Abstract
Objective
Over the past decade, myocardial triglyceride content has become an accepted biomarker for chronic metabolic and cardiac disease. The purpose of this study was to use proton (hydrogen 1)-magnetic resonance spectroscopy (1H-MRS) at 3Tesla (3 T) field strength to assess potential gender-related differences in myocardial triglyceride content in healthy individuals.
Methods
Cardiac MR imaging was performed to enable accurate voxel placement and obtain functional and morphological information. Double triggered (i.e., ECG and respiratory motion gating) 1H-MRS was used to quantify myocardial triglyceride levels for each gender. Two-sample t-test and Mann-Whitney U-test were used for statistical analyses.
Results
In total, 40 healthy volunteers (22 male, 18 female; aged >18 years and age matched) were included in the study. Median myocardial triglyceride content was 0.28% (interquartile range [IQR] 0.17–0.42%) in male and 0.24% (IQR 0.14–0.45%) in female participants, and no statistically significant difference was observed between the genders. Furthermore, no gender-specific difference in ejection fraction was observed, although on average, male participants presented with a higher mean ± SD left ventricular mass (136.3 ± 25.2 g) than female participants (103.9 ± 16.1 g).
Conclusions
The study showed that 1H-MRS is a capable, noninvasive tool for acquisition of myocardial triglyceride metabolites. Myocardial triglyceride concentration was shown to be unrelated to gender in this group of healthy volunteers.
Keywords: Cardiac, magnetic resonance imaging, 1H-Magnetic resonance spectroscopy (1H-MRS), myocardium, triglycerides, metabolism, gender
Introduction
A number of studies have demonstrated the potential of proton (hydrogen 1)-magnetic resonance spectroscopy (1H-MRS) for the noninvasive assessment of myocardial triglyceride content.1,2 The availability of high field (3 T) magnetic resonance (MR) systems in clinical practice has facilitated precise measurements of myocardial tissue metabolites, as signal intensity is augmented with field strength.3,4
The use of double triggered (i.e., electrocardiogram [ECG] and respiratory motion gating) 1H-MRS increases the potential clinical application of the technique, particularly for cardiac examinations.5 Indeed, a strong correlation between in vivo 1H-MRS and ex vivo myocardial triglyceride measurements has been demonstrated.6
Over the past decade, in vivo evaluation of myocardial triglyceride content has become an accepted biomarker for chronic metabolic and cardiac disease.5,7,8 Body mass index (BMI), hepatic triglyceride content, visceral fat and metabolic disease have been shown to be related to myocardial triglyceride concentration.8 However, little information is available about possible differences in myocardial triglyceride concentrations between men and women.9
The aim of this present study was to analyse the concentration of myocardial triglyceride metabolites in healthy volunteers, to assess any potential gender difference.
Subjects and methods
Study population
Male and female healthy volunteers ≥ 18 years of age were included in this study. Exclusion criteria included history of cardiac disease, metabolic disease (in particular diabetes mellitus), presence of implanted devices possibly limiting cardiac imaging and spectroscopy, pregnancy and fear of confined spaces.
The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants and the study protocol was approved by the ethics committee of the University of Würzburg, Würzburg, Germany.
Cardiac imaging and spectroscopy
Double-triggered high-field cardiac magnetic resonance imaging (MRI) and 1H-MRS were performed on all participants using a 3 T magnet (MAGNETOM Trio®, Siemens AG Healthcare Sector, Erlangen, Germany) equipped with a 12-channel phased array coil. Participants were scanned in the supine position. All volunteers underwent a fasting period of ≥2 h prior to imaging. No intravenous (i.v.) contrast agent or any other medication was administered to the study cohort.
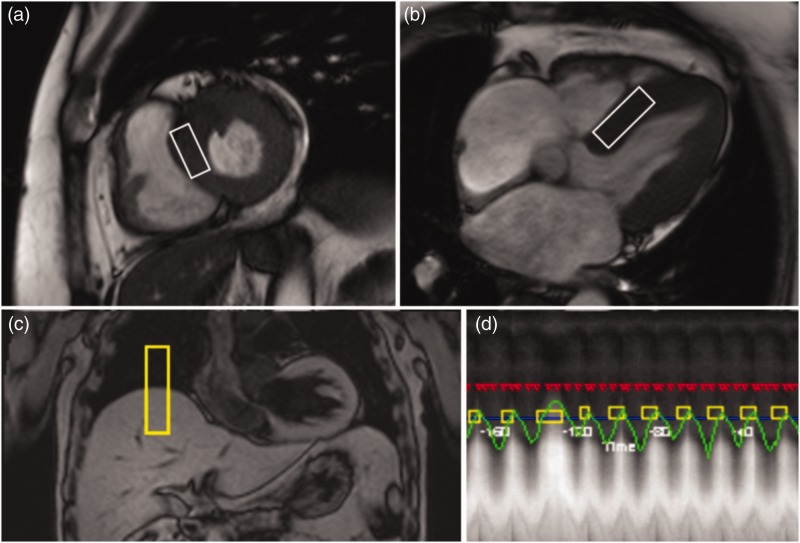
To facilitate precise voxel placement in the desired cardiac cycle, as well as evaluation of left ventricular (LV) mass and LV function (expressed as ejection fraction) parameters, conventional dynamic steady-state free precession cine images in the short-axis and four-chamber views were acquired prior to cardiac 1H-MRS. Double-triggered cardiac single voxel 1H-MR spectra (voxel size 6 ml) were obtained from the interventricular septum as described previously,5 thus avoiding contamination by epicardial fat (Figure 1a, 1b). Respiratory motion gating (navigator positioned on the lung-liver interface of the right hemidiaphragma) allowed unrestricted breathing during spectroscopic data acquisition (Figure 1c, 1d). Single voxel 1H-MRS was performed at end-systole and end-expiration using a point-resolved spectroscopy sequence with an echo time of 35 ms and a repetition time of at least one heartbeat. A set of 32 single data acquisitions was averaged for each spectrum.
Figure 1.
Myocardial voxel localization in the interventricular septum for 1H-MRS acquisition, guided by the short-axis (a) and four-chamber (b) views. Respiratory motion gating navigator positioned on the lung-liver interface of the right hemidiaphragma (c) allows unrestricted breathing during spectroscopic data acquisition (d).
Spectral analysis
Data from 1H-MRS were analysed using commercially available software (Spectroscopy Evaluation®, Siemens AG Healthcare Sector, Erlangen, Germany). For each calculation two spectra (with and without water suppression) were obtained from identical voxel positions. The water signal from the non water-suppressed spectra was used as an internal reference for relative quantification of myocardial triglyceride resonance. Chemical shift of the myocardial triglyceride metabolites on 1H-MRS was measured in parts per million (ppm).
Determination of resonance signals was supported by previous experience at our centre. The water signal was set to a ∂-value of 4.7 ppm, consecutively invoking protons in methylene groups to appear at 1.3 ppm, as well as protons in methyl groups at 0.9 ppm. The areas under the curve (AUC) for both triglyceride peaks were summated for the calculation of total myocardial triglyceride content. In accordance with previous studies, relative myocardial triglyceride was calculated by expressing the myocardial metabolite signal as a percentage of the unsuppressed water signal (myocardial triglyceride/water resonance ratio).5,10–12
Statistical analyses
All statistical analyses were performed using statistical software (IBM SPSS® Statistics for Mac, Version 21.0, Armonk, NY, USA). Kolmogorov–Smirnov test was used for evaluation of normal distribution. Mann-Whitney U-test was used to determine potential gender differences in myocardial triglyceride content. Two sample t-test was used to determine potential gender differences in age, BMI, LV mass and ejection fraction. For myocardial triglyceride content, data were presented as median, interquartile range (IQR) and full range; for age, BMI, LV mass and ejection fraction, data were presented as mean ± SD. A P-value of < 0.05 was considered statistically significant.
Results
In total, 40 healthy volunteers (22 men and 18 women) were included in the study. The characteristics of all participating individuals, assorted according to gender are shown in Table 1. All participants successfully underwent morphological and spectroscopic imaging. As expected, parameters of LV function (i.e., ejection fraction) (men, 63.2 ± 7.0% [range 50–78%]; women, 65.2 ± 5.2% [range 56–76%]) and LV mass (men, 136.3 ± 25.2 g [range 70–173 g]; women, 103.9 ± 16.1 g [range 79–134 g]) fell within well-established standard values.7,13
Table 1.
Characteristics of the healthy volunteers in this study assessing potential gender-related differences in myocardial triglyceride content using 1H-Magnetic Resonance Spectroscopy.
| Characteristic | Male volunteers | Female volunteers | Statistical significance |
|---|---|---|---|
| Patients, n | 22 | 18 | |
| Age, mean ± SD, years | 31.9 ± 7.9 | 31.6 ± 11.0 | n.s. |
| BMI, mean ± SD, kg/m2 | 22.9 ± 3.4 | 21.5 ± 3.6 | n.s. |
| Ejection fraction, mean ± SD, % | 63.3 ± 7.0 | 65.2 ± 5.2 | n.s. |
| LV mass, mean ± SD, g | 136.3 ± 25.2 | 103.9 ± 16.1 | P < 0.01 |
| Myocardial triglyceride, median, % | 0.28 | 0.24 | n.s. |
| (IQR) | (0.17–0.42) | (0.14–0.45) | |
| (Range) | (0.10–2.30) | (0.10–2.10) |
SD, standard deviation; BMI, body mass index; LV, left ventricle; IQR, interquartile range; n.s, not statistically significant
Both gender groups presented with similar mean ± SD age and BMI values. The BMI ranged between 18.5–33.5 kg/m2 in men and 18.1–31.8 kg/m2 in women. Comparing male with female volunteers, no gender-specific differences in ejection fraction were observed while on average, men presented with a higher LV mass than women (p < 0.01).
Spectroscopic measurements of myocardial triglyceride content
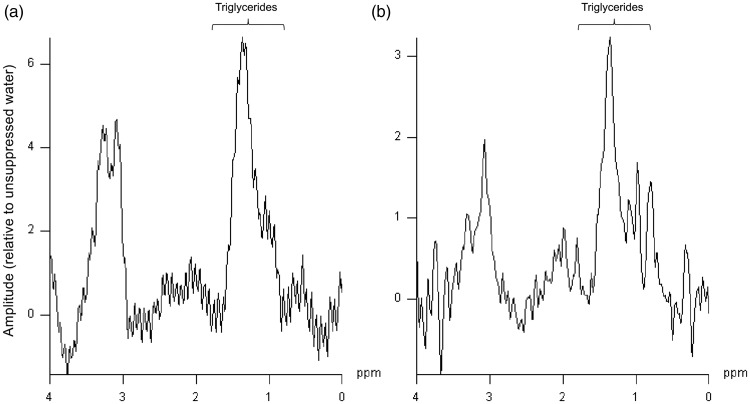
Myocardial 1H-MRS was successfully performed in all participants. Figure 2 shows typical examples of myocardial 1H-MR spectra from a 22-year-old man (Figure 2a) and a 24-year-old woman (Figure 2b) with normal BMI values.
Figure 2.
Typical examples of proton (hydrogen 1)-magnetic resonance spectroscopy (1H-MRS) from healthy 22-year-old male (a) and 24-year-old female (b) volunteers. Both volunteers showed similar myocardial triglyceride content.
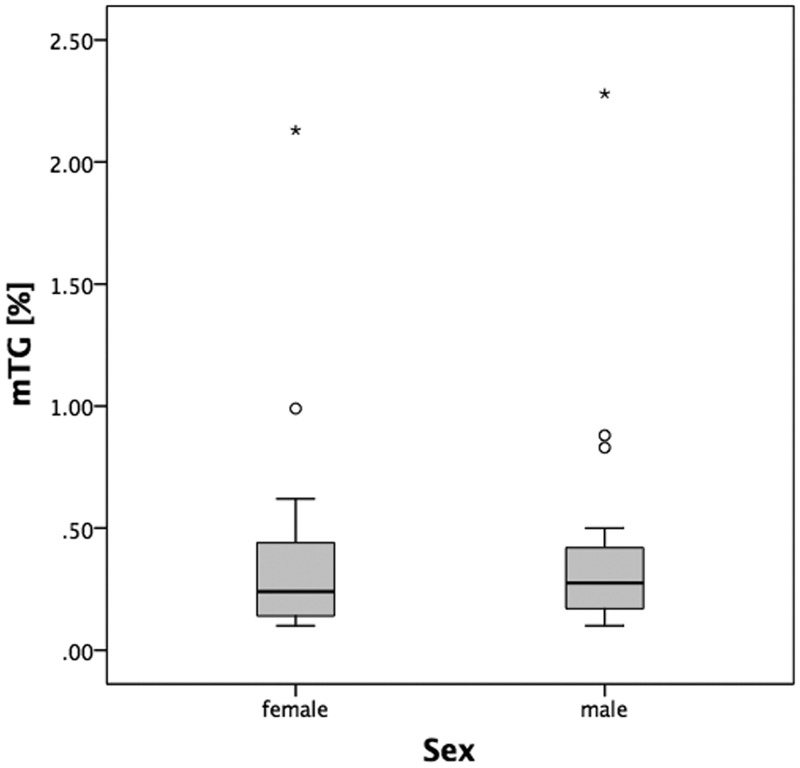
The gender-independent averaged myocardial triglyceride values were mean ± SD, 0.41 ± 0.47%; median 0.25% (IQR 0.16–0.43%). There was no statistically significant difference in myocardial triglyceride content between male (0.28%; IQR 0.17–0.42% [range 0.1–2.3%]) and female volunteers (0.24%; IQR 0.14–0.45% [range 0.1–2.1%]) (Table 1 and Figure 3).
Figure 3.
Myocardial triglyceride (mTG) content (expressed as mTG/water resonance ratio in %) was similar in both genders.
Discussion
The present study used 1H-MRS at 3 T to determine potential gender-related differences in the myocardial lipid content in healthy individuals. Normal relative myocardial triglyceride values, as well as concentration ranges, were assessed for both genders. The results from this study showed that myocardial triglyceride concentration was not related to gender.
The voxel size of 20 × 15 × 20 mm3 enabled an accurate voxel placement in the interventricular septum. As described previously, this voxel position is associated with minimal risk of acquiring false-positive results that might arise from lipid contamination of the voxel by epicardial fat.5,7 The method itself, as well as the clinical application of 1H-MRS, has made substantial progress throughout the past decade. The benefits of respiratory navigator technique, which significantly improves the reproducibility of in vivo myocardial triglyceride determination using a 1.5 T scanner, have been described.5
The role and influence of myocardial triglyceride in the human body and especially its impact on heart function have been studied intensively by cardiac 1H-MRS.7,8,11,12,14 For example, myocardial steatosis was found to be an independent predictor of diastolic function in humans.14 Moreover, pathological conditions, such as obesity and diabetes mellitus type II, have been shown to be related to myocardial triglyceride content.8,14 However, few studies have examined the influence of gender on myocardial triglyceride distribution in humans.9
Although there were a number of individuals from both sexes with myocardial triglyceride values in excess of 2%, overall, the gender-independent mean myocardial triglyceride values obtained within this study population (0.41 ± 0.47%) were in accordance with previous data.8,10 For example, in one study by McGavock et al., the myocardial triglyceride levels in lean subjects (mean BMI 23 kg/m2) was found to be mean ± SD 0.46% ± 0.30.8 However, in another study by a different group, the mean gender-dependent myocardial triglyceride concentrations (i.e., 6.7% in men and 12.3% in women) were much higher than values observed in this present study.9 Nevertheless, by comparison with the present study which used 1H-MRS at 3 T, the previous study acquired data at a lower field strength of 1.5 T, which may have influenced the measurement of myocardial triglyceride values.9 Perhaps this could in part be due to a reduced spectral resolution, as well as a lower signal-to-noise ratio at the low field strength.
The present study had some limitations. Although not statistically significant, a minor imbalance in the average BMI was observed in the study population, with a higher mean BMI being observed in the male group. As myocardial triglyceride content is thought to be related to BMI, this may well have influenced the levels in this group. Therefore, by comparison with the findings from this present study, accurately BMI-matched gender cohorts may well show a lower myocardial triglyceride content in male subjects. Further research is required to explore this hypothesis.
Myocardial 1H single voxel spectra were obtained from the interventricular septum only. As a result, data from the present study may not be representative for the entire left ventricle. Furthermore, one must be aware that, following the approach of most former 1H-MRS studies, the analyses of cardiac metabolism within this study are semiquantitative, and rely on the determination of triglyceride/water resonance ratios.1,2,6 Absolute quantification of myocardial lipid concentration would need myocardial biopsy, which is not without risk. Nevertheless, a high specificity of in vivo intramyocardial triglyceride measurement in the human heart has been demonstrated.6 Finally, please note that individual subjects within this study cohort also serve as healthy controls in other 1H-MRS studies undertaken by our group. Consequently, data obtained from study subjects may be evaluated with respect to other issues in additional 1H-MRS studies.
In conclusion, the study confirmed that 1H-MRS is a reliable, noninvasive, and sensitive tool for the measurement of myocardial lipids (which have already been established as important biomarkers of myocardial metabolism). Using 1H-MRS, the results from this study showed that myocardial triglyceride concentration was independent of gender in this group of healthy volunteers. The gender-specific myocardial triglyceride values obtained in this study may be valuable as reference values in future studies evaluating metabolic disorders using magnetic resonance spectroscopy, and also may be useful in routine clinical practice.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Reingold JS, McGavock JM, Kaka S, et al. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab 2005; 289: E935–E939. [DOI] [PubMed] [Google Scholar]
- 2.den Hollander JA, Evanochko WT, et al. Observation of cardiac lipids in humans by localized 1H magnetic resonance spectroscopic imaging. Magn Reson Med 1994; 32: 175–180. [DOI] [PubMed] [Google Scholar]
- 3.Abgragam A. Principles of Nuclear Magnetism, Oxford: Clarendon Press, 1961. [Google Scholar]
- 4.Vlaardingerbroek MT. Magnetic Resonance Imaging: Theory and Practice, 3rd ed Berlin, Heidelberg, NewYork: Springer-Verlag, 2003. [Google Scholar]
- 5.van der Meer RW, Doornbos J, Kozerke S, et al. Metabolic imaging of myocardial triglyceride content: reproducibility of 1H MR spectroscopy with respiratory navigator gating in volunteers. Radiology 2007; 245: 251–257. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor RD, Xu J, Ewald GA, et al. Intramyocardial triglyceride quantification by magnetic resonance spectroscopy: in vivo and ex vivo correlation in human subjects. Magn Reson Med 2011; 65: 1234–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczepaniak LS, Dobbins RL, Metzger GJ, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med 2003; 49: 417–423. [DOI] [PubMed] [Google Scholar]
- 8.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007; 116: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Yang L, Yang J, et al. Gender-specific normal levels of myocardial metabolites determined by localized 1H-magnetic resonance spectroscopy. J Int Med Res 2012; 40: 1507–1512. [DOI] [PubMed] [Google Scholar]
- 10.Petritsch B, Köstler H, Machann W, et al. Non-invasive determination of myocardial lipid content in Fabry disease by 1H-MR Spectroscopy. Rofo 2012; 184: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 11.Beer M. Cardiac spectroscopy: techniques, indications and clinical results. Eur Radiol 2004; 14: 1034–1047. [DOI] [PubMed] [Google Scholar]
- 12.van der Meer RW, Rijzewijk LJ, Diamant M, et al. The ageing male heart: myocardial triglyceride content as independent predictor of diastolic function. Eur Heart J 2008; 29: 1516–1522. [DOI] [PubMed] [Google Scholar]
- 13.Riley-Hagan M, Peshock RM, Stray-Gundersen J, et al. Left ventricular dimensions and mass using magnetic resonance imaging in female endurance athletes. Am J Cardiol 1992; 69: 1067–1074. [DOI] [PubMed] [Google Scholar]
- 14.Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008; 52: 1793–1799. [DOI] [PubMed] [Google Scholar]