Abstract
Objective
To compare the efficacy and safety of two different doses of celecoxib and diclofenac in the treatment of Norwegian patients with ankylosing spondylitis.
Methods
In this 12-week, double-blind, non-inferiority trial patients were randomized to 200 mg once daily (qd) celecoxib, 400 mg qd celecoxib, or 50 mg three times daily (tid) diclofenac. The primary objective compared patients’ assessments of Global Pain Intensity, measured on a visual analogue scale.
Results
A total of 330 patients were randomized (200 mg celecoxib, n = 107; 400 mg celecoxib, n = 108; diclofenac, n = 115). Least squares mean changes in Global Pain Intensity at 12 weeks were −25.8 mm, −30.6 mm and −28.2 mm, respectively. Both celecoxib treatment groups were non-inferior to diclofenac. More patients in the 400 mg celecoxib group met the Assessments in Ankylosing Spondylitis 20 responder criteria at Week 12 (60.2%) than in the celecoxib 200 mg (51.4%) and the diclofenac 50 mg (57.4%) groups. Adverse events were mild-to-moderate in severity, with dyspepsia and diarrhoea the most commonly reported.
Conclusions
Celecoxib and diclofenac both provided pain reduction, in addition to improvements in disease activity and functional capacity, in patients with ankylosing spondylitis.
Keywords: COX-2 inhibitors, ankylosing spondylitis, non-steroidal anti-inflammatory drugs, celecoxib
Introduction
Ankylosing spondylitis (AS) is a chronic, debilitating form of arthritis primarily affecting the spine; it is characterized by axial skeletal stiffness and inflammation at the attachment sites of tendons and ligaments to bone. Pain, fatigue, limited mobility and stiffness are among the multiple health issues that are common in patients with AS. Over time, the affected bones can become fused.1 Although the precise cause is not known, the human leukocyte antigen B27 (HLA-B27) gene marker is involved in around 90% of cases in Caucasian patients.2 AS affects young people and diagnosis typically occurs at ∼26 years of age. Men are more often affected than women, with a ratio of roughly 2:1.1. Approximately 80% of patients develop the first symptoms of AS before they are 30 years old; less than 5% of patients first present with symptoms after the age of 45.2 Although there is no cure for AS, treatments are available to manage the symptoms.
Treatment for all AS patients should include non-pharmacological therapy, specifically an exercise component to help maintain posture and range of motion.3 Non-steroidal anti-inflammatory drugs (NSAIDs), including cyclo-oxygenase-2 (COX-2)-selective NSAIDs, are recommended as first-line drug treatment for AS patients with pain and stiffness, according to the Ankylosing Spondylitis Assessment Study Working Group/European League Against Rheumatism 2010 guidelines.4 Continuous treatment with NSAIDs is preferred for patients with persistently active symptomatic disease. The population of patients with AS is younger than that of patients who are traditionally treated with long-term NSAID therapy, such as those with osteoarthritis and other related conditions. Care must be taken when these medicines are used, as gastrointestinal (GI), renal and cardiovascular toxicities are well documented.3 Not all NSAIDs have been studied in patients with AS, and the GI tolerability of different NSAIDs varies when they are used in a long-term setting.5 There is a need to document efficacy of a variety of NSAIDs to provide treatment options for patients with AS.
Celecoxib was approved in the USA in 2005 for the relief of the signs and symptoms of AS;6 it received European Union approval in 2007.7 Celecoxib was shown to be effective in significantly relieving pain and improving function in patients with AS in a 6-week trial and two 12-week studies.8–10 In addition, in one of these trials,9 long-term follow-up of patients who were prescribed a variable dose of celecoxib highlighted that continuous treatment with celecoxib reduced radiological progression in the spine of patients with AS in comparison with on-demand usage.11 Post-hoc analysis from the same trial suggests that the benefit may be greatest in patients with raised levels of C-reactive protein (CRP) at baseline,12 a finding corroborated by results from an independent German cohort population.13
The trial presented here assessed the efficacy and safety of two doses of celecoxib, 200 mg once daily (qd) and 400 mg qd, compared with diclofenac 50 mg three times daily (tid) for the treatment of AS in a Norwegian population.
Patients and methods
Study design
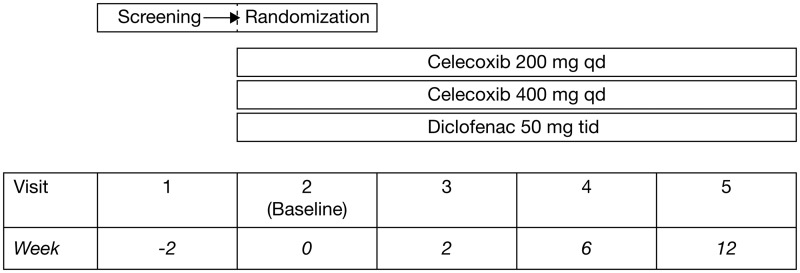
This randomized, double-blind, double-dummy comparative clinical trial of patients with AS was conducted in 27 centres in Norway between September 2002 and November 2004. The trial followed the design outlined in Figure 1. There were five visits: screening (visit 1); randomization (visit 2/baseline); assessment visits at 2, 6 and 12 weeks (visits 3, 4 and 5, respectively). Following a washout period, patients were required to exhibit flare at visit 2, as shown by a Global Pain Intensity score of ≥40 mm on a visual analogue scale (VAS) and worsening by at least 30% compared to that recorded at the screening visit. At that time, patients were randomized to one of the three treatment arms in a 1:1:1 manner, according to a computer-generated randomization schedule generated by the sponsor. Efficacy evaluations were conducted in the intention-to-treat (ITT) population; safety evaluations were conducted in the safety population. Both the ITT and safety populations included all patients who were randomized and received at least one dose of study drug.
Figure 1.
Overview of study design for a Norwegian trial comparing the efficacy and safety of celecoxib 200 and 400 mg once daily (qd) and diclofenac 50 mg three times daily (tid) in patients with ankylosing spondylitis.
The study was conducted in compliance with the ethical principles originating in, or derived from, the Declaration of Helsinki14 and in compliance with an independent ethics committee, informed consent regulations and International Congress on Harmonisation Good Clinical Practice Guidelines. In addition, all local regulatory requirements were followed. Written informed consent was obtained prior to the patients entering the study (e.g. before initiation of protocol-specific procedures). The trial was registered retrospectively on ClinicalTrials.gov in August 2015. Ongoing and new trials conducted by Pfizer are proactively registered on ClinicalTrials.gov since the Food and Drug Administration Amendment Act of September 2007 (this present trial pre-dated these requirements).
Study population
Patients aged 18–75 years with a clinical diagnosis of AS according to modified New York criteria15 (clinical and radiological) were eligible for participation in the trial. Patients who were exhibiting acute peripheral articular disease (excluding hips and shoulders) and/or ongoing extra-articular signs (e.g. cardiac involvement) were not eligible. All eligible patients must have had active symptoms requiring daily treatment with NSAIDs during the 30 days prior to study entry. Other exclusion criteria were: ulcerative colitis or Crohn’s disease; endoscopy-confirmed gastroduodenal ulcer within the past year and/or continued GI bleeding; cardiac, renal and/or hepatic disease; coagulation disorders or history of asthma and known hypersensitivity to celecoxib, NSAIDs or sulphonamide medication. Aspirin ≤ 160 mg/day for cardioprotection, methotrexate < 15 mg/week and occasional paracetamol (≤2000 mg/day, including during the screening period) were allowed.
Treatment
The three study treatment arms were 200 mg qd celecoxib, 400 mg qd celecoxib and 50 mg tid diclofenac. Celecoxib capsules were administered orally as 200 mg capsules in two different daily doses as a once-daily regimen: 200 mg and 400 mg. Diclofenac 50 mg tablets were administered orally three times daily. Use of placebo (tablets/capsules) was made only to achieve double blinding. The treatment period was 12 weeks.
Assessments
The primary objective was to compare patients’ assessments of Global Pain Intensity (measured on a VAS) at 12 weeks, in the 200 mg or 400 mg celecoxib groups versus the 50 mg diclofenac group. A secondary objective was to compare patients’ assessments of Global Pain Intensity in the two celecoxib dosage groups with those in the diclofenac group at 2 and 6 weeks. Other secondary objectives included comparison of the following at 12 weeks: nocturnal pain (VAS); Bath Ankylosing Spondylitis Functional Index (BASFI); Bath Ankylosing Spondylitis Disease Activity Index (BASDAI); physician’s Global Assessment of Disease Activity (VAS); patient’s Global Assessment of Disease Activity (VAS). Assessments of safety measures, including GI symptoms, were also among the secondary objectives. CRP was measured and recorded as a biological marker of inflammation.
A responder analysis was also performed at Week 12. Assessments in Ankylosing Spondylitis (ASAS) 20 analysis considered a patient to be a responder if he/she demonstrated improvement of 20% from baseline and absolute improvement of at least 10 mm on a 0–100 mm scale in at least three of the following four assessments: (i) Patients’ Global Assessment of Disease Activity by VAS scale (0–100 mm); (ii) Patients’ Global Pain Intensity by VAS scale (0–100 mm); (iii) Functionality Index by BASFI (0–100 mm); (iv) inflammation (the mean of the last two VAS scores for morning stiffness intensity and duration in BASDAI).
For the remaining domain, a patient must have shown an absence of deterioration of at least 20% and an absolute change of at least 10 mm on a 0–100 mm scale.
Statistical analyses
Sample size calculations were based on pairwise comparisons of each of the celecoxib treatment groups against the 50 mg tid diclofenac treatment group. Assuming the Global Pain Intensity VAS difference between diclofenac and at least one of the celecoxib arms to be >8 mm, at 80% statistical test power and a significance level of 0.025 in each test, 150 patients per treatment group were required. To accommodate a withdrawal rate of 6%, the study was designed to enrol 160 patients in each group (n = 150/0.93), to achieve a total of 480 patients.
An analysis of covariance (ANCOVA) model with centre and treatment as fixed effects and baseline value as a covariate was used to compare and test the primary endpoint. Comparisons of 50 mg tid diclofenac versus 200 mg qd celecoxib and 50 mg tid diclofenac versus 400 mg qd celecoxib were adjusted using the Dunnett–Hsu Test. The mean difference between treatment groups was estimated using the least squares means from the ANCOVA model, and 95% Dunnett–Hsu confidence intervals were computed.
If the analysis failed to reject the null hypothesis of equality on the primary endpoint, a non-inferiority approach was used. Non-inferiority was declared if the upper bound of the 95% two-sided confidence interval for celecoxib minus diclofenac was <10 mm for either celecoxib dose group. The ANCOVA model was used to test secondary endpoints; the Tukey–Kramer test was used for pairwise comparisons between treatment groups; and Fisher’s exact test was used to make pairwise comparisons between treatment groups for the responder analysis (ASAS 20).
The study was terminated early due to difficulties with recruitment (only 330 of the planned 480 patients were randomized). The decision to terminate the study was made prior to unblinding and was independent of the data. Prior to unblinding, the statistical analysis plan was amended from equivalence to non-inferiority. The power of the trial at this time was estimated to be approximately 86% and a new non-inferiority bound was also added (10 mm compared with the original 8 mm initially specified for the original power calculation).
Results
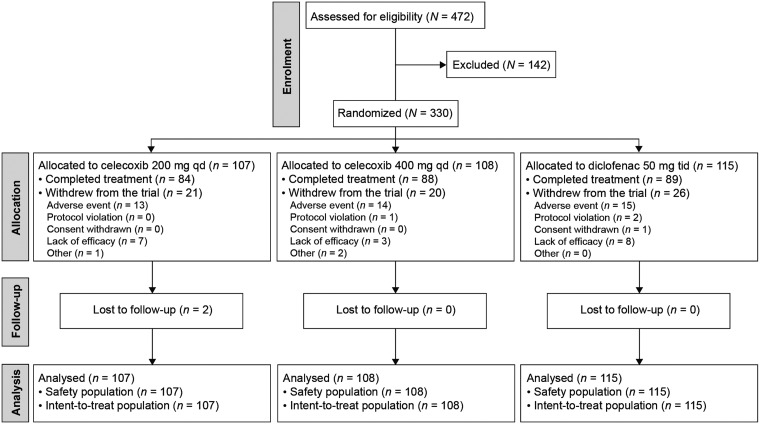
The majority of the 330 patients randomized and enrolled in the study completed treatment (84/107 [78.5%] 200 mg celecoxib, 88/108 [81.5%] 400 mg celecoxib, 89/115 [77.4%] diclofenac). Withdrawal rates due to lack of efficacy were low in all treatment groups (seven of 107 [6.5%] 200 mg celecoxib, three of 108 [2.8%] 400 mg celecoxib, eight of 115 [7.0%] diclofenac; Figure 2). No statistically significant between-group differences were observed in the incidence of withdrawal due to lack of efficacy. Most of the patients were Caucasian (99.7%) and male (72.4%), with a mean (SD) age of 43.8 (±10.3) years. The mean time since diagnosis was 10.3 (±8.8) years, and the majority of patients were positive for HLA-B27 (92.1%). Disease characteristics were similar across treatment groups (data not shown).
Figure 2.
Patient disposition in a Norwegian trial comparing the efficacy and safety of celecoxib 200 and 400 mg once daily (qd) and diclofenac 50 mg three times daily (tid) in treatment of ankylosing spondylitis.
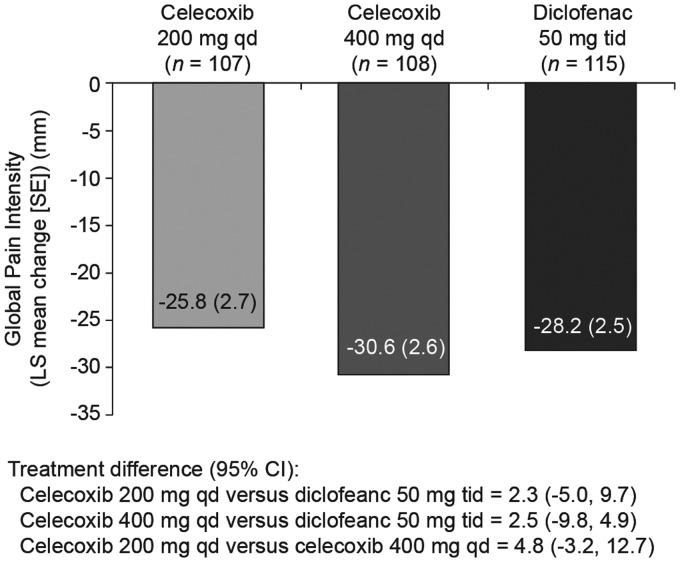
Global Pain Intensity decreased similarly between baseline and Week 12 in all treatment groups (mean baseline values 66.3 and 63.1 for 200 mg celecoxib and 400 mg celecoxib, respectively, and 67.0 for diclofenac; data not shown). Non-inferiority (based on non-inferiority margin of 10 mm) of both celecoxib treatment groups versus diclofenac was demonstrated (Figure 3). There were no statistically significant between-group differences in Global Pain Intensity at Weeks 2 and 6 (data not shown). Improvements from baseline to Week 12 were observed in all three treatment groups for Nocturnal Pain Intensity, BASDAI and both the Physicians’ and Patients’ Global Assessment of Disease Severity (Table 1), but there were no statistically significant between-group differences in any parameter.
Figure 3.
Change from baseline in Global Pain Intensity at Week 12 in Norwegian patients with ankylosing spondylitis treated with celecoxib 200 or 400 mg daily (qd) or diclofenac 50 mg three times daily (tid): intention-to-treat population. LS mean, least squares mean.
Table 1.
Changes from baseline in secondary efficacy endpoints at Week 12 in Norwegian patients with ankylosing spondylitis stratified according to treatment group (celecoxib 200 or 400 mg daily [qd] or diclofenac 50 mg three times daily [tid]): intention-to-treat population.
| Parameter | Celecoxib |
Diclofenac group | |
|---|---|---|---|
| 200 mg group | 400 mg group | ||
| Nocturnal pain, n | 107 | 108 | 115 |
| Baseline | 61.3 ± 24.2 | 57.9 ± 23.3 | 62.0 ± 21.7 |
| Week 12 | 35.9 ± 26.3 | 27.6 ± 23.4 | 34.4 ± 25.7 |
| LS mean change from baselinea | −25.9 ± 2.5 | −33.1 ± 2.5 | −28.0 ± 2.4 |
| Difference in LS mean (95% CI) | |||
| Celecoxib vs diclofenaca,b,c | 2.1 (−5.3, 9.5) | −5.1 (−12.5, 2.3) | – |
| Celecoxib 200 mg vs celecoxib 400 mga,b,c | 7.2 (−0.4, 14.7) | – | |
| BASFI, n | 107 | 108 | 113 |
| Baseline | 48.1 ± 21.8 | 45.5 ± 22.1 | 48.3 ± 20.1 |
| Week 12 | 34.0 ± 21.2 | 29.4 ± 22.7 | 30.8 ± 20.0 |
| LS mean change from baselinea | −14.9 ± 1.8 | −18.2 ± 1.8 | −18.1 ± 1.7 |
| Difference in LS mean (95% CI) | |||
| Celecoxib vs diclofenaca,b,c | 3.2 (−2.1, 8.6) | −0.04 (−5.4, 5.3) | – |
| Celecoxib 200 mg vs celecoxib 400 mga,b,c | 3.3 (−2.2, 8.7) | – | |
| BASDAI, n | 107 | 107 | 114 |
| Baseline | 58.4 ± 20.7 | 52.8 ± 19.3 | 56.2 ± 18.8 |
| Week 12 | 40.6 ± 21.0 | 33. ± 21.6 | 37.5 ± 21.3 |
| LS mean change from baselinea | − 17.5 ± 1.9 | −20.8 ± 1.9 | −19.5 ± 1.8 |
| Difference in LS mean (95% CI) | |||
| Celecoxib vs diclofenaca,b,c | 2.0 (−3.7, 7.6) | −1.3 (−7.0, 4.3) | – |
| Celecoxib 200 mg vs celecoxib 400 mga,b,c | 3.3 (−2.5, 9.1) | – | |
| Physician’s Global Assessment of Disease Severity, n | 107 | 108 | 114 |
| Baseline | 58.3 ± 16.6 | 55.2 ± 17.0 | 59.0 ± 16.3 |
| Week 12 | 36.6 ± 18.7 | 33.1 ± 20.0 | 35.6 ± 20.7 |
| LS mean change from baselinea | −21.1 ± 2.0 | −23.5 ± 1.9 | −22.9 ± 1.9 |
| Difference in LS mean (95% CI) | |||
| Celecoxib vs diclofenaca,b,c | 1.8 (−4.0, 7.5) | −0.7 (−6.5, 5.1) | – |
| Celecoxib 200 mg vs celecoxib 400 mga,b,c | 2.5 (−3.4, 8.3) | – | |
| Patient’s Global Assessment of Disease Severity, n | 105 | 107 | 112 |
| Baseline | 65.9 ± 19.6 | 62.6 ± 21.3 | 67.5 ± 18.0 |
| Week 12 | 43.4 ± 24.7 | 37.2 ± 25.4 | 40.7 ± 26.7 |
| LS mean change from baselinea | −23.0 ± 2.7 | −28.1 ± 2.7 | −26.5 ± 2.6 |
| Difference in LS mean (95% CI) | |||
| Celecoxib vs diclofenaca,b,c | 3.5 (−4.4, 11.4) | −1.5 (−9.4, 6.4) | – |
| Celecoxib 200 mg vs celecoxib 400 mga,b,c | 5.0 (−3.0, 13.0) | – | |
| ASAS 20 responders, n (%)d | 107 | 108 | 115 |
| Responders at Week 12 | 55 (51.4) | 65 (60.2) | 66 (57.4) |
Data presented as: n or n (%) patients; 95% CI; mean ± SD (baseline and Week 12 data); mean ± SEM (LS mean change from baseline).
Derived from analysis of covariance with baseline as a covariate, and treatment and centre as factors.
Calculated as difference between treatment groups in change from baseline. A negative difference indicates a numerical superiority of celecoxib over diclofenac.
Tukey–Kramer multiple comparison procedure used to generate confidence interval and P-value.
Pairwise comparisons between treatment groups at Week 12 were made using Fisher’s exact test.
No significant between-group differences (200 mg qd celecoxib vs diclofenac, 400 mg celecoxib vs diclofenac, or 200 mg celecoxib vs 400 mg celecoxib; P ≥ 0.05).
BASFI, Bath Ankylosing Spondylitis Functional Index; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CI, confidence interval; LS mean, least squares mean; ASAS, Assessments in Ankylosing Spondylitis.
More patients in the 400 mg celecoxib group met the ASAS 20 responder criteria at Weeks 2, 6 and 12 (58.3%, 60.2% and 60.2%, respectively) than in the 200 mg celecoxib (57.9%, 50.5% and 51.4%, respectively) and diclofenac (54.8%, 54.8% and 57.4%, respectively) treatment groups. There were no statistically significant between-group differences at Week 12 (Table 1).
Treatment-emergent adverse events were reported for 176 patients. The incidence of adverse events was slightly higher for the diclofenac treatment group (56%) than the 200 mg celecoxib (52%) and 400 mg celecoxib (52%) groups. The majority of adverse events were mild-to-moderate in severity. The most commonly occurring adverse events (≥2% of patients in any treatment group) were dyspepsia and diarrhoea (Table 2). Drug-related adverse events (as judged by the investigator) were observed in 43% of diclofenac-treated patients, 38% of 200 mg celecoxib-treated patients and 29% of 400 mg celecoxib-treated patients.
Table 2.
Summary of adverse events in Norwegian patients with ankylosing spondylitis stratified according to treatment group (celecoxib 200 or 400 mg daily [qd] or diclofenac 50 mg three times daily [tid]): safety population.
| Adverse event | Celecoxib |
Diclofenac group n = 115 | |
|---|---|---|---|
| 200 mg group n = 107 | 400 mg group n = 108 | ||
| Adverse events | 56 (52) | 56 (52) | 64 (56) |
| Drug-related adverse events | 41 (38) | 31 (29) | 49 (43) |
| Drug withdrawn due to adverse events | |||
| Temporarily | 1 (0.9) | 0 (0) | 3 (2.6) |
| Permanently | 12 a (11.2) | 14 (13.0) | 15 (13.0) |
| Serious adverse events | 1 (0.9) | 2 (1.9) | 2 (1.7) |
| Deaths | 0 (0) | 0 (0) | 0 (0) |
| Adverse events occurring in ≥2% of patients in any treatment group (system organ class)b | |||
| Gastrointestinal disorders | |||
| Abdominal discomfort | 3 (2.8) | 3 (2.8) | 3 (2.6) |
| Abdominal distension | 2 (1.9) | 3 (2.8) | 3 (2.6) |
| Abdominal pain NOS | 7 (6.5) | 3 (2.8) | 2 (1.7) |
| Diarrhoea | 5 (4.7) | 4 (3.7) | 8 (7.0) |
| Dyspepsia | 10 (9.3) | 6 (5.6) | 13 (11.3) |
| Flatulence | 2 (1.9) | 0 (0) | 3 (2.6) |
| Gastrointestinal disorder NOS | 3 (2.8) | 1 (0.9) | 0 (0) |
| Nausea | 4 (3.7) | 4 (3.7) | 8 (7.0) |
| General/administration site disorders | |||
| Fatigue | 4 (3.7) | 4 (3.7) | 3 (2.6) |
| Infection and infestations | |||
| Nasopharyngitis | 4 (3.7) | 6 (5.6) | 2 (1.7) |
| Nervous system disorders | |||
| Dizziness | 1 (0.9) | 2 (1.9) | 4 (3.5) |
| Headache | 4 (3.7) | 5 (4.6) | 4 (3.5) |
| Skin and subcutaneous tissue disorders | |||
| Pruritus | 3 (2.8) | 1 (0.9) | 2 (1.7) |
Data presented as n (%) patients.
One patient withdrew due to an adverse event of headache; however, onset of the headache was prior to the first dose of study medication and it was therefore not considered to be treatment emergent. Because the investigator listed the patient’s reason for discontinuation as ‘due to an adverse event’, it remains as such within patient disposition.
If a patient had > 1 adverse event within a system organ class, that patient was counted once in the overall incidence for that system organ class.
NOS, not otherwise specified.
A total of 41 patients experienced treatment-emergent adverse events that resulted in withdrawal of study medication (12 in the 200 mg celecoxib group, 14 in the 400 mg celecoxib group, 15 in the diclofenac group). Severe adverse events were experienced by five patients (1.5%) during the trial: one (0.9%) patient in the 200 mg celecoxib group, two (1.9%) in the 400 mg celecoxib group and two (1.7%) in the diclofenac group. No severe adverse events in the celecoxib groups were considered by the investigator to be related to the study medication. In the diclofenac group, two patients experienced severe adverse events considered by the investigator to be related to study medication: one patient experienced dizziness of moderate severity for which the drug was permanently withdrawn; another patient reported severe abdominal pain and continued in the study. No deaths were observed during the study.
Data regarding the change from baseline to Week 12 in CRP, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are shown in Table 3. Mean transaminase shifts from baseline to Week 12 were observed for patients who were treated with diclofenac, with ALT and AST increasing by 9.2 ± 20.0 U/l (range −35.0 to 115.0) and 2.1 ± 8.6 U/l (range −24.0 to 45.0), respectively. Increases in transaminases were not observed for patients in the 200 mg celecoxib and 400 mg celecoxib groups (Table 3). In the celecoxib treatment groups there were similar proportions of patients with abnormal ALT levels at baseline and at Week 12. However, in the diclofenac treatment group the number of patients who had abnormal ALT levels rose from seven (6.1%) at baseline to 21 (18.3%) at Week 12 (Table 3).
Table 3.
Abnormal serum chemistry and mean change in serum values from baseline to Week 12 in Norwegian patients with ankylosing spondylitis, stratified according to treatment group (celecoxib 200 or 400 mg daily [qd] or diclofenac 50 mg three times daily [tid]): safety population.
| Parameter | Celecoxib |
Diclofenac group | |
|---|---|---|---|
| 200 mg group | 400 mg group | ||
| C-reactive protein | |||
| N | 107 | 108 | 115 |
| Abnormal serum chemistry, baseline | 31 (29.0) | 41 (38.0) | 41 (35.7) |
| Abnormal serum chemistry, Week 12 | 32 (29.9) | 36 (33.3) | 36 (31.3) |
| Change from baseline to Week 12, mg/l | 0.8 ± 5.5 | −1.3 ± 12.3 | 0.7 ± 8.6 |
| N a | 99 | 102 | 107 |
| Alanine aminotransferase | |||
| N | 107 | 108 | 115 |
| Abnormal serum chemistry, baseline | 11 (10.3) | 10 (9.3) | 7 (6.1) |
| Abnormal serum chemistry, Week 12 | 11 (10.3) | 6 (5.6) | 21 (18.3) |
| Change from baseline to Week 12, U/l | −1.7 ± 12.9 | −1.8 ± 11.5 | 9.2 ± 20.0 |
| N a | 98 | 99 | 107 |
| Aspartate aminotransferase | |||
| N | 107 | 108 | 115 |
| Abnormal serum chemistry, baseline | 2 (1.9) | 1 (0.9) | 2 (1.7) |
| Abnormal serum chemistry, Week 12 | 3 (2.8) | 0 (0) | 2 (1.7) |
| Change from baseline to Week 12, U/l | −0.1 ± 7.6 | −0.6 ± 5.7 | 2.1 ± 8.6 |
| N a | 98 | 101 | 106 |
Data presented as: n or n (%) patients; mean ± SEM (change from baseline to Week 12).
Number of intention-to-treat patients with baseline and Week 12 measures.
Discussion
The findings of this 12-week comparative trial are consistent with those of other clinical studies that demonstrate the clinical efficacy of celecoxib and diclofenac in treating patients with AS.8–10 Despite difficulties encountered with slow recruitment in this trial (with only 330 of an anticipated 480 patients randomized), statistical consideration of the results was possible across a range of primary and secondary measures.
The primary results of this study indicated that celecoxib, at both 200 mg and 400 mg qd, and diclofenac 50 mg tid, were effective in treating Norwegian patients with AS. No difference was observed between the two doses of celecoxib versus diclofenac in terms of efficacy. Global Pain Intensity decreased similarly in all three treatment groups between baseline and Week 12, with no statistically significant difference between either of the celecoxib groups and the maximum licensed daily dose of diclofenac. There were, however, numerical treatment differences favouring celecoxib 400 mg for some secondary parameters. Suggestions, if not statistical evidence, of incremental efficacy with a total daily dose of 400 mg of celecoxib compared with 200 mg have also been reported in a 12-week trial comparing celecoxib 200 mg qd and 400 mg qd with naproxen 500 mg twice daily (bid),9 and in a second study comparing 200 mg qd and 200 mg bid with diclofenac slow release 75 mg bid.12
In contrast to the other clinical trial data comparing celecoxib and diclofenac in AS, there was no consistent evidence in the present study that continuous use of NSAIDs over 12 weeks has a lowering effect on levels of CRP. Most trial data for celecoxib and traditional NSAIDs suggest that, as a broad class of medicines, NSAIDs reduce CRP in the AS population;8–10 however, this contrasts with the findings from a 24-week study of patients with rheumatoid arthritis (RA), which showed approximate 2 mg/ml increases in CRP with both celecoxib (200 mg bid) and diclofenac (75 mg bid).16 Given that elevated CRP has been postulated to correlate with severity of disease in AS17 and that studies suggest the disease-delaying benefits may be greatest in patients with raised CRP,13 further research is warranted to corroborate these findings.
The types of adverse events most commonly reported in this study are consistent with the recognized toxicities of NSAIDs, be they traditional or COX-2 selective agents. Overall, there were fewer treatment-emergent adverse events among patients treated with celecoxib than in those treated with diclofenac. The majority of treatment-emergent adverse events were not attributed to study treatment by the investigators. The incidence of the most common GI adverse events, dyspepsia and diarrhoea, was higher in patients treated with diclofenac compared with those who received either dose of celecoxib. In a similarly designed 12-week trial undertaken in the same disease model, where the slow-release formulation of diclofenac (75 mg bid) was used, the drug was associated with significantly greater incidences of GI toxicity than reported with either celecoxib 100 mg bid or 200 mg bid.8 Similar significant differences in GI tolerability between celecoxib and diclofenac favouring celecoxib have been observed in other chronic disease models (osteoarthritis and RA) using both the slow-release16 and standard-release formulations of diclofenac.18 For all other system organ classes there were relatively few adverse events, suggesting that this class of medicine is fairly well tolerated in this patient population, who were of relatively young age (∼44 years old).
Mean increases in transaminases that were seen after 12 weeks’ treatment with diclofenac were not observed in patients treated with either dose of celecoxib. While the majority of these changes in liver enzyme levels fall within clinically normal ranges, the increase in the number of patients with an abnormal ALT at study end compared with baseline (21 versus seven) in the diclofenac treatment group is consistent with the toxicity recognized in a previous meta-analysis.19
The trial has a number of limitations. It was terminated early due to challenges with recruitment (only 330 of an anticipated 480 patients were randomized). Despite this, the number of patients randomized was sufficient to demonstrate non-inferiority with a good degree of certainty. The trial could also be criticized for not having a placebo arm; however, trials have been conducted in this disease area that were of placebo and active comparator design9–10 and comparator only,8,20 with the latter becoming more common as NSAIDs have become well recognized in disease area guidelines.3 An active comparator only design could also be considered more realistic for this painful debilitating condition where not offering pharmacotherapy is an unrealistic option.
The results of this 12-week study demonstrate that celecoxib 200 mg and 400 mg qd were similarly effective to diclofenac 50 mg tid in treating the symptoms of AS. Improvements in the primary and secondary endpoints were numerically greater for the 400 mg versus the 200 mg qd dose of celecoxib; however, none of the differences reached statistical significance. Patients may respond differently to different NSAIDs; the results of this study may be useful when choices of therapy for AS patients are being made.
Acknowledgement
Editorial support was provided by Kate Bradford, PhD, of PAREXEL, and was funded by Pfizer Inc.
Declaration of Conflicting Interest
Chris Walker is an employee of Pfizer Ltd. Margaret N. Essex, Chunming Li and Peter W. Park are all employees of Pfizer Inc.
Funding
This study was sponsored by Pfizer Inc.
References
- 1.McVeigh CM, Cairns AP. Diagnosis and management of ankylosing spondylitis. BMJ 2006; 333: 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldtkeller E, Khan MA, van der Heijde D, et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int 2003; 23: 61–66. [DOI] [PubMed] [Google Scholar]
- 3.Braun J, van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011; 70: 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zochling J, van der Heijde D, Burgos-Vargas R, et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2006; 65: 442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niculescu L, Li C, Huang J, et al. Pooled analysis of GI tolerability of 21 randomized controlled trials of celecoxib and nonselective NSAIDs. Curr Med Res Opin 2009; 25: 729–740. [DOI] [PubMed] [Google Scholar]
- 6.FDA Approves New Use for Pfizer’s Celebrex. http://www.drugs.com/news/fda-approves-new-pfizer-s-celebrex-1497.html (2005, accessed 25 November 2014).
- 7.Europe approves new use for Pfizer’s Celebrex drug. http://www.reuters.com/article/2007/02/23/pfizer-celebrex-idUSL237742220070223 (2007, accessed 25 November 2014).
- 8.Sieper J, Klopsch T, Richter M, et al. Comparison of two different dosages of celecoxib with diclofenac for the treatment of active ankylosing spondylitis: results of a 12-week randomised, double-blind, controlled study. Ann Rheum Dis 2008; 67: 323–329. [DOI] [PubMed] [Google Scholar]
- 9.Barkhuizen A, Steinfeld S, Robbins J, et al. Celecoxib is efficacious and well tolerated in treating signs and symptoms of ankylosing spondylitis. J Rheumatol 2006; 33: 1805–1812. [PubMed] [Google Scholar]
- 10.Dougados M, Behier JM, Jolchine I, et al. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum 2001; 44: 180–185. [DOI] [PubMed] [Google Scholar]
- 11.Wanders A, Heijde Dv, Landewé R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005; 52: 1756–1765. [DOI] [PubMed] [Google Scholar]
- 12.Kroon F, Landewé R, Dougados M, et al. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis 2012; 71: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 13.Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis 2012; 71: 1616–1622. [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2000; 284: 3043–3045. [PubMed]
- 15.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984; 27: 361–368. [DOI] [PubMed] [Google Scholar]
- 16.Emery P, Zeidler H, Kvien TK, et al. Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparison. Lancet 1999; 354: 2106–2111. [DOI] [PubMed] [Google Scholar]
- 17.Benhamou M, Gossec L, Dougados M. Clinical relevance of C-reactive protein in ankylosing spondylitis and evaluation of the NSAIDs/coxibs’ treatment effect on C-reactive protein. Rheumatology (Oxford) 2010; 49: 536–541. [DOI] [PubMed] [Google Scholar]
- 18.McKenna F, Borenstein D, Wendt H, et al. Celecoxib versus diclofenac in the management of osteoarthritis of the knee. Scand J Rheumatol 2001; 30: 11–18. [DOI] [PubMed] [Google Scholar]
- 19.Soni P, Shell B, Cawkwell G, et al. The hepatic safety and tolerability of the cyclooxygenase-2 selective NSAID celecoxib: pooled analysis of 41 randomized controlled trials. Curr Med Res Opin 2009; 25: 1841–1851. [DOI] [PubMed] [Google Scholar]
- 20.Huang F, Gu J, Liu Y, et al. Efficacy and safety of celecoxib in Chinese patients with ankylosing spondlyitis: a 6-week randomised, double blind study with 6-weeks open-label extension treatment. Current Therapeutic Research 2014; 76: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]