Abstract
Objective
To evaluate the beneficial effects of hydroxysafflor yellow A (HSYA) on monocrotaline (MCT)-induced pulmonary arterial hypertension (PAH) in rats, and to investigate the main pathophysiological mechanism of HSYA in preventing development of MCT-induced PAH.
Methods
Four groups (control, control with HSYA treatment, MCT-exposed, and MCT-exposed with HSYA treatment) were evaluated at day 28 following MCT exposure. Haemodynamic measurements, right ventricular hypertrophy, morphometry, inflammatory cytokines and oxidant expression were assessed.
Results
HSYA significantly reduced haemodynamic changes, right ventricular hypertrophy and morphometric changes induced by exposure to MCT. HYSA also suppressed MCT-induced inflammation and oxidative stress in rat pulmonary tissue.
Conclusions
Experimental MCT-induced PAH may be reduced by HSYA treatment, and the mechanism may involve suppression of inflammation and oxidative stress.
Keywords: Chinese medicine, hydroxysafflor, inflammation, monocrotaline, oxidant, pulmonary artery hypertension, safflower
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease with a poor prognosis, characterized by remodelling of the pulmonary vasculature and increased arterial resistance, resulting in right ventricular failure and death.1 Efforts to improve the prognosis in patients with PAH have led to therapeutic options in three pharmacological classes: endothelin receptor antagonists, phosphodiesterase type-5 inhibitors, and prostanoids, all of which have provided some improvements, mainly in terms of symptomatic relief.2 Despite new therapy options, there remains no cure for PAH and prognosis is poor. Thus, there is a need for new or alternative approaches to control PAH.3 Alternative approaches to treatment of diseases in general, such as traditional Chinese medicines, which have been used in China, Japan, and other Asian countries for centuries, are attracting increasing interest in Western countries.4,5
Although the exact mechanism underlying PAH is not fully understood, studies using monocrotaline (MCT)-induced models of PAH suggest that inflammatory status and oxidative stress are the main contributors to disease pathogenesis;6 pulmonary vascular remodelling can be attenuated through inhibition of pulmonary inflammatory cell proliferation and leukocyte infiltration;7 and alleviating oxidative stress can prevent pulmonary vascular remodeling.8
Injectable Safflower extract is widely used in Chinese medicine, particularly for the treatment of cerebrovascular and cardiovascular diseases,9 and has been approved as a medicinal drug by the State Food and Drug Administration since 2005.10 Hydroxysafflor yellow A (HSYA), which is the main chemical component of safflower, has been shown to possess many pharmacological activities. For example, HSYA has been demonstrated to ameliorate proliferation of fibroblast cells in vitro;11 antagonize binding of platelet activating factor to its receptor; produce antithrombotic effects; inhibit platelet aggregation; and exhibit cardio-protective, and neuro-protective effects in rat models.12–15 Most importantly, many beneficial effects of HSYA are considered to be related to its antioxidant16 and anti-inflammatory function.17 In a rat model of alcohol-induced liver injury, HSYA apparently decreased the levels of reactive oxygen species and malondialdehyde (MDA) in liver tissue, ameliorated the severity of long-term alcohol-induced liver damage, and improved the liver architecture.18 HSYA has also been shown to downregulate levels of inflammatory mediators including tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6, thus exhibiting a beneficial effect on a mouse model of acute pulmonary injury.19 Whether HSYA could protect against PAH remains unclear, however, the present authors hypothesise that HSYA might protect against PAH-associated pulmonary vascular remodelling via inhibition of inflammation and oxidative stress. Thus, the purpose of the present study was to investigate the protective effect of HSYA in a MCT-induced PAH model.
Materials and methods
Study population and experimental design
Specific pathogen-free male Wistar rats (aged 6 weeks; weight, 200–220 g) were purchased from Silikejingda experimental animals Corporation (Changsha, China). Rats were housed in 10 polycarbonate cages with wood sawdust bedding, under climate-controlled conditions on a 12-h light/12-h dark cycle. Each cage held 4–5 rats with free access to food and water. Animals were allowed 1 week to adjust to the new environment prior to start of the experiment, then randomly divided, using a computer generated randomization schedule, into four groups: (1) control group (n = 10) with no study treatment; (2) MCT + HSYA group (n = 10) treated with a single dose of 60 mg/kg MCT subcutaneous (s.c.) injection, followed by 10 mg/kg HSYA intraperitoneal (i.p.) injection, once daily; (3) MCT group (n = 10) treated with a single dose of 60 mg/kg MCT s.c. injection, followed by equivalent HSYA volume of sterile saline i.p. injection, once daily; (4) control + HSYA group (n = 10) with no MCT exposure and treated with 10 mg/kg HSYA i.p. injection, once daily. The numbers for each sample were determined by analysis of variance20 based on the results of pre-experiment. Researchers (XH, ZZ) responsible for sample processing and analyses were blinded to the experimental groups. HSYA was provided by Huahuikaide Pharmaceutical Co., Ltd (Shanxi, China) with > 95% purity, and dissolved in 0.9% saline to a concentration of 5 mg/ml for injection. MCT (Sigma-Aldrich Co, St. Louis, MO, USA) was dissolved in 1 N HCL, then the pH was adjusted to 7.4 with 1 N NaOH, as described previously.21 To perform analyses at 28 days following MCT exposure, as described in the following sections, all rats were anaesthetized by pentobarbital and sacrificed.
The study was approved by the ethical committee of Hunan Normal University, Changsha, China. All animal experiment protocols were performed under the Hunan Provincial People’s Hospital institutional guidelines of animal welfare.
Haemodynamic measurements
Haemodynamic measurements were performed on all rats in the four groups. On day 28 following MCT exposure, right ventricular systolic pressure, mean pulmonary arterial pressure, right ventricular end diastolic pressure and heart rate (HR) were recorded. Rats were anaesthetized with 40 mg/kg sodium pentobarbital i.p. injection, and supplementary anaesthesia (20 mg/kg pentobarbital i.p. injection) was added when necessary. Rats were placed on a heater to keep body temperature constant at 37℃. As described previously,22 a 3.5 French umbilical vessel catheter (Bound Tree Medical, Chicago, IL, USA) angled to 90° over the distal 1 cm and curved slightly at the tip, was introduced into the right external jugular vein. With the angle directed anteriorly, the catheter was inserted 2.5 cm proximally, which placed the tip of the catheter into the right atrium. The catheter was then rotated 90° counter-clockwise and inserted 1 cm further, which placed the tip of the catheter into the right ventricle. Finally, an additional 1.5 cm was further advanced so that the tip of the catheter was placed into the pulmonary artery. Placement at each stage was confirmed by respective pressure contours. Haemodynamic values were automatically calculated using a Cardiomax III physiological data acquisition system (Columbus Instruments, Columbus, OH, USA). Following haemodynamic measurements, the thorax of each rat was opened, and the heart was used to detect right ventricular hypertrophy measurements, the left lung was processed for histological evaluation and the right lung was homogenized for analysis of oxidative stress and inflammatory indices.
Right ventricular hypertrophy measurements
On day 28 following MCT exposure, following haemodynamic measurements, the hearts of all 40 rats were collected and right ventricular hypertrophy was measured by Fulton’s index as previously described.23 Hearts were isolated, flushed with 0.154 M saline, and dissected to separate the right ventricle from the left ventricle plus septum. As indices of right ventricle hypertrophy, the ratios of right ventricle weight/left ventricle plus septum weight were determined.
Morphometric analysis
Isolated left lungs, collected following haemodynamic measurements, were inflated, fixed with 10% formalin, dehydrated by alcohol gradient and then embedded in paraffin using standard techniques. Embedded tissues were longitudinally cut into 4 µm-thick sections, and stained with haematoxylin and eosin for morphometric analysis. The slides were evaluated by light microscopy at 400 × magnification, and the researchers (XH, ZZ) who assessed the extent of vascular remodelling were blinded to the grouping. Ten randomly chosen fields containing terminal arterioles (50–150 µm external diameters) from each slide were captured. The areas bounded by external elastic lamina and the internal elastic lamina of each vessel were measured. The percent wall thickness of the arterioles were calculated by the following formulas as wall thickness (%) = 100 × (1–internal elastic lamina/external elastic lamina). The degree of vascular muscularization was evaluated by a scale of 1–3, of which: 1 = no muscularization, not occluded; 2 = partial muscularization, not fully occluded; 3 = muscularization, fully occluded, as described previously.24
Inflammatory status
Total RNA was extracted from the right lung tissue of each rat, using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. Total mRNA was reverse transcribed into cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Haematologic Technologies, USA) in a 20 µl reaction volume containing total 1.0 µg RNA, oligo(dT)15 primer, dNTPs, and diethylpyrocarbonate H2O. The reverse transcription reaction was performed for 50 min in a 42℃ water bath, and terminated by heating to 95℃ for 5 min. The resultant cDNA was used as the template for amplification of IL-1β, TNF-α, and IL-6 by semi-quantitative polymerase chain reaction (PCR). The cDNA was also used to amplify β-actin in separate tubes during each PCR reaction as an internal control (GenBank sequence ID, rat actin beta NM031144). Each 25 µl reaction contained 200 ng of cDNA template (in 2.0 µl), and either IL-1β, TNF-α, IL-6 or β-actin forward and reverse primers (0.5 µl each), 2 × PCR Mastermix (12.5 µl; TianGen, Beijing, China), and ddH2O (9.5 µl). The primer sets used were as follows (based on GenBank sequences): IL-1β forward 5′-TGA CCC ATG TGA GCT GAA AG-3′ and reverse 5′-AAC TAT GTC CCG ACC ATT GC-3′, yielding 367 base-pair (bp) products; TNF-α forward 5′-CAC CAT GAG CAC GGA AAG CA-3′ and reverse 5′-GCA ATG ACT CCA AAG TAG ACC-3′, yielding 692 bp products; IL-6 forward 5′-TCC TAC CCC AAC TTC CAA TGC TC-3′ and reverse 5′-TTG GAT GGT CTT GGT CCT TAG CC-3′, yielding 79 bp products; and β-actin forward 5′-ACC ACA GCT GAG AGG GAA ATC G-3′ and reverse 5′-AGA GGT CTT TAC GGA TGT CAA CG-3′, yielding 281 bp products, respectively. The PCR conditions were as follows, performed using a BioRad thermal cycler (Hercules, CA, USA): IL-1β, 94℃ for 4 min followed by 35 cycles of 94℃ for 30 s, 55℃ for 30 s, and 72℃ for 40 s; TNF-α, 95℃ for 5 min followed by 35 cycles of 95℃ for 30 s, 56℃ for 30 s, and 72℃ for 40 s; IL-6, 94℃ for 5 min followed by 35 cycles of 95℃ for 30 s, 54℃ for 30 s, and 72℃ for 40 s. The subsequent PCR products were loaded into 1.5% agarose gels with 1% ethidium bromide for electrophoresis, and checked against a DNA ladder marker. Images were captured using Image Master VDS (Amersham Pharmacia Biotech, UK), and densitometric analysis was performed using Image Master 1D Elite software, version 2.0.1 (Amersham Pharmacia Biotech). Results are presented in relative terms as arbitrary units (AU) normalized to β-actin.
Oxidative stress
Isolated pulmonary tissues from all rats were homogenized in 1 ml saline (0.154 M). The homogenates were then centrifuged at 1000 g at 4℃ for 15 min, and the supernatants were collected to detect the indices reflecting oxidative stress. MDA concentration and superoxide dismutase (SOD) activity were determined using commercially available kits (Thibabituric Acid reacting substance production and Hydroxylamine colorimetry kit, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. Tissue 8-hydroxydesoxyguanosine (8-OHdG) levels were measured using an enzyme-linked immunosorbent assay kit (8-OHdG check; BlueGene Biotech Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. Protein concentration was measured using the Bradford method (Bradford Protein Assay Kit, Beijing Leagene Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instructions, and the results were expressed per weight of protein.25,26
Statistical analyses
Data are presented as mean ± SD, and SPSS software, version 12.0 (SPSS, Chicago, IL, USA) was used for data analyses. Between-group differences in measurement data were analysed by one-way analysis of variance. Between-group differences in vascular muscularization were analysed by χ2-test. Two-sided tests were used throughout, and a P value <0.05 was considered statistically significant.
Results
HSYA treatment alleviated haemodynamic measurements and right ventricular hypertrophy in MCT-induced PAH
Right ventricular systolic pressure and mean pulmonary arterial pressure were significantly increased in the MCT group (97.86 ± 40.28 mmHg and 24.89 ± 11.77 mmHg, respectively) versus the control group (31.97 ± 14.25 mmHg and 15.26 ± 9.17 mmHg, respectively; P < 0.05; Figure 1(A)). MCT-induced right ventricular systolic pressure and mean pulmonary arterial pressure were significantly reduced by HSYA treatment (62.31 ± 33.48 mmHg and 17.41 ± 9.28 mmHg, respectively) versus MCT alone (P < 0.05; Figure 1(A)). Compared with the control group, right ventricular systolic pressure remained significantly higher in the MCT + HSYA group (62.31 ± 33.48 mmHg versus 31.97 ± 14.25 mmHg, P < 0.05) and mean pulmonary arterial pressure also remained significantly higher in the MCT + HSYA group (17.41 ± 9.28 mmHg versus 15.26 ± 9.17 mmHg, P > 0.05). There were no statistically significant differences between the control group and control + HYSA group in terms of right ventricular systolic pressure and mean pulmonary arterial pressure.
Figure 1.
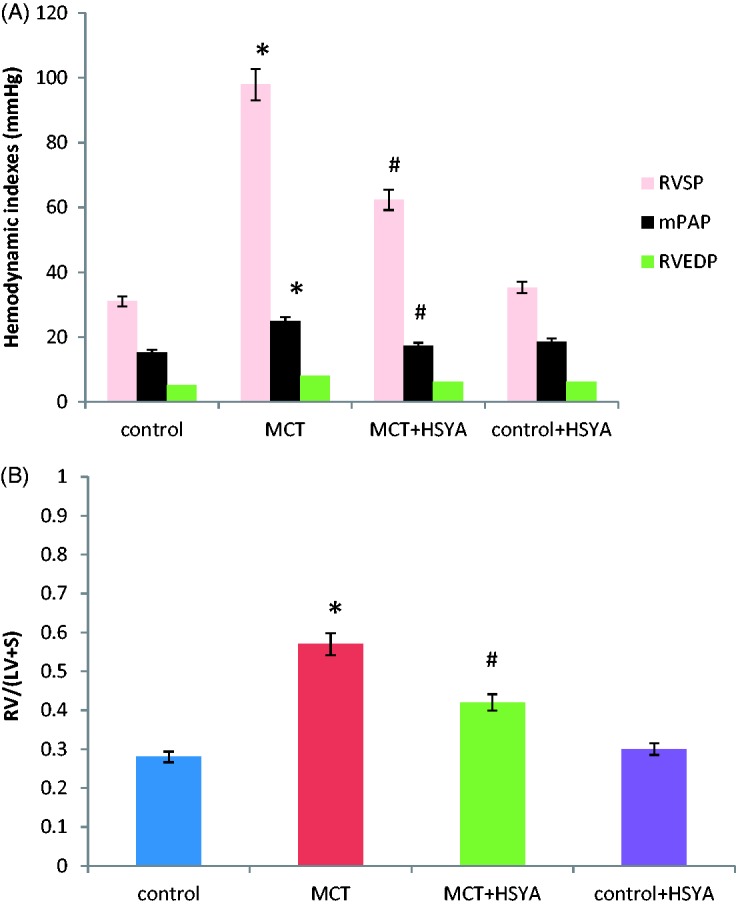
Effects of hydroxysafflor yellow A (HSYA) treatment on monocrotaline (MCT)-induced pulmonary arterial hypertension in rats, showing: (A) HSYA treatment effects on haemodynamic indices; and (B) HSYA treatment effects on right ventricular hypertrophy. RVSP, right ventricular systolic pressure; mPAP, mean pulmonary arterial pressure; RVEDP, right ventricular end diastolic pressure; RV/(LV + S), right ventricular weight to left ventricular plus septal weight ratio. Data presented as mean ± SD; *P < 0.05 versus control group; #P < 0.05 versus MCT group (one-way analysis of variance)
MCT significantly increased the right ventricle weight/(left ventricle plus septum weight) ratio versus the control group (0.57 ± 0.17 versus 0.28 ± 0.09, respectively; P < 0.01). The increase in right ventricle weight/(left ventricle plus septum weight) ratio was significantly lower in the MCT + HYSA treated group (0.42 ± 0.11) versus MCT alone (P < 0.05; Figure 1(B)). Compared with the control group, right ventricle weight/(left ventricle plus septum weight) ratio remained higher in the MCT + HSYA group (0.42 ± 0.11 versus 0.28 ± 0.09, P < 0.05). There was no statistically significant difference in right ventricular end diastolic pressure between the four study groups.
There were no statistically significant differences in HR between the control, MCT, MCT + HSYA and control + HSYA groups (417.8 ± 45.1, 499.5 ± 50.2, 461.3 ± 39.7 and 410.5 ± 32.3 beats per min, respectively).
HSYA treatment alleviated pulmonary vascular remodelling in MCT-induced PAH
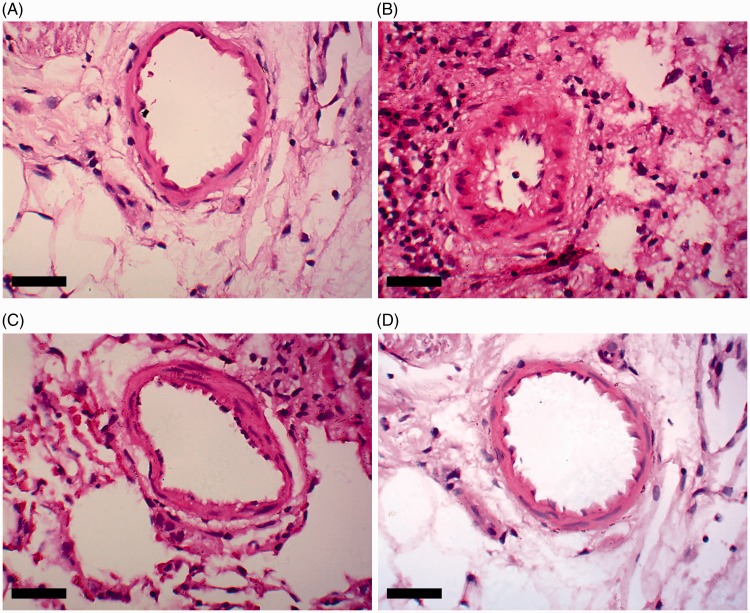
Histological examination of pulmonary tissue (Figure 2(A), control; (B), MCT alone; (C), MCT + HSYA; and (D), control + HYSA) showed an increase in thickness of the pulmonary artery wall in tissue from rats treated with MCT, and the lumen appeared stenosed or occluded (Figure 2(B)). In addition, large numbers of inflammatory cells were observed to have infiltrated the pulmonary tissue in the MCT treated group (Figure 2(B)). These pathological changes appeared to be attenuated in tissue from the MCT + HSYA group (Figure 2(C)). Mean percent wall thickness of pulmonary arterioles was significantly increased in rats treated with MCT compared with the control group (50.16 ± 18.39% versus 33.97 ± 9.74%, respectively; P < 0.05; Figure 2(E)). In contrast, in the MCT + HSYA group, percent wall thickness was significantly reduced (39.85 ± 10.72%) compared with the MCT group (P < 0.05), but the value remained higher than control group (39.85 ± 10.72% versus 33.97 ± 9.74%, P < 0.05). The effects of HSYA on pulmonary vascular remodelling were further demonstrated by the percentage of non-muscularized, partially muscularized and fully muscularized arterioles (Figure 2(F)). MCT was associated with increased levels of fully muscularized arterioles (68.32 ± 22.59%) compared with the control group (8.17 ± 3.97%; P < 0.01). MCT was also observed to cause a decrease in non-muscularized arterioles (11.69 ± 6.65%) compared with the control group (74.36 ± 21.22%; P < 0.01). In the MCT + HSYA group, the percentage of fully muscularized arterioles was reduced (48.07 ± 25.26%) and the percentage of non-muscularized arterioles was increased (37.9 ± 19.48%) versus the MCT group (both P < 0.05). Compared with the control group, the percentage of fully muscularized arterioles remained higher in the MCT + HSYA group (48.07 ± 25.26% versus 8.17 ± 3.97%, P < 0.01) and the percentage of non-muscularized arterioles remained lower (37.9 ± 19.48% versus 74.36 ± 21.22%, P < 0.05).
Figure 2.
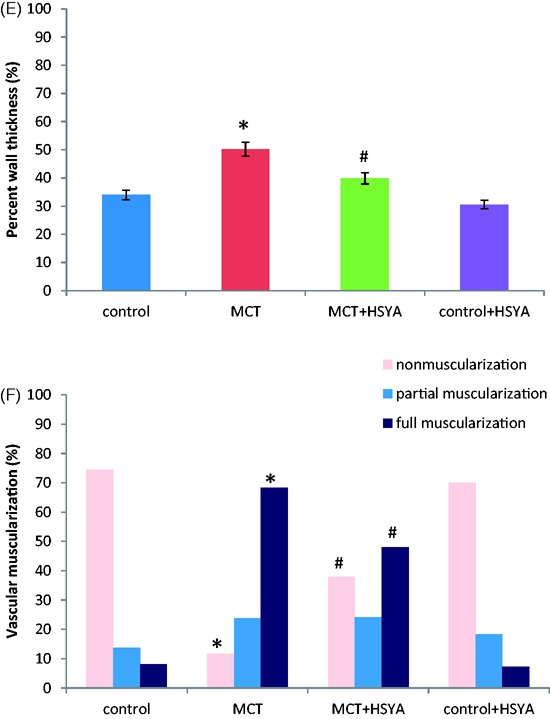
Effects of hydroxysafflor yellow A (HSYA) treatment on monocrotaline (MCT)-induced pulmonary arterial hypertension-related pulmonary vascular remodelling in rats, showing: (A–D) representative photomicrographs of haematoxylin and eosin stained pulmonary arterioles in (A) control group, (B) MCT group, (C) MCT + HSYA group and (D) control + HSYA group; (E) HSYA attenuated the MCT-induced increase in pulmonary arteriole wall thickness; and (F) HSYA attenuated MCT-induced vascular muscularization in PAH by increasing nonmuscularization and decreasing full muscularization. Nonmuscularization, arterioles not occluded; Partial muscularization, arterioles not fully occluded; Full muscularization, arterioles fully occluded. Data presented as mean ± SD; magnification × 400; scale bars = 50 µm; *P < 0.05 versus control group; #P < 0.05 versus MCT group (one-way analysis of variance or χ2-test)
HSYA treatment suppressed inflammatory status in MCT-induced PAH
Relative levels of IL-1β, IL-6 and TNF-α mRNA were significantly increased in the MCT group compared with the control group (11.45 ± 6.85 versus 6.20 ± 2.42, 16.72 ± 9.92 versus 7.34 ± 3.07, and 6.49 ± 3.18 versus 3.16 ± 1.43, respectively; all P < 0.05; Figure 3A–C). In the MCT + HSYA group, relative levels of IL-1β, IL-6 and TNF-α was were significantly reduced compared with the MCT group (7.06 ± 3.31 versus 11.45 ± 6.85, 10.29 ± 4.83 versus 16.72 ± 9.92, and 4.66 ± 2.17 versus 6.49 ± 3.18, respectively; all P < 0.05; Figure 3A–C). Compared with the control group, values of IL-1β, IL-6 and TNF-α mRNA in the MCT + HSYA group remained higher (IL-1β, 7.06 ± 3.31 versus 6.20 ± 2.42, P > 0.05; IL-6, 10.29 ± 4.83 versus 7.34 ± 3.07, P < 0.05; and TNF-α, 4.66 ± 2.17 versus 3.16 ± 1.43, P < 0.05, respectively). There were no statistically significant differences in IL-1β, IL-6 and TNF-α mRNA levels in the control + HYSA group versus the control group.
Figure 3.
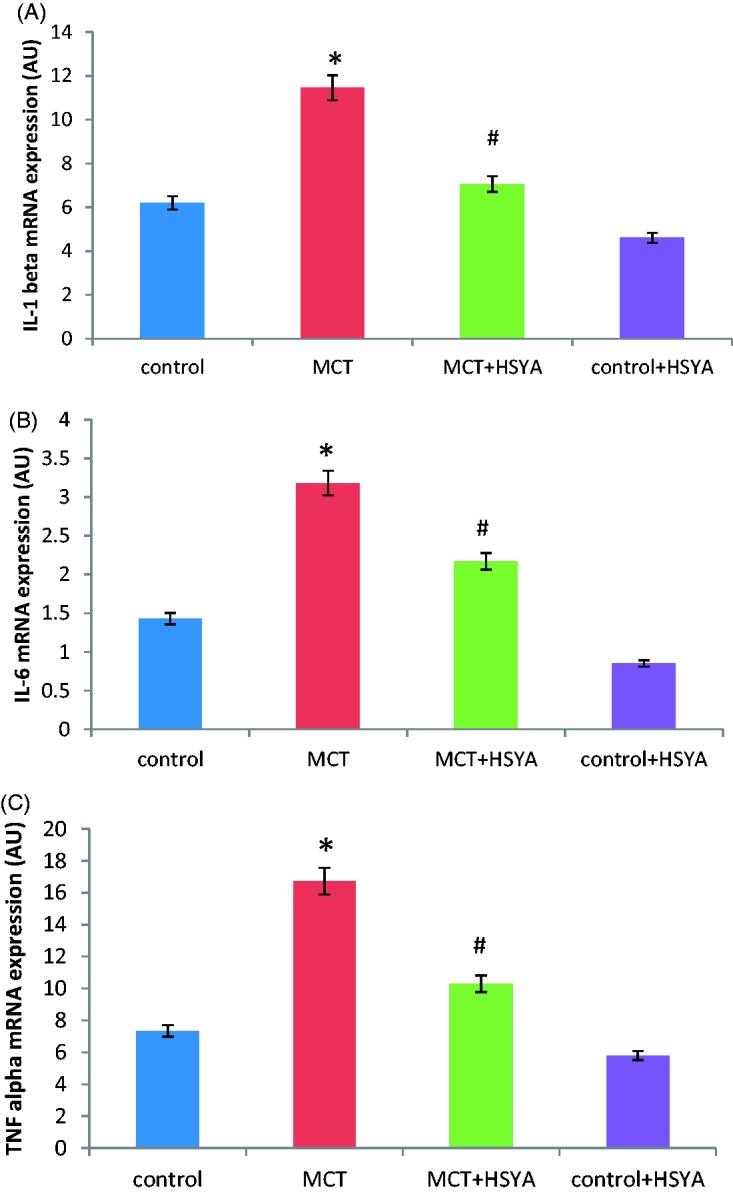
Effects of hydroxysafflor yellow A (HSYA) treatment on monocrotaline (MCT)-induced pulmonary arterial hypertension-related pulmonary mRNA expression of (A) interleukin (IL)-1β; (B) IL-6; and (C) TNF-α in rat tissue. AU, arbitrary units; data presented as mean ± SD; *P < 0.05 versus control group; #P < 0.05 versus MCT group (one-way analysis of variance)
HSYA treatment decreased MDA and 8-OHdG concentration and increased SOD activity in MCT-induced PAH
Concentrations of MDA and 8-OHdG were higher in the MCT group than in the control group (MDA, 2.18 ± 1.05 versus 0.75 ± 0.48 nmol/mg protein, P < 0.05, Figure 4A; and 8-OHdG, 71.67 ± 32.28 versus 37.33 ± 17.38 ng/g protein, P < 0.01, Figure 4B). SOD activity was lower in the MCT group compared with the control group (40.25 ± 11.24 versus 67.57 ± 22.26 U/mg protein, P < 0.05, Figure 4C). MDA levels were lower in the MCT + HSYA group (1.15 ± 0.92 mmol/mg protein), 8-OHdG concentrations were lower in the MCT + HSYA group (45.65 ± 27.53 ng/g protein) and SOD activity was increased in the MCT + HSYA group (55.71 ± 15.30 u/mg protein) versus the MCT group (all P < 0.05 versus MCT; Figure 4). Compared with the control group, concentrations of MDA and 8-OHdG in the MCT + HSYA group remained higher (MDA, 1.15 ± 0.92 mmol/mg protein versus 0.75 ± 0.48 nmol/mg protein, P < 0.05; 8-OHdG, 45.65 ± 27.53 ng/g protein versus 37.33 ± 17.38 ng/g protein, P < 0.05) and SOD activity remained lower (SOD, 55.71 ± 15.30 u/mg protein versus 67.57 ± 22.26 U/mg protein, P < 0.05, respectively). No statistically significant difference was observed between the control group and the control + HSYA group in terms of MDA, 8-OHdG, and SOD (Figure 4).
Figure 4.
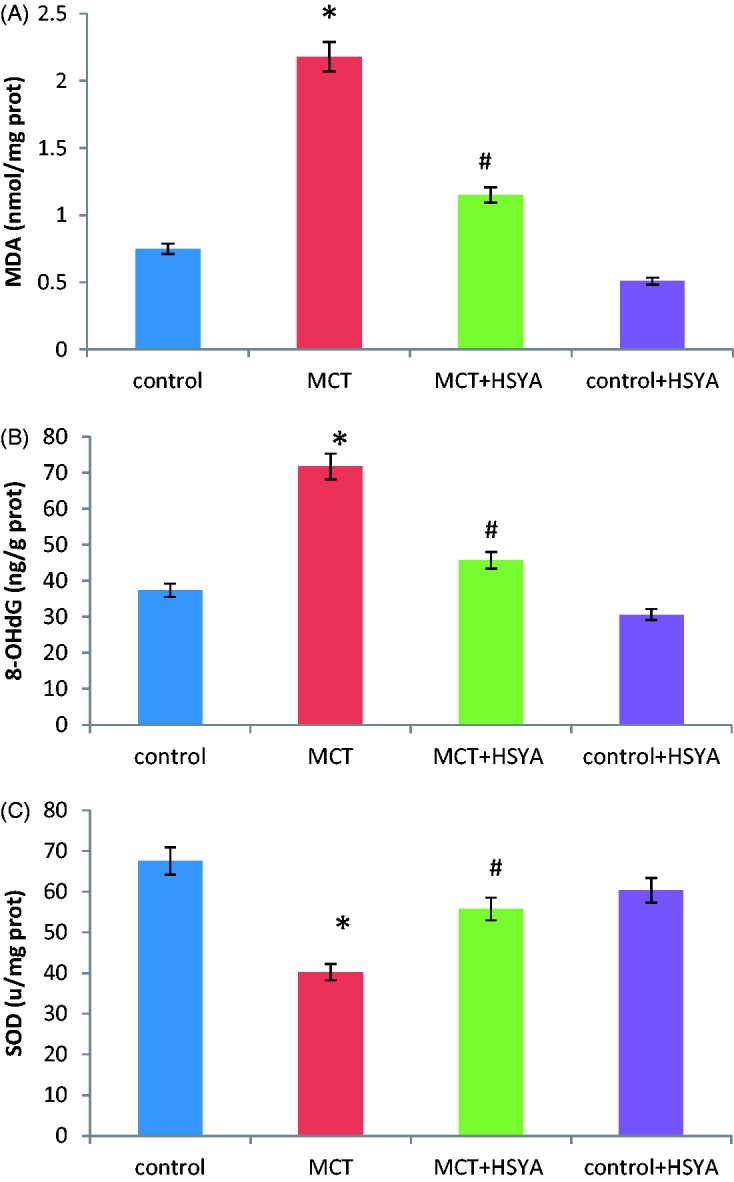
Effects of hydroxysafflor yellow A (HSYA) treatment on monocrotaline (MCT)-induced pulmonary arterial hypertension-related oxidative stress in rat tissue. HSYA treatment was associated with (A) decreased malondialdehyde (MDA) levels; (B) decreased 8-hydroxydesoxyguanosine (8-OHdG) concentration; and (C) upregulated superoxide dismutase (SOD) activity compared with MCT alone. Data presented as mean ± SD; *P < 0.05 versus control group; #P < 0.05 versus MCT exposed group (one-way analysis of variance)
Discussion
In the present study, the beneficial effects of HSYA in a MCT-induced rat model of PAH were investigated. HSYA was shown to inhibit vascular remodelling, and thus attenuate the development of MCT-induced PAH. Morphometric analysis demonstrated that HSYA decreased MCT-induced vascular wall thickness and the degree of muscularization. Compared with the MCT exposed group, haemodynamic measurements including right ventricular systolic pressure and mean pulmonary arterial pressure were markedly decreased in the HYSA treated group, and right ventricular hypertrophy was attenuated. The effects of HYSA were also demonstrated to be accompanied by suppression of inflammatory status and oxidative stress in pulmonary tissue, shown by reduced levels of IL-1β, IL-6 and TNF-α, and alleviation of the oxidative stress indices SOD, MDA, 8-OHdG.
The pathology of PAH is complicated, and inflammatory status and oxidative stress may play key roles in humans and in experimental animal models. Infiltration of inflammatory mediators contributes to the development of oxidative stress,27 and the increase of oxidants can induce either the infiltration of inflammatory cells or inflammatory-cell release of cytokines.28,29 Interactions of the two components results in cell proliferation and vascular remodelling, and obliteration of the pulmonary arterial lumen, eventually leading to PAH.30 Thus, there remains a need to develop strategies that simultaneously target the two components leading to PAH.31 Carthamus tinctorius L. (commonly known as safflower) is well established as a Chinese herbal medicine that contains yellow and red pigments, and has long been used clinically in the treatment of cardiovascular and cerebrovascular diseases.32 HSYA, one of the active ingredients of the yellow pigment of safflower, is a well-known natural medicine with anti-inflammatory and antioxidant activities.33 An investigation of the beneficial effects of HSYA during acute pulmonary injury induced by lipopolysaccharide in mice34 showed that treatment with HSYA could significantly alleviate inflammatory status, and suppress the increase of myeloperoxidase activity in pulmonary tissue. Furthermore, the physiological mechanisms of HSYA in the treatment of cerebral ischemia disease have been shown,10 and the anti-inflammatory and anti-oxidant effects of HSYA have also been demonstrated. Thus, HSYA was selected as a candidate drug in the present study, to investigate the effects on MCT-induced PAH. The present study showed that HSYA alleviated the development of MCT-induced PAH, and inflammatory status and oxidative stress in tissues from rats treated with MCT and HSYA were decreased compared with exposure to MCT alone.
Inflammation is a major contributor to the development of PAH, therefore, suppression of inflammatory process may be beneficial in preventing the progress of PAH.35,36 Cytokines play a role in almost every step of the inflammatory process. In particular, IL-1β and TNF-α have been associated with the accumulation of extracellular matrix proteins, and IL-6 has been linked to the proliferation of smooth muscle cells, which are all involved in pulmonary vascular remodelling in various types of PAH.37 One study concluded that inhibiting the expression of IL-6 could prevent the progression of muscularization and proliferative arteriopathy in PAH.38 Another study showed that suppression of TNF-α could attenuate MCT-induced PAH.39 Blocking IL-1 signalling may also have beneficial effects in the treatment of PAH.40 These inflammatory cytokines appear to be so pivotal that they could serve as biomarkers of disease progression or as therapeutic targets. A study into the protective effects of HSYA on acute pulmonary injury, found that HSYA normalized the expression of inflammatory cytokines, including TNF-α and IL-1β, and significantly decreased the number of infiltrating inflammatory cells.41 The anti-inflammatory effect of HSYA was also demonstrated in a study that showed inflammatory mediators, including IL-1β and TNF-α, were significantly reduced in a HSYA-treated group.42 The effect of HSYA on PAH, however, was not reported. In order to evaluate the role of inflammatory status in the development of PAH, the present study investigated IL-1β, IL-6, and TNF-α expression in pulmonary tissue, and demonstrated increased IL-1β, IL-6 and TNF-α levels in pulmonary tissues from MCT-treated rats, supporting the role of inflammatory status in PAH pathogenesis. In MCT plus HSYA-treated rats, the present study found that cytokines significantly decreased compared with MCT-treated rats, and these effects were accompanied by attenuated pulmonary vascular remodelling. Inflammatory status in an MCT-induced PAH model is not invariable, and comprises an initial asymptomatic inflammatory phase, followed by a less inflammatory symptomatic phase just before the onset of pulmonary vascular remodelling.43 The present study showed the effectiveness of HSYA therapy in MCT-induced PAH, and the effect may relate to its immunosuppressive function, however, the way in which HSYA influences the entire inflammatory stage remains unclear, and will be the subject of further investigation by the present authors.
Suppression of oxidative stress is also important in preventing the development of PAH,44,45 and studies in rat models of MCT-induced pulmonary hypertension have shown that oxidative stress impairs the pulmonary vascular endothelium and leads to smooth muscle cell proliferation.46,47 Another study demonstrated that reduction of oxidative stress biomarkers was associated with amelioration of clinical symptoms in patients with PAH.48 Thus, drugs with antioxidant effects may be beneficial in the treatment of PAH. 8-OHdG is a product of DNA oxidative damage caused by reactive oxygen species,49 and could be a new biomarker to assess DNA oxidative damage and oxidative stress. MDA is the ultimate product of unsaturated lipid peroxidation,50 and measurement of MDA in the blood can provide information on cellular membrane injury caused by excessive generation of free radicals. SOD, an important antioxidant enzyme that regulates oxidative tissue damage, catalyses the dismutation of two superoxide radicals to hydrogen peroxide and oxygen.51 Thus, 8-OHdG, MDA and SOD are the most frequently used biomarkers of oxidative stress in clinical or animal experiments.52–54 An investigation of the protective effects of HSYA on cultured rat cardiomyocytes exposed to anoxia/reoxygenation demonstrated that HSYA produced protective effects through increased SOD activity and decreased MDA production.16 In the present study, 8-OHdG and MDA levels were found to be increased and SOD activity was decreased in the pulmonary tissue of MCT-induced PAH in rats. Treatment with HSYA significantly decreased the 8-OHdG and MDA content and increased SOD activity compared with MCT alone, suggesting that these factors may contribute to the antioxidative effects of HSYA.
The results of the present study are limited by several factors. First, the sample number is relatively small, and would need to be increased in further studies. Secondly, protein levels of inflammatory and oxidative indices were not measured, and should be investigated in future studies to support the results of the present study, which showed changes in mRNA levels. Thirdly, overall inflammatory and oxidative stress were not assessed during the development of MCT-induced PAH, but only at one time point, and HSYA treatment initiated at different stages of PAH pathogenesis should be investigated. Fourthly, the MCT-induced PAH model may not be fully representative of PAH in humans, and thus the effectiveness of HSYA treatment should be evaluated in other models of PAH with different aetiologies.
In conclusion, the present study demonstrated that HSYA treatment reduced haemodynamic deterioration and pulmonary vascular remodelling in MCT-induced PAH. HSYA may have potential as a supplementary treatment in PAH through attenuation of disease pathogenesis. The present findings provide new insight into the potential role of reducing inflammation and oxidation in the treatment of PAH. Further studies are required to examine the effects of HSYA in treating established PAH, in addition to attenuating PAH pathogenesis. Moreover, clinical studies are required to explore the effectiveness of HSYA in patients with PAH.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Ren Shu fund from Hunan Provincial People’s Hospital (No. 2006-60) and by general grants from building ‘111’ talents team of Hunan Provincial People’s Hospital, and funded by Changsha Municipal Science and Technology Bureau (No. k1307039-31).
References
- 1.McLaughlin W, Shah SJ, Souza R, et al. Management of pulmonary arterial hypertension. J Am Coll Cardiol 2015; 65: 1976–1997. [DOI] [PubMed] [Google Scholar]
- 2.Montani D, Chaumais MC, Guignabert C, et al. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther 2014; 141: 172–191. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Manes A. New treatment strategies for pulmonary arterial hypertension: hopes or hypes? J Am Coll Cardiol 2013; 62: 1101–1102. [DOI] [PubMed] [Google Scholar]
- 4.Li XM, Huang CK, Zhang TF, et al. The Chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J Allergy Clin Immunol 2000; 106: 660–668. [DOI] [PubMed] [Google Scholar]
- 5.Newman DJ, Gragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod 2007; 70: 461–477. [DOI] [PubMed] [Google Scholar]
- 6.Kolli MB, Manne ND, Para R, et al. Cerium oxide nanoparticles attenuate monocrotaline induced right ventricular hypertrophy following pulmonary arterial hypertension. Biomaterials 2014; 35: 9951–9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luan Y, Chao S, Ju ZY, et al. Therapeutic effects of baicalin on monocrotaline-induced pulmonary arterial hypertension by inhibiting inflammatory response. Int Immunopharmacol 2015; 26: 188–193. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed LA, Obaid AA, Zaki HF, et al. Naringenin adds to the protective effect of L- arginine in monocrotaline-induced pulmonary hypertension in rats: favorable modulation of oxidative stress, inflammation and nitric oxide. Eur J Pharm Sci 2014; 62: 161–170. [DOI] [PubMed] [Google Scholar]
- 9.Kong D, Xia W, Zhang Z, et al. Safflower yellow injection combined with conventional therapy in treating unstable angina pectoris: a meta-analysis. J Tradit Chin Med 2013; 33: 553–561. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Peng JH, Zhang XN. A clinical study of Safflower Yellow injection in treating coronary heart disease angina pectoris with Xin-blood stagnation syndrome. Chin J Integr Med 2005; 11: 222–225. [in Chinese, English abstract]. [DOI] [PubMed] [Google Scholar]
- 11.Liu SX, Zhang Y, Wang YF, et al. Upregulation of heme oxygenase-1 expression by hydroxysafflor yellow A conferring protection from anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int J Cardiol 2012; 160: 95–101. [DOI] [PubMed] [Google Scholar]
- 12.Sun X, Wei X, Qu S, et al. Hydroxysafflor yellow A suppresses thrombin generation and inflammatory responses following focal cerebral ischemia- reperfusion in rats. Bioorg Med Chem Lett 2010; 20: 4120–4124. [DOI] [PubMed] [Google Scholar]
- 13.Han SY, Li HX, Ma X, et al. Protective effects of purified safflower extract on myocardial ischemia in vivo and in vitro. Phytomedicine 2009; 16: 694–702. [DOI] [PubMed] [Google Scholar]
- 14.Koyama N, Suzuki K, Furukawa Y, et al. Effects of safflower seed extract supplementation on oxidation and cardiovascular risk markers in healthy human volunteers. Br J Nutr 2009; 101: 568–575. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu M, Takahashi T, Komatsu M, et al. Antioxidant and neuroprotective activities of Mogami-benibana (safflower, Carthamus tinctorius Linne). Neurochem Res 2009; 34: 795–805. [DOI] [PubMed] [Google Scholar]
- 16.Duan JL, Wang JW, Guan Y, et al. Safflor yellow A protects neonatal rat cardiomyocytes against anoxia/reoxygenation injury in vitro. Acta Pharmacol Sin 2013; 34: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan LH, Chen J, Li L, et al. Protective effects of Carthamus tinctorius injection on isoprenaline-induced myocardial injury in rats. Pharm Biol 2011; 49: 1204–1209. [DOI] [PubMed] [Google Scholar]
- 18.He Y, Liu Q, Li Y, et al. Protective effects of hydroxysafflor yellow A (HSYA) on alcohol-induced liver injury in rats. J Physiol Biochem 2015; 71: 69–78. [DOI] [PubMed] [Google Scholar]
- 19.Liu YL, Liu YJ, Liu Y, et al. Hydroxysafflor yellow A ameliorates lipopolysaccharide-induced acute lung injury in mice via modulating toll-like receptor 4 signaling pathways. Int Immunopharmacol 2014; 23: 649–657. [DOI] [PubMed] [Google Scholar]
- 20.Rosner B. Fundamentals of Biostatistics. 7th Ed. Brooks/Cole, 2010, pp.302–303.
- 21.Kang KK, Ahn GJ, Sohn YS, et al. Da-8159, a new PDE5 inhibitor, attenuates the development of compensatory right ventricular hypertrophy in a rat model of pulmonary hypertension. J Int Med Res 2003; 31: 517–528. [DOI] [PubMed] [Google Scholar]
- 22.Xu D, Guo H, Xu X, et al. Exacerbated pulmonary arterial hypertension and right ventricular hypertrophy in animals with loss of function of extracellular superoxide dismutase. Hypertension 2011; 58: 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tual L, Morel OE, Favret F, et al. Carvedilol inhibits right ventricular hypertrophy induced by chronic hypobaric hypoxia. Pflugers Arch 2006; 452: 371–379. [DOI] [PubMed] [Google Scholar]
- 24.Abe K, Shimokawa H, Morikawa K, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res 2004; 94: 385–393. [DOI] [PubMed] [Google Scholar]
- 25.Huang YJ, Zhang BB, Ma N, et al. Nitrative and oxidative DNA damage as potential survival biomarkers for nasopharyngeal carcinoma. Med Oncol 2011; 28: 377–384. [DOI] [PubMed] [Google Scholar]
- 26.Boskos CS, Liacos C, Korkolis D, et al. Thymidine phosphorylase to dihydropyrimidine dehydrogenase ratio as a predictive factor of response to preoperative chemoradiation with capecitabine in patients with advanced rectal cancer. J Surg Oncol 2010; 102: 408–412. [DOI] [PubMed] [Google Scholar]
- 27.Escobar J, Pereda J, Arduini A, et al. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: a key role for protein phosphatases. Curr Pharm Des 2009; 15: 3027–3042. [DOI] [PubMed] [Google Scholar]
- 28.Lugrin J, Rosenblatt-Velin N, Parapanov R, et al. The role of oxidative stress during inflammatory processes. Biol Chem 2014; 395: 203–230. [DOI] [PubMed] [Google Scholar]
- 29.Richards GA, White H, Grimmer H, et al. Increased oxidants and reduced antioxidants in irradiated parenteral nutrition solutions may contribute to the inflammatory response. J Intensive Care Med 2009; 24: 252–260. [DOI] [PubMed] [Google Scholar]
- 30.Crosswhite P, Sun Z. Nitric oxide, oxidative stress and inflammation in pulmonary arterial hypertension. J Hypertens 2010; 28: 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csiszar A, Labinskyy N, Olson S, et al. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension 2009; 54: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asgarpanah J, Kazemivash N. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin J Integr Med 2013; 19: 153–159. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Xue C, Dong F, et al. Hydroxysafflor yellow a attenuates small airway remodeling in a rat model of chronic obstructive pulmonary disease. Biol Pharm Bull 2014; 37: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 34.Sun CY, Pei CQ, Zang BX, et al. The ability of hydroxysafflor yellow a to attenuate lipopolysaccharide-induced pulmonary inflammatory injury in mice. Phytother Res 2010; 24: 1788–1795. [DOI] [PubMed] [Google Scholar]
- 35.Groth A, Vrugt B, Brock M, et al. Inflammatory cytokines in pulmonary hypertension. Respir Res 2014; 15: 47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Chami H, Hassoun PM. Immune and inflammatory mechanisms in pulmonary arterial hypertension. Prog Cardiovasc Dis 2012; 55: 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabinovitch M, Guignabert C, Humbert M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014; 115: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009; 104: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Zuo XR, Wang YY, et al. Monocrotaline-induced pulmonary arterial hypertension is attenuated by TNF-α antagonists via the suppression of TNF-α expression and NF-kβ pathway in rats. Vascul Pharmacol 2013; 58: 71–77. [DOI] [PubMed] [Google Scholar]
- 40.Lawrie A, Hameed AG, Chamberlain J, et al. Paigen diet-fed apolipoprotein E knockout mice develop severe pulmonary hypertension in an interleukin-1-dependent manner. Am J Pathol 2011; 179: 1693–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y, Wang L, Jin M, et al. Hydroxysafflor yellow A alleviates early inflammatory response of bleomycin-induced mice lung injury. Biol Pharm Bull 2012; 35: 515–522. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z, Wu Z, Zhu X, et al. Hydroxy- safflor yellow A inhibits neuroinflammation mediated by Aβ1-42 in BV-2 cells. Neurosci Lett 2014; 562: 39–44. [DOI] [PubMed] [Google Scholar]
- 43.Price LC, Montani D, Tcherakian C, et al. Dexamethasone reverses monocrotaline-induced pulmonary arterial hypertension in rats. Eur Respir J 2011; 37: 813–822. [DOI] [PubMed] [Google Scholar]
- 44.Ogura S, Shimosawa T, Mu S, et al. Oxidative stress augments pulmonary hypertension in chronically hypoxic mice overexpressing the oxidized LDL receptor. Am J Physiol Heart Circ Physiol 2013; 305: 155–162. [DOI] [PubMed] [Google Scholar]
- 45.Sutendra G, Bonnet S, Rochefort G, et al. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med 2010; 2: 44ra58–44ra58. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed LA, Obaid AA, Zaki HF, et al. Role of oxidative stress, inflammation, nitric oxide and transforming growth factor-beta in the protective effect of diosgenin in monocrotaline-induced pulmonary hypertension in rats. Eur J Pharmacol 2014; 740: 379–387. [DOI] [PubMed] [Google Scholar]
- 47.Dorfmuller P, Chaumais MC, Giannakouli M, et al. Increased oxidative stress and severe arterial remodeling induced by permanent high-flow challenge in experimental pulmonary hypertension. Respir Res 2011; 12: 119–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan YF, Zhang R, Jiang X, et al. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc Res 2013; 99: 395–403. [DOI] [PubMed] [Google Scholar]
- 49.Black CN, Bot M, Scheffer PG, et al. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015; 51: 164–175. [DOI] [PubMed] [Google Scholar]
- 50.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 2005; 15: 316–328. [DOI] [PubMed] [Google Scholar]
- 51.Maier CM, Chan PH. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist 2002; 8: 323–334. [DOI] [PubMed] [Google Scholar]
- 52.Niimi K, Yasui T, Hirose M, et al. Mitochondrial permeability transition pore opening induces the initial process of renal calcium crystallization. Free Radic Biol Med 2012; 52: 1207–1217. [DOI] [PubMed] [Google Scholar]
- 53.Deng Y, Zhang Y, Zhang R, et al. Mice in vivo toxicity studies for monohaloacetamides emerging disinfection byproducts based on metabolomic methods. Environ Sci Technol 2014; 48: 8212–8218. [DOI] [PubMed] [Google Scholar]
- 54.Chang D, Zhang X, Rong S, et al. Serum antioxidative enzymes levels and oxidative stress products in age-related cataract patients. Oxid Med Cell Longev 2013; 2013: 587826–587826. [DOI] [PMC free article] [PubMed] [Google Scholar]