin treatment showing improved healing versus sham-treated group; and (D) day 7 of 100 mg/kg/day naringenin treatment showing almost normal skin with full hair
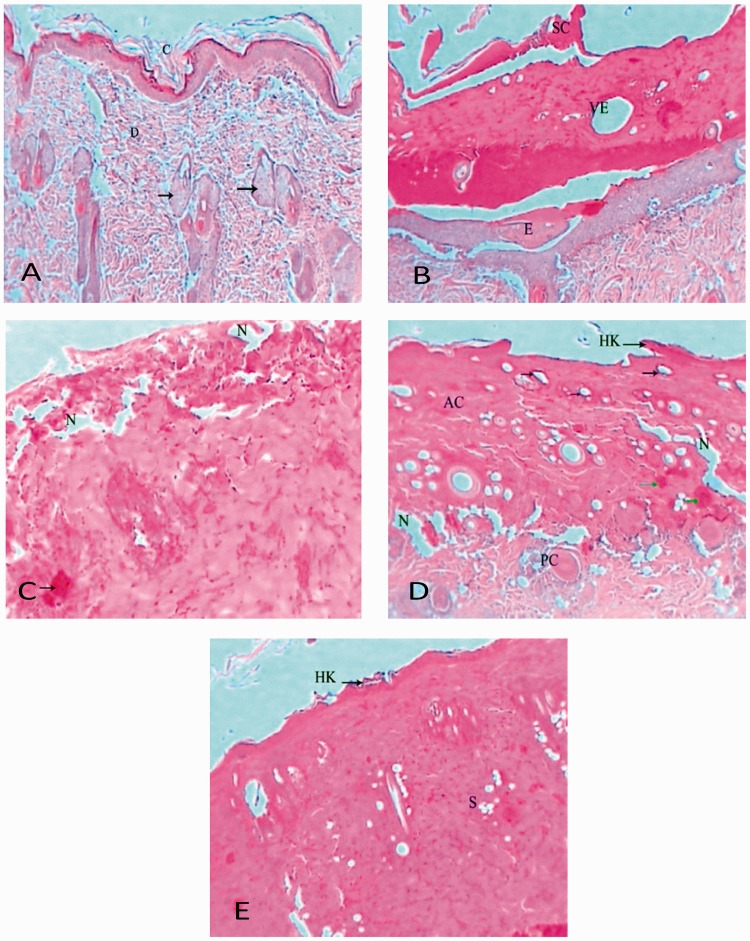
Normal (uninjured control) rat dermal thin skin sections showed normal architecture comprising: superficial stratum corneum with dead squamous cells composed mainly of keratin; stratum granulosum with 3–5 layers of flattened cells filled with keratin granules; stratum spinosum with synthesis of abundant keratin; and cellular and well-vascularized dermis with many sebaceous glands and hair follicles (Figure 4(A)). Dermal sections of rat skin from day 7 of the sham-treated group showed scar formation, acanthosis, hyperkeratosis, vascular ectasia, epidermis separation from the dermis, oedema beneath the dermis, bleeding and severe inflammation within the dermis, a wide scar, inflammation beneath the scar and granular parakeratosis (Figure 4(B)). Dermal sections of rat skin from day 7 of the 25 mg/kg/day oral naringenin treated group showed degenerated epidermis with inflammatory cells, ulceration, wide necrotic areas, bleeding foci, scattered inflammatory cells next to inflammatory foci, and granular parakeratosis (Figure 4(C)). Dermal sections of rat skin from day 7 of the 50 mg/kg/day oral naringenin treated group showed marked pathological changes represented by acanthosis, hyperkeratosis, vascular ectasia, necrosis, bleeding foci and inflammation in the epidermis and dermis, granular parakeratosis, degenerated sebaceous glands, wide necrotic areas and keratin cysts surrounded by infiltrative cells (Figure 4(D)). Dermal sections of rat skin from day 7 of the 100 mg/kg/day oral naringenin treated group showed some changes manifested by acanthotic epidermis, bleeding foci, focal spongiosis and minor hyperkeratosis, aggregations of infiltrative cells in the superficial area of the epidermis, vascular ectasia, granular parakeratosis and degenerated sebaceous gland (Figure 4(E)).
Figure 4.
Representative photomicrographs of dermal histopathological sections showing effect of oral naringenin treatment over 7 days in male Wistar rats with thermally-induced burn injury: (A) control uninjured rat; (B) day 7 of sham treatment; (C) day 7 of 25 mg/kg/day naringenin treatment; (D) day 7 of 50 mg/kg/day naringenin treatment; and (E) day 7 of 100 mg/kg/day naringenin treatment. AC, acanthosis; PC, Pacinian corpuscle; SE, sebaceous gland; VE, vascular ectasia; N, necrosis; HK, hyperkeratosis; E, oedema; D, dermis; C, stratum corneum; G, stratum granulosum; and S, stratum (haematoxylin & eosin stain, original magnification × 400)
Discussion
Metabolic alterations through oxidative processes are strongly associated with thermal injury of the skin, in which free radicals are produced via common biological pathways.12 Variation in the production of free radicals following use of antioxidants appears to play a significant role in the treatment of burn wounds.13 The present study demonstrated that thermal injury of the skin resulted in oxidative stress and increased serum proinflammatory biomarkers. The present study also indicated that naringenin was capable of reducing the effects of thermal injury, shown by significant reduction in skin TBARS levels, and increased GSH levels and antioxidant enzyme activities, compared with sham-treated controls. The burn-associated increased inflammatory markers, which play a pivotal role in thermal injury, were also decreased by naringenin treatment, and histological evaluation of the skin demonstrated a protective effect of naringenin against thermal injury.
Thermal injury is a painful process that causes damage in local tissue, mainly through skin oedema, and produces significant systemic effects involving different organs.14,15 Proinflammatory cytokines are released immediately, and for the first few days, following thermal injury, and are associated with increased synthesis of acute phase proteins.16,17 Experimental rodent models do not necessarily follow the exact clinical pathways observed in humans however, resulting in contradictory published reports. One study found that blood IL-6 and TNF-α concentrations were not markedly increased at 1–24 h following 20% total body surface area burn in mice.18 Rats with 20% total body surface area burns showed significant increases in blood IL-6 and TNF-α levels at days 3 and 7 post-burn.19 In another experimental rat thermal burn model, plasma inflammatory interleukins were markedly elevated during 7 post-burn days.20 Interleukins were found to play an important role in sepsis pathophysiology in patients with burns.21,22 In present study, a considerable elevation in serum TNF-α and interleukin levels was observed 8 days following 20% thermal injury (treatment day 7). In addition, other inflammatory biomarkers including serum NO, caspase-3, LTB4, PGE2 and NF-κB were markedly increased in the present thermal-burn wounded rats, and naringenin treatment significantly reduced the elevated levels of serum cytokines. These results suggest that naringenin has a preservative function against thermal-injury associated inflammation.
Several studies have demonstrated that inflammatory markers such as IL-1 and TNF-α are released by tissue macrophages and monocytes in response to any noxious event.23 Similarly, serum TNF-α levels were increased in burned animals in the present study, suggesting that amelioration of burn-associated oxidative injuries by naringenin may involve inhibition of a wide range of proinflammatory mediators generated by leukocytes and macrophages. The present data showed that oxidative stress was induced during 8 days following thermal-burn injury in rats. These results concur with other studies reporting increased lipid peroxidation within 2 weeks following burn injuries and decreased antioxidant enzyme activities associated with a disturbance in the microcirculation, exhaustion of the antioxidant system, and oxidative stress.24 Moreover, stimulated leukocytes are documented to produce free radicals, which in turn trigger the intensity of inflammation and lipid peroxidation.25,26
Phenolic compounds, including naringenin, are characterized by suppressive effects on cancerous cell proliferation, via inhibition of growth factor cascades, such as NF-κB.27 In addition, naringenin has been shown to attenuate the induction of NF-κB following inactivation of TNF-α, irrespective of antioxidant properties.28 In the present study, thermal-burn wounded rats treated with naringenin for 7 days showed markedly reduced lipid peroxidation and an enhanced antioxidant system in skin samples, supporting the antioxidative potential of naringenin against thermal injury-induced oxidative stress, and concurring with other published reports.29,30 Since proinflammatory mediators are prompted by free radicals, the use of antioxidants would be expected to suppress cytokine release. In the current investigation, naringenin showed strong antioxidant effects, lowering the deleterious generation of reactive oxygen species and inhibiting TNF-α. Naringenin has been demonstrated to inhibit superoxide anion and cytokine production in in vitro and in vivo studies,31 and also revealed to inhibit NF-κB activation in lipopolysaccharide-induced inflammation. The present author concludes that naringenin may have the ability to inhibit proinflammatory cytokines causing the attenuation of NF-κB activity.
Although the aims of current study were achieved, the results may be limited by several factors. First, the effect of topically applied naringenin was not investigated, due to lack of a suitable pharmaceutical laboratory to provide topical naringenin. Secondly, further investigations should use appropriate techniques to determine the molecular expression of inflammatory and oxidative stress biomarkers to support the present results. Thirdly, determination of the wound-associated expression of several factors that stimulate the angiogenesis, such as vascular endothelial growth factor, would add more impact to the study.
In conclusion, the present findings suggest that oral naringenin supplementation may be beneficial for burn patients, showing marked potential against thermal burn-induced oxidative stress in the present animal model. Further investigations are warranted to elucidate the potential mechanism of action of naringenin.
Acknowledgments
The author is thankful to Dr Khalid E Ibrahim for estimating the histopathological changes in skin tissues.
Declaration of Conflicting Interests
The author declared that there is no conflict of interest.
Funding
This study was supported by a grant from King Abdulaziz City for Science and Technology (KACST; Grant No. AT-34-205).
References
- 1.Sener G, Sehirli AO, Satiroglu H, et al. Melatonin improves oxidative organ damage in a rat model of thermal injury. Burns 2002; 28: 419–425. [DOI] [PubMed] [Google Scholar]
- 2.Madaghiele M, Demitri C, Sannino A, et al. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2014; 2: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillory AN, Clayton RP, Herndon DN, et al. Cardiovascular dysfunction following burn injury: what we have learned from rat and mouse models. Int J Mol Sci 2016; 17: 53–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham E. Effects of stress on cytokine production. Methods Achiev Exp Pathol 1991; 14: 45–62. [PubMed] [Google Scholar]
- 5.Sevaljevic L, Glibetic M, Poznanovic G, et al. Thermal injury-induced expression of acute-phase proteins in rat liver. Burns Incl Therm Inj 1988; 14: 280–286. [DOI] [PubMed] [Google Scholar]
- 6.Kataranovski M, Magic Z, Pejnovic N. Early inflammatory cytokine and acute phase protein response under the stress of thermal injury in rats. Physiol Res 1999; 48: 473–482. [PubMed] [Google Scholar]
- 7.Dahiya P. Burns as a model of SIRS. Front Biosci (Landmark Ed) 2009; 14: 4962–4967. [DOI] [PubMed] [Google Scholar]
- 8.Agay D, Andriollo-Sanchez M, Claeyssen R, et al. Interleukin-6, TNF-alpha and interleukin-1 beta levels in blood and tissue in severely burned rats. Eur Cytokine Netw 2008; 19: 1–7. [DOI] [PubMed] [Google Scholar]
- 9.Patel K, Singh GK, Patel DK. A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med 2014. doi: 10.1007/s11655-014-1960-x. [DOI] [PubMed] [Google Scholar]
- 10.Park HY, Kim GY, Choi YH. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-κB and inhibiting mitogen-activated protein kinases. Int J Mol Med 2012; 30: 204–210. [DOI] [PubMed] [Google Scholar]
- 11.Walker HL, Mason AD., Jr A standard animal burn. J Trauma 1968; 8: 1049–1051. [DOI] [PubMed] [Google Scholar]
- 12.Ward PA, Till GO. Pathophysiologic events related to thermal injury of skin. J Trauma 1990; 30: S75–S79. [DOI] [PubMed] [Google Scholar]
- 13.Al-Jawad FH, Sahib AS, Al-Kaisy AA. Role of antioxidants in the treatment of burn lesions. Ann Burns Fire Disasters 2008; 21: 186–191. [PMC free article] [PubMed] [Google Scholar]
- 14.Yamashita Y, Jeschke MG, Wolf SE. Differential expression of hepatocyte growth factor in liver, kidney, lung, and spleen following burn in rats. Cytokine 2000; 12: 1293–1298. [DOI] [PubMed] [Google Scholar]
- 15.Hilton G. Emergency. Thermal burns. Am J Nurs 2001; 101: 32–34. [DOI] [PubMed] [Google Scholar]
- 16.Struzyna J, Pojda Z, Braun B, et al. Serum cytokine levels (IL-4, IL-6, IL-8, G-CSF, GM-CSF) in burned patients. Burns 1995; 21: 437–440. [DOI] [PubMed] [Google Scholar]
- 17.Dehne MG, Sablotzki A, Hoffmann A, et al. Alterations of acute phase reaction and cytokine production in patients following severe burn injury. Burns 2002; 28: 535–542. [DOI] [PubMed] [Google Scholar]
- 18.Kawakami M, Kaneko N, Anada H, et al. Measurement of interleukin-6, interleukin-10, and tumor necrosis factor-alpha levels in tissues and plasma after thermal injury in mice. Surgery 1997; 121: 440–448. [DOI] [PubMed] [Google Scholar]
- 19.Deveci M, Eski M, Sengezer M, et al. Effects of cerium nitrate bathing and prompt burn wound excision on IL-6 and TNF-alpha levels in burned rats. Burns 2000; 26: 41–45. [DOI] [PubMed] [Google Scholar]
- 20.Caldwell FT, Jr., Graves DB, Wallace BH. The effect of indomethacin on the cytokine cascade and body temperature following burn injury in rats. Burns 1999; 25: 283–294. [DOI] [PubMed] [Google Scholar]
- 21.Drost AC, Burleson DG, Cioffi WG, Jr., et al. Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. J Trauma 1993; 35: 335–339. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Endo S, Inada K. Plasma cytokine levels in patients with severe burn injury–with reference to the relationship between infection and prognosis. Burns 1996; 22: 587–593. [DOI] [PubMed] [Google Scholar]
- 23.Singh RP, Agarwal R. Flavonoid antioxidant silymarin and skin cancer. Antioxid Redox Signal 2002; 4: 655–663. [DOI] [PubMed] [Google Scholar]
- 24.Dubinina EE. The role of reactive oxygen species as signal molecules in tissue metabolism in oxidative stress. Vopr Med Khim 2001; 47: 561–581[in Russian, English abstract]. [PubMed] [Google Scholar]
- 25.Lazarenko VA, Lyashev YD, Shevchenko NI. Effect of a synthetic indolicidin analogue on lipid peroxidation in thermal burns. Bull Exp Biol Med 2014; 157: 447–449. [DOI] [PubMed] [Google Scholar]
- 26.Bekyarova G, Tancheva S, Hristova M. Protective effect of melatonin against oxidative hepatic injury after experimental thermal trauma. Methods Find Exp Clin Pharmacol 2009; 31: 11–14. [DOI] [PubMed] [Google Scholar]
- 27.Xu C, Chen J, Zhang J, et al. Naringenin inhibits angiotensin II-induced vascular smooth muscle cells proliferation and migration and decreases neointimal hyperplasia in balloon injured rat carotid arteries through suppressing oxidative stress. Biol Pharm Bull 2013; 36: 1549–1555. [DOI] [PubMed] [Google Scholar]
- 28.Pinho-Ribeiro FA, Zarpelon AC, Mizokami SS, et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J Nutr Biochem 2016; 33: 8–14. [DOI] [PubMed] [Google Scholar]
- 29.Ali R, Shahid A, Ali N, et al. Amelioration of Benzo[a]pyrene-induced oxidative stress and pulmonary toxicity by Naringenin in Wistar rats: A plausible role of COX-2 and NF-κB. Hum Exp Toxicol 2016. doi: 10.1177/0960327116650009. [DOI] [PubMed] [Google Scholar]
- 30.Roy S, Ahmed F, Banerjee S, et al. Naringenin ameliorates streptozotocin-induced diabetic rat renal impairment by downregulation of TGF-β1 and IL-1 via modulation of oxidative stress correlates with decreased apoptotic events. Pharm Biol 2016; 54: 1616–1627. [DOI] [PubMed] [Google Scholar]
- 31.Pinho-Ribeiro FA, Zarpelon AC, Fattori V, et al. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016; 105: 508–519. [DOI] [PubMed] [Google Scholar]