Abstract
Transcranial direct current stimulation (tDCS) can alter cortical excitability, and has been effective in treating some neurological disorders. This case report describes the use of tDCS in a 13-year-old female who developed bilateral hearing impairment after brainstem encephalitis when she was 6 years old. Her auditory function was more impaired in her right ear than her left. Anodal stimulation (1 mA) was applied for 10 min to the left auditory cortex once per day for 4 consecutive days to improve her right ear speech discrimination score. Sustained and significant improvement in maximum speech discrimination was observed after the four tDCS treatments. To our knowledge, this is the first case report of improvement in speech discrimination after anodal stimulation of the auditory cortex. These results encourage further studies investigating the beneficial effects of tDCS in patients with hearing impairments.
Keywords: Transcranial direct current stimulation, auditory cortex, hearing impairment, speech discrimination, encephalitis
Introduction
Retrocochlear hearing loss can occur following damage in the brainstem and temporal lobe due to a stroke, brain tumour or auditory nerve disease. Patients with retrocochlear hearing loss characteristically perform poorly on speech discrimination compared with pure tone audiometry.1 Hearing aids and cochlear implants can improve communication in patients with hearing impairments;2 however, recovery of speech discrimination after retrocochlear hearing loss is challenging, and new treatments are needed.
Transcranial direct current stimulation (tDCS) is a noninvasive brain stimulation technique that can alter the excitability of the human cortex.3 Anodal and cathodal tDCS increase and decrease cortical excitability respectively,4 and by altering cortical excitability, motor learning acquisition and retention can be enhanced.5,6 We report a patient with bilateral hearing impairment after brainstem encephalitis, whose speech discrimination was significantly improved after anodal tDCS.
Case report
A 13-year-old Japanese girl with no congenital disorders and no personal or family history of hearing impairment developed brainstem encephalitis at 6 years old. She recovered without paralysis or movement disorder, but her bilateral hearing function was impaired. Magnetic resonance imaging showed no abnormalities, but an auditory brainstem response test showed that I-V waves were not recognized after 105 dB stimulation of either ear. Pure tone audiometry showed a 40 dB hearing threshold in the right ear and 20 dB hearing threshold in the left ear. Maximum speech discrimination scores were ∼20% for the right ear and ∼50% for the left ear. The patient did not use hearing aids or have cochlear implants.
Anodal tDCS was performed to improve the patient’s right ear auditory function. The protocol was approved by the Ethics Committee of Tohoku University Hospital (reference no. 2013-2-40), and the patient and her parents provided written informed consent. A three-dimensional navigation system (Brainsight™, Rogue Research Inc, Montreal, QC, Canada) was used to help localize specific anatomic structures in the brain to pinpoint the patient’s auditory cortex. The anode was placed over the left auditory cortex.
Stimulation (Eldith DC Stimulator™, neuroConn GmbH, Ilmenau, Germany) was applied for 10 min (1 mA) with the anode positioned over the left auditory cortex and the cathode positioned over the contralateral supraorbital region. The patient was instructed to rest during tDCS. Anodal stimulation of the left auditory cortex was performed once a day for 4 consecutive days. Pure tone audiometry and speech audiometry tests were given to the patient three times (before tDCS, immediately after the first stimulation and 4 days after the last tDCS session). Pure tone audiometry materials were used to define the average hearing threshold, as in previous studies.7,8 Japanese monosyllabic word lists, which have been used in previous studies,9,10 were used for the speech discrimination tests. Maximum speech discrimination was assessed using analysis of variance (ANOVA) with repeated measures. Posthoc analysis was performed with Bonferroni’s correction.
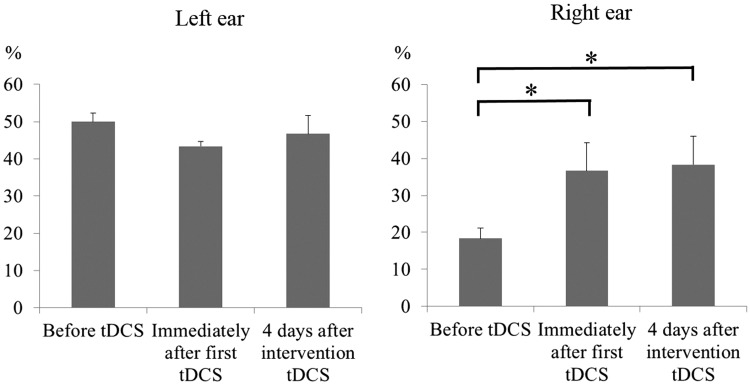
There were no adverse side-effects during the course of the study. Figure 1 shows the maximum speech discrimination after stimulation. A repeated-measures ANOVA for maximum speech discrimination in the right ear showed a significant effect of time (F [2,4] = 13.3; P = 0.017). Posthoc testing revealed that speech discrimination in the right ear improved immediately after a single stimulation (P = 0.039). This effect was still evident 4 days after the fourth tDCS treatment (P = 0.029; Figure 1). In contrast, there was no significant difference in maximum speech discrimination in the left ear. There were no significant changes in pure tone audiometry for either ear (right ear: mean 40.0 ± 2.2 SD dB before tDCS, 37.5 ±1.3 dB immediately after the first session, 42.1 ± 5.0 dB 4 days after tDCS; left ear: 22.1 ± 1.4 dB before tDCS, 20.9 ± 3.6 dB immediately after the first session, 23.8 ±4.3 dB 4 days after tDCS).
Figure 1.
Maximum speech discrimination tests in a 13-year-old Japanese girl with bilateral hearing impairment after brainstem encephalitis conducted after anodal transcranial direct current stimulation (tDCS) of her left auditory cortex, 1 mA for 10 min once a day for 4 days.
*P < 0.05; repeated-measures analysis of variance.
Discussion
Anodal tDCS delivered to the primary auditory cortex on 4 consecutive days improved speech discrimination in a patient with hearing impairment. Although studies have suggested that anodal tDCS can improve random gap detection test results in healthy subjects,11 and reduce tinnitus intensity,12 to our knowledge there have been no reports of using tDCS to improve speech audiometry in patients with hearing impairments.
When anodal tDCS was administered to the left auditory area, there was no improvement in hearing in the left ear; the dominant acoustic tract from the left auditory area was to the right ear, where significant improvement in hearing was observed. This finding supports the hypothesis that increased auditory cortex excitability elicited by anodal tDCS might improve auditory function in the contralateral ear. However, anodal tDCS improved the patient’s speech discrimination but not their pure tone audiometry scores. As a putative mechanism, anodal tDCS over the auditory cortex might improve speech discrimination related to language function by enhancing activity in Wernicke’s area adjacent to the auditory cortex, which is thought to play a role in understanding speech.13,14 The improvement of auditory function after anodal tDCS might be consistent with a study in which anodal tDCS applied over the bilateral auditory cortex improved gap detection task performance.11 Conversely, anodal tDCS over the right auditory cortex worsened frequency discrimination and auditory pitch learning in healthy subjects.15,16 Heimrath et al.17 reported that anodal tDCS over the auditory cortex worsened the gap detection threshold in healthy control subjects. Although these findings of tDCS effects are controversial, this may be due to the use of different stimulation protocols and evaluation parameters, and the response to anodal tDCS over the auditory cortex may depend on an individual’s auditory ability. Future studies should question whether the effects of anodal tDCS over the auditory cortex vary according to the severity of the patient’s hearing impairment. Such evaluations might also demonstrate speech discrimination scores improved in the present case because the baseline score was worse.
A further important finding in this study is that the improvement in speech discrimination lasted for 4 days after the final anodal tDCS intervention. Auditory function testing immediately after tDCS may have induced a learning effect. To evaluate the mechanism of any learning effect induced by tDCS over the auditory cortex in more detail, future studies should administer the auditory task during and immediately after tDCS.
This patient’s results should be considered in the context of two major limitations. First, we did not compare anodal tDCS with sham stimulation, and second, electrophysiological examinations such as auditory brainstem response and functional neuroimaging are needed, to clarify how tDCS led to an improved hearing function.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported by JSPS Grant-in-Aid for Scientific Research (no. 25242054).
References
- 1.Starr A, Picton TW, Sininger Y, et al. Auditory neuropathy. Brain 1996; 119(Pt 3): 741–753. [DOI] [PubMed] [Google Scholar]
- 2.Cardon G, Sharma A. Central auditory maturation and behavioral outcome in children with auditory neuropathy spectrum disorder who use cochlear implants. Int J Audiol 2013; 52: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeuchi N, Izumi S. Noninvasive brain stimulation for motor recovery after stroke: mechanisms and future views. Stroke Res Treat 2012; 2012. Article ID: 584727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000; 527(Pt 3): 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saucedo Marquez CM, Zhang X, Swinnen SP, et al. Task-specific effect of transcranial direct current stimulation on motor learning. Front Hum Neurosci 2013; 7: 333–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vollmann H, Conde V, Sewerin S, et al. Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimul 2013; 6: 101–107. [DOI] [PubMed] [Google Scholar]
- 7.Sugiura S, Yasue M, Sakurai T, et al. Effect of cerumen impaction on hearing and cognitive functions in Japanese older adults with cognitive impairment. Geriatr Gerontol Int 2014; 14(Suppl. 2): 56–61. [DOI] [PubMed] [Google Scholar]
- 8.Ashitani M, Ueno C, Doi T, et al. Clinical features of functional hearing loss with inattention problem in Japanese children. Int J Pediatr Otorthinolaryngol 2011; 75: 1431–1435. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda S, Fukushima K, Toida N, et al. Monosyllable speech perception of Japanese hearing aid users with prelingual hearing loss: implications for surgical indication of cochlear implant. Int J Pediatr Otorhinolaryngol 2003; 67: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 10.Hosoi H, Tsuta Y, Murata K, et al. Suggestion audiometry for non-organic hearing loss (pseudohypoacusis) in children. Int J Pediatr Otorhinolaryngol 1999; 47: 11–21. [DOI] [PubMed] [Google Scholar]
- 11.Ladeira A, Fregni F, Campanha C, et al. Polarity-dependent transcranial direct current stimulation effects on central auditory processing. PLoS One 2011; 6: e25399–e25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garin P, Gilain C, Van Damme JP, et al. Short- and long-lasting tinnitus relief induced by transcranial direct current stimulation. J Neurol 2011; 258: 1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin L, Bear D. Time to understand. A case study of word deafness with reference to the role of time in auditory comprehension. Brain 1974; 97: 373–384. [DOI] [PubMed] [Google Scholar]
- 14.Cappa S, Cavallotti G, Vignolo LA. Phonemic and lexical errors in fluent aphasia: correlation with lesion site. Neuropsychologia 1981; 19: 171–177. [DOI] [PubMed] [Google Scholar]
- 15.Tang MF, Hammond GR. Anodal transcranial direct current stimulation over auditory cortex degrades frequency discrimination by affecting temporal, but not place, coding. Eur J Neurosci 2013; 38: 2802–2811. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita R, Andoh J, Zatorre RJ. Polarity-specific transcranial direct current stimulation disrupts auditory pitch learning. Front Neurosci 2015; 9: 174–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heimrath K, Kuehne M, Heinze HJ, et al. Transcranial direct current stimulation (tDCS) traces the predominance of the left auditory cortex for processing of rapidly changing acoustic information. Neuroscience 2014; 261: 68–73. [DOI] [PubMed] [Google Scholar]