Abstract
Objective
To explore working memory and the ability to process different emotional stimuli in patients with first-onset and untreated minor (mild or moderate) depression.
Methods
Patients with first-onset and previously untreated minor depression, and healthy controls, were enrolled. Using a modified Sternberg working memory paradigm to investigate the combined effects of emotional stimuli with working memory, participants were exposed to experimental stimuli comprising pictures that represented positive, neutral and negative emotions. Working memory ability was measured using reaction time and accuracy, and emotion-processing ability was measured using pupil diameter.
Results
Out of 36 participants (18 patients with minor depression and 18 controls), there were no statistically significant between-group differences in response time and accuracy. Positive stimuli evoked changes in pupil diameter that were significantly smaller in patients with minor depression versus controls, but changes in pupil diameter evoked by negative stimuli were not significantly different between the two groups.
Conclusions
Healthy subjects showed a stronger emotional response to positive emotional stimuli than patients with first onset and previously untreated minor depression, but there were no differences in response to negative emotions. There were no statistically significant between-group differences in terms of speed of cognitive response, but this may have been due to the relatively small samples sizes assessed. Studies with larger sample populations are required to further investigate these results.
Keywords: Depression, emotion, eye movement, pupil diameter, working memory
Introduction
Patients with depression are thought to insufficiently process positive information and excessively process negative information,1 tending to ignore happy emotional stimuli while preferentially processing negative emotional stimuli.2–4 In addition, a study in non-clinical populations demonstrated that depression diminishes the capability of imagining future positive outcomes and strengthens the ability to imagine negative outcomes.5 Patients with affective disorders also present cognitive dysfunction in areas such as working memory, attention and learning.6–11
Cognitive dysfunction in depression is primarily characterized by executive dysfunction resulting from frontal lobe damage, and memory disorders resulting from temporal lobe damage.12 Depression has been shown to significantly impair attention and word memory,13 however, although patients with depression are shown to have impaired free recall, their cued recall and recognition are comparable to those of healthy individuals.14 A study using the Sternberg working memory paradigm15 found that capacity for working memory was decreased in patients with untreated major depressive disorder.16 Using a verbal version of the n-back task,17 compared with healthy individuals, patients with major depressive disorder exhibited reduced accuracy and slower reaction times on 1-, 2-, and 3-back tasks, however, there was no significant between-group difference at the baseline 0-back level.18 Patients with major depressive disorder were also shown to be significantly impaired in terms of performance (accuracy and reaction time) on a 2-back task, compared with healthy individuals.19 Using a symbol n-back experimental paradigm, individuals with major depressive disorder were demonstrated to have significant memory impairments and exhibit lower accuracy versus healthy controls on 0-, 1-, and 2-back task levels, but not on a baseline 3-back level.20 The same study also found that patients’ reaction times were significantly longer at all levels versus healthy controls.20 Brain structure research into emotion and working memory focuses on the amygdala in the medial temporal lobe (the key brain structure for emotional memory, representing the core of the entire emotional memory neural network), and the hippocampus (which represents memory organization).21 The amygdala and hippocampus work together via two independent systems when dealing with emotion and memory,22 however, in patients with depression, cognitive dysfunction and resulting memory impairment, this collaboration is difficult to identify.
Many findings from studies into working memory remain inconsistent, possibly due to differing experimental paradigms and materials, however, all have demonstrated that working memory is impaired in patients with depression. Most cognitive impairment research has focused on patients with mild or moderate depressive symptoms, with little research regarding first-onset and untreated depressive disorder. In patients with depression, cognitive dysfunction, such as reduced working memory, is thought to occur because of cognitive control impairment.23 The prefrontal lobe and anterior cingulate (including ventrolateral prefrontal cortex and dorsolateral prefrontal cortex) comprise the centre of cognitive control and executive function.24 In healthy individuals, activation of the prefrontal cortex leads to reduced activity in the negative emotion processing regions, such as the amygdala,25 suggesting that this cognitive control region has a regulatory role in processing negative emotions. Patients with depression who completed a colour-word Stroop task,26 which requires cognitive control, were shown to have a greater probability of error and significantly decreased event-related potential N2 and N450 amplitudes compared with healthy controls, and these changes occurred in the left dorsolateral prefrontal and dorsal anterior cingulate regions.27 In a digital sorting task that required cognitive control, patients with depression exhibited significantly decreased dorsolateral prefrontal cortex activity compared with healthy controls, suggesting that brain disorders related to cognitive control functions in patients with depression result in a failure to effectively adjust and control emotional processing regions.28 Patients with depression, who were directed to successively complete two independent tasks,29 showed excessive activation of the emotion processing brain regions during an emotion processing task versus healthy controls, whereas during a digital scheduling task, cognitive control brain regions were insufficiently activated. When emotion processing was combined with task performance in an experimental paradigm,30 brain regions involved in emotional processing were revealed to be excessively activated in patients with depression, whereas cognitive control brain regions were insufficiently activated. Cognitive control and emotional processing brain regions are thought to control and regulate each other,31 and major depressive disorder is often accompanied by working memory deficits caused by competition for resources generated between emotional and cognitive processing. Decreased cognitive control ability leads to inadequate distribution of cognitive and attention resources for cognitive tasks.32
Despite a large number of studies into emotional damage and working memory in depression, several deficiencies exist: most studies focus on major depressive disorder, with few studies into first-onset and untreated depressive disorder (mild/moderate depressive symptoms); the majority of studies examine emotional processing and working memory issues as separate elements, rather than in combination; previous studies have focused on the working memory system (executive function, executive function templates, and voice and visual space templates), with less attention to the memory function system (memory encoding and retrieval); and research has focused on emotional processing functions, with limited studies investigating processing capacity for external emotional stimuli.
Research into working memory suggests that patients with major depressive disorder, whose work and daily life has been affected, do not have complete damage to working memory function.14,18,20 Based on the different manifestations of work, studies and lives of patients with non-severe versus severe depression, the present authors hypothesized that working memory is partially and mildly damaged in patients with non-severe depression. Some studies suggest that cognitive dysfunction does not improve with remission of depression,6,33–36 which infers that there would be no relationship between cognitive impairment and depression severity.36–39 In addition, patients with major depression show impairments in cognitive control and executive functions,6,7,29,33,40 and this decreased ability to process information should affect cognition ability and behaviour. Patients with depression tend to insufficiently process positive information but excessively process negative information,1 therefore, the present authors hypothesized that the performance of emotionally impaired patients with first-onset and untreated depressive disorder is characterized by a reduced capacity for external positive-emotion processing and excessive capacity for negative-emotion processing.
In the present study, patients with first-onset and previously untreated minor depression were compared with healthy subjects, using a modified Sternberg working memory paradigm15,41 that combined emotional stimuli with working memory to investigate emotional experience and working memory abilities. Working-memory ability was measured using reaction time and accuracy, and emotion-processing ability was measured by changes in pupil diameter, as mental activities associated with emotions can lead to changes in pupil size.42,43
Patients and methods
Study population
This prospective case-control study sequentially recruited patients with first-onset and previously untreated depressive disorder (minor depression group) attending the outpatient clinic at Beijing Anding Hospital, Capital Medical University, Beijing, China. Sex-, age- and education-matched healthy subjects were recruited from the local population in Beijing via advertisements, and the study was conducted between December 2013 and March 2015. Patients with depression were screened by a psychiatrist (LF and BbF) using the Mini International Neuropsychiatric Interview44 and diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria.45
Inclusion criteria for patients with minor depression were as follows: (1) aged 16–60 years and right-handed; (2) met the DSM-IV diagnostic criteria for depression and diagnosed with mild or moderate depression; (3) scored < 24 on the 17-item Hamilton Depression Rating Scale (HDRS),46 which was used to assess the degree of depression; (4) were undergoing treatment for the first time and had no history of taking antidepressants or drugs that affected nervous system function prior to treatment; (5) had no suicidal tendencies and could work, study and live normally; and (6) had normal or corrected-to-normal vision with no colour blindness or other eye disease, and could complete the eye movement experiment. Exclusion criteria included concomitant delirium, dementia and other cognitive disorders, and substance misuse. Inclusion criteria for the healthy controls were as follows: (1) aged 16–60 years and right-handed; (2) scored < 5 on a self-assessment based on the quick inventory of depressive symptomatology-self report (QIDS-SR16)47 and <4 on the Beck Depression Inventory;48 (3) had no history of mental illness; and (4) had normal or corrected-to-normal vision with no colour blindness or other eye disease.
This study was approved by the Ethics committee of Beijing Anding Hospital, Capital Medical University, China. All participants provided written informed consent, and received compensation for their participation.
Experimental materials
Sixty pictures were selected from the International Affective Picture System49 based on the level of valence and arousal. Positive pictures were selected to have high valence and high arousal levels (mean valence level 7.31 ± 0.44, mean arousal level 5.54 ± 0.44), and negative pictures had low valence and high arousal (mean valence level 2.79 ± 0.51, mean arousal level 5.97 ± 0.44). Neutral pictures were images of daily necessities, such as life and work scenes, buildings, transportation, and geometry figures that did not easily induce emotion (mean valence level 5.18 ± 0.17, mean arousal level 3.23 ± 0.22). All of the pictures were processed using Adobe Photoshop software, version 6.0 (Adobe Systems Corporation, San Jose, CA, USA); and the size (7.94 × 7.94 cm), grey scale and resolution were consistent between the pictures.
Experimental paradigm and procedure
The present study applied a modified Sternberg working memory paradigm,15,41 and substituted three types of pictures (to stimulate positive, neutral and negative emotions) for the string of numbers conventionally used as cues. To sufficiently induce the participants’ emotions, each cue included four pictures of the same type of emotional category, which were presented to the subjects once. The experiment included three types of cue: positive, negative and neutral emotion pictures. The target stimulus was a picture, and participants were asked to determine whether the target picture had appeared in the cue.
The experimental procedure followed several steps. First, a ‘+’ sign appeared at the center of a 17-inch liquid crystal display screen (1024 × 768 resolution) for 0.5 s to indicate that the stimulus pictures would appear. The cue was presented for 10 s, and participants were instructed to carefully watch the pictures and remember them. After 5 s for memory retention, a target picture appeared on the screen, and participants were asked to determine whether it had appeared in the cue. If it had appeared in the cue, participants were instructed to click the left button; otherwise, they clicked the right button. Finally, a ‘*’ symbol appeared on the screen, indicating that the participants should rest for 2 s. After this time, the next trial began.
The experimental equipment comprised a corneal reflection eye tracker (Tobii T120 Eye Tracker; Tobii Technology, Danderyd, Sweden), with a sampling frequency of 120 Hz. The experimental materials were displayed on the liquid crystal display screen using a Microsoft IE6.0 browser (Microsoft Corporation, Seattle, USA), and the distance between the display screen and participants was 60 cm. As the cue was presented, the Tobii T120 eye tracker recorded pupil diameter at each fixation point as participants viewed the pictures, and reaction time and accuracy as participants responded throughout the task. Participants were required to keep their heads still, and there were no other physical constraints.
To study the brain’s ability to process positive and negative emotional stimuli, differences in pupil diameter changes evoked by positive and negative emotional pictures were analysed in patients with minor depression and control groups using the following calculations:
Assuming that there were m fixations in task j of participant i, the pupil diameter size of the k (k = 1, 2,…, m) fixation point Fijk is PTijk in participant i, which was calculated from the following equation
| (1) |
where Leftijk is the left pupil diameter of the fixation and Rightijk is the right pupil diameter of the fixation. Thus, the mean pupil diameter in task j for participant i was as follows:
| (2) |
Assuming the PSi is the change in pupil diameter evoked by the r positive emotional picture stimuli of participant i based on equation (1) and equation (2), the pupil diameter change for the l negative emotional picture stimuli, PNi, would then be calculated by the following two equations:
| (3) |
| (4) |
where the PRi is the mean pupil diameter of participant i evoked by all of the neutral emotion picture stimuli.
Statistical analyses
Data are presented as mean ± SD, and statistical analyses were conducted using SPSS software, version 20.0 (SPSS, Chicago, IL, USA). Reaction time and accuracy were analysed based on emotional category (positive, neutral or negative). Pupil diameter changes (difference in pupil diameter between emotional and emotion-neutral states) were analysed based on type of emotion (positive or negative). A mixed-model analysis of variance (ANOVA) with 2 (group: minor depression, healthy controls) × 3 (within-subject factor stimulus material: positive, neutral, negative) repeated measures was conducted to analyse accuracy and reaction time. Using a two-sample t-test, between-group differences in pupil diameter changes were compared. In addition, a Bayesian analysis was conducted,50 and Pearson’s correlation coefficient was used to assess pupil diameter changes that correlated with accuracy and reaction time.
To correct for outliers, trials for which reaction times were either > 3 SD from each participant’s mean (calculated separately for each trial type) or <400 ms were excluded (a standard procedure used to trim reaction-time data), which removed approximately 1.25% of the trials. The resulting means for the corrected trials were used in the present analyses. A P-value < 0.05 was considered to be statistically significant.
Results
Demographic and clinical data
A total of 18 patients with first-onset and previously untreated minor depressive disorders and 18 healthy controls were included, and the two groups were matched by sex, age and education. In the minor depression group (8 male and 10 female patients), mean age was 34.28 ± 10.12 years, mean number of years in education was 13.61 ± 3.52, mean HDRS score was 16.06 ± 4.35, and mean QIDS-SR16 score was 16.56 ± 3.35. In the healthy controls (9 male and 9 female participants), mean age was 33.56 ± 8.48 years, mean number of years in education was 14.50 ± 3.45, and mean QIDS-SR16 score was 2.50 ± 1.58.
Between-group comparisons of working memory reaction time
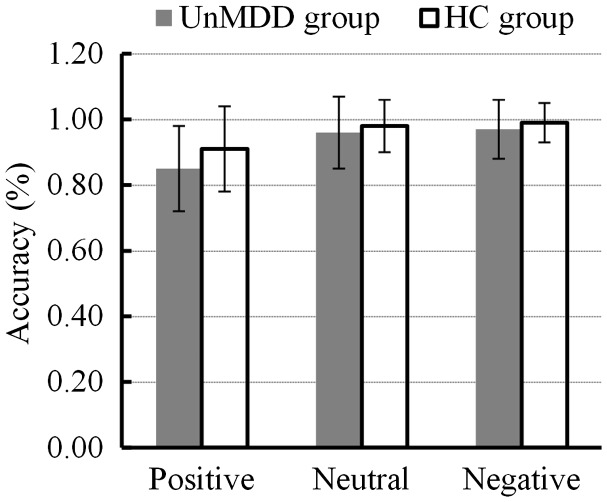
Reaction times for positive, neutral and negative pictures between the minor depression and healthy control groups are shown in Figure 1. A mixed-model ANOVA with 2 (group: minor depression, healthy controls) × 3 (emotional category: positive, neutral, negative) repeated measures analysis of reaction time revealed that there was no statistically significant overall effect of emotional category (F [2, 33] = 0.231; P = 0.601), and no significant interaction effect (F [2, 33] = 0.598, P = 0.451). There was also no statistically significant overall effect in terms of group (F [1, 34] = 3.571, P = 0.067). A follow-up independent t-test (positive, t [34] = 1.965, P = 0.058; neutral, t [34] = 1.009, P = 0.320; and negative, t [34] = 1.903, P = 0.066] also showed that there was no statistically significant difference in terms of response time between healthy controls and patients with minor depression.
Figure 1.
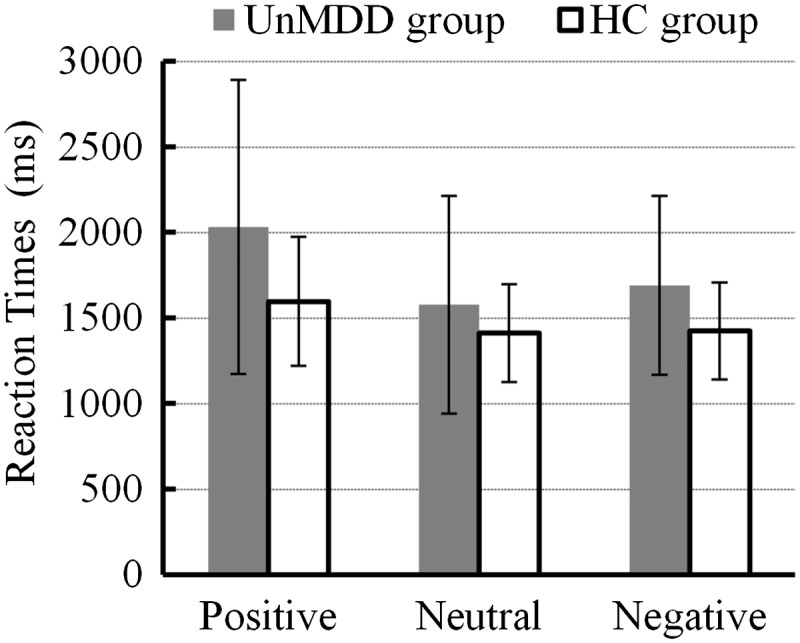
Comparison of working memory reaction time for different emotional stimuli (positive, neutral or negative) between patients with first onset and previously untreated minor depression (n = 18) and age-, sex-, and educationally-matched healthy controls (n = 18); UnMDD, first onset and previously untreated minor depression; HC, heathy controls.
Between-group comparisons of working memory accuracy for different emotion categories
To address whether any difference in reaction time between the two study groups could be due to insufficient encoding, maintaining and/or retrieval the difference in between-group accuracy was assessed. Between group comparisons of accuracy in the positive, neutral and negative emotion categories are shown in Figure 2. A mixed-model ANOVA with 2 (group: minor depression, healthy controls) × 3 (emotional category: positive, neutral, negative) repeated measures analysis of accuracy showed that there was no statistically significant overall effect of emotional category (F [2, 33] = 1.411, P = 0.212), no significant interaction effect (F [2, 33] = 1.204, P = 0.213), and no significant overall effect in terms of group (F [1, 34] = 2.700, P = 0.101), indicating that there were no between-group differences in working memory task performance.
Figure 2.
Comparison of working memory accuracy for different emotional stimuli (positive, neutral or negative) between patients with first onset and previously untreated minor depression (n = 18) and age-, sex-, and educationally-matched healthy controls (n = 18); UnMDD, first onset and previously untreated minor depression; HC, heathy controls.
Change in pupil diameter
Analysis of pupil diameter in response to neutral stimuli showed that mean pupil diameter in the minor depression group was 3.71 ± .56 mm, and in the healthy controls was 3.81 ± .45 mm, with no statistically significant between-group difference (t [34] = −0.518, P = 0.609).
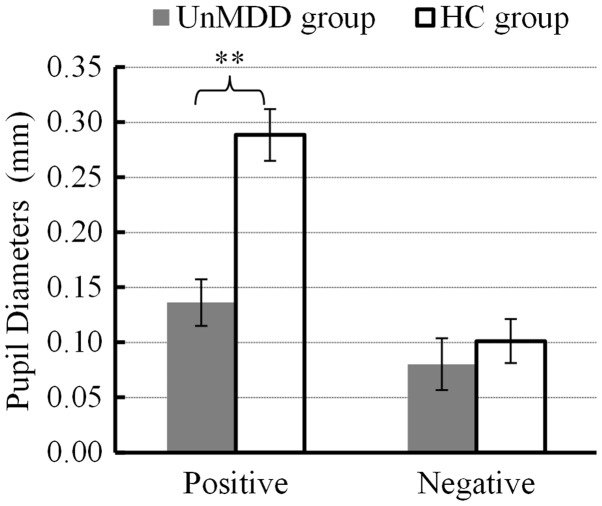
Using a mixed-model ANOVA to compare mean change in pupil diameter induced by positive and negative emotion pictures (Figure 3), revealed a statistically significant difference in overall effect of pupil diameter change between the groups (F [1, 34] = 13.254, P < 0.005). An independent-samples t-test showed that there were statistically significant between-group differences in changes of pupil diameter induced by positive emotion (t [34] = 4.823, P < 0.000, P Bayes [H0] = 0.002, Bayes Factor = 621.18), and these changes were significantly higher in the healthy controls. There were no statistically significant between-group differences in change of pupil diameter induced by negative emotion (t [34] = 0.686, P = 0.497, P Bayes (H0) = 0.721, Bayes Factor = 2.59).
Figure 3.
Changes in pupil diameter in response to positive and negative emotional experiences in patients with first onset and previously untreated minor depression (n = 18) and age-, sex-, and educationally-matched healthy controls (n = 18). Data presented as mean ± SD; **P < 0.005 (mixed-model analysis of variance); UnMDD, first onset and previously untreated minor depression; HC, heathy controls.
Relationship between change in pupil diameter and working memory accuracy/reaction time
Pearson’s correlation coefficient analyses to explore whether changes in pupil diameter were associated with working memory reaction time and accuracy (Table 1), revealed that in patients with minor depression, change in pupil diameter evoked by positive emotional stimuli was correlated with memory accuracy regarding positive information (r = 0.448, P = 0.048), whereas change in pupil diameter evoked by negative emotional stimuli was not significantly correlated with memory accuracy regarding negative information (P = 0.062). No other statistically significant correlations were observed.
Table 1.
Pearson’s correlation coefficient analysis of the association between change in pupil diameter, and working memory reaction time and accuracy in patients with first onset and previously untreated minor depression (n = 18) and age-, sex-, and educationally-matched healthy controls (n = 18).
| Study parameter |
|||
|---|---|---|---|
| Study group | Change in pupil diameter (stimulus type) | Accuracy | Reaction time |
| Minor depression | Positive stimuli | 0.448a | −0.395 |
| Negative stimuli | −0.322b | 0.235 | |
| Healthy control | Positive stimuli | 0.165 | −0.191 |
| Negative stimuli | −0.172 | 0.128 | |
P = 0.048; bP = 0.062.
Discussion
Reaction time and accuracy are thought to be behavioural indicators of working memory damage in patients with major depressive disorders.16,18 In the present study, overall working memory capacity (reaction time and accuracy) of patients with minor depression was not significantly different from healthy controls, suggesting that working memory function (including encoding, maintaining and retrieval) in patients with minor depression may not be impaired. These results were inconsistent with the authors’ hypothesis that working memory is partially and mildly damaged in patients with non-severe depression, but were consistent with a study that found patients with major depressive disorder had a normal capacity for cued recall and recognition.14 The present study showed that mean reaction times in patients with minor depression were not significantly different from healthy controls, however, this lack of statistical significance may have been due to the small sample size investigated. The present results suggest that information processing in general may not be slower in patients with minor depression versus healthy individuals, however, further investigation with a larger sample size is required to confirm this.
Expansion and narrowing of pupil diameter has been shown to reflect emotional changes in humans.42,43 Positive pictorial stimuli, containing happy emotional information, will cause a happy emotional experience, resulting in increased pupil diameters. Negative pictorial stimuli, containing sad or frightening emotional information, will evoke unpleasant emotional experiences, resulting in decreased pupil diameters. Different emotional experiences are the result of different emotional processing activities in the brain and the direct reactions to those activities. The present study found that when patients with minor depression viewed positive pictures, changes in pupil diameter were significantly smaller than those of healthy individuals, but there was no such between-group difference when participants viewed negative pictures. These results suggest that patients with minor depression have limitations in processing information from external positive visual stimuli, which may result in an insufficient ability to experience pleasurable emotions. Patients with minor depression did not present limitations in processing information from external negative visual stimuli, however, suggesting that the negative emotional experience did not differ from healthy individuals in the present study. This finding partially concurred with the authors’ hypothesis that patients with first-onset, untreated depression have a reduced capacity for external positive-emotion processing and excessive capacity for negative-emotion processing. Two factors may explain this partial agreement: First, the present research included patients with first-onset and previously untreated mild or moderate depressive symptoms; and secondly, changes in pupil diameter during an emotional experience were calculated using the participants’ pupil diameters during their own emotional experiences minus their pupil diameters in a neutral emotional state, which reflected the results of processing external emotional stimuli (the inherent unconscious negative automatic processing of patients with depression was excluded). For healthy individuals, pupil diameter size during an emotional state is the result of external emotional stimulation,42,43 and pupil diameter size during a neutral experience reflects the normal pupil diameter.51 In patients with depression, pupil diameter during the emotional state is the summation of pupil diameter caused by external emotional stimulation and pupil changes caused by internal negative emotion processing.51 Pupil size during the neutral state is the summation of pupil diameter during normal behaviour and pupil changes caused by internal unconscious negative automatic processing. Thus, from the view of cognitive subtraction, regardless of healthy control or depression group, changes in pupil diameter can only reflect the processing of external emotional stimulation.
In patients with depression, imaging studies have shown that negative emotion-processing brain regions are enhanced, with excessive processing of negative emotions compared with healthy controls.52 This result52 is produced by direct comparisons between patients with depression and healthy controls, reflecting the additional inherent and automatic unconscious negative emotional processing in patients with depression, and does not contradict the conclusions that patients with minor depression do not tend to handle external negative visual stimuli excessively. Thus, patients with depression tend to process external negative stimuli based on their inherent automatic processing of negative stimuli, which strengthens the activities of negative-processing brain regions in patients with depression compared with healthy individuals.52 Furthermore, changes in pupil diameter can only reflect the processing of external negative stimuli and the experience this evokes. The subjective experience of patients with severe depression is shown to be reduced for both positive and negative emotional stimuli, whereas the subjective experience of patients with mild depression is reduced only for positive emotional stimuli.53 The manifestations of emotional damage in patients with severe depression have been shown to indicate a reduced ability to process positive emotions and maintain the activity of brain areas, whereas negative emotional processing and brain activity maintenance are normal.54 These studies support the present finding that emotional damage in patients with non-severe depression results from a lack of positive emotion processing capacity rather than excessive negative emotion processing.
Anhedonia, the diminished capacity to experience pleasure, is related to dysregulation of the brain’s reward systems, and together with sensitivity to pressure, is thought be an endophenotypic characteristic for major depression.55 The present study showed that, although patients with minor depression and healthy controls did not differ in terms of negative emotional experience, patients with minor depression had a significantly lower positive emotional experience compared with healthy controls. This finding suggests that anhedonia is not only an endophenotypic feature of major depressive disorders, but also of moderate or mild depressive disorders: That is, anhedonia may be one of the core symptoms of depression.
Finally, analysis of the correlation between degree of emotional experience and working memory in the present study showed that change in pupil diameter evoked by positive stimuli in patients with minor depression was strongly correlated with memory accuracy in relation to positive information. The positive emotional experience in patients with minor depression was significantly lower than in healthy controls, suggesting that the lower the positive emotional experience, the lower the memory accuracy of positive information. In contrast, changes in pupil diameter evoked by negative stimuli were not significantly correlated with memory accuracy relating to negative information; the lack of significance may have been due to the relatively small sample size investigated. Studies of mood-congruent memory have suggested that, compared with healthy controls, patients with depression remember more negative stimuli that are consistent with their depressive mood, and remember less positive stimuli that are inconsistent with their mood.56 Increased memory for negative information with mood-congruency and decreased memory for positive information with mood-inconsistency are basic manifestations of memory capacity in patients with depression.57,58 The present results are consistent with published studies, and showed that non-major depression also had a mood-congruent memory effect, which may indicate that mood-congruent memory effects are one of the core features of depression.
The present study has several limitations: First, comparisons were only made between patients with first onset and previously untreated minor depression and healthy controls, and the present results were indirectly compared with results of previous findings in patients with major depression, which may affect the interpretation of the findings; Secondly, the limited sample size may have affected the statistical validity. For example, correlation analyses may not have resulted in an unbiased estimate of probability, although the sample size in the present study was comparable to a similar published eye-tracking study;59 Thirdly, the study of cognitive control and executive function was based on analysis of cognitive behaviours (reaction time and accuracy) rather than direct imaging of brain function. Future research should include a larger sample size, a comparator group of patients with severe depression, and provide imaging evidence, such as functional magnetic resonance imaging data.
The present study revealed that first-onset and previously untreated patients with mild or moderate depressive symptoms may have different working memory responses compared with healthy controls. In terms of emotional experience, healthy controls showed a stronger emotional response to positive emotions than patients with minor depression, whereas there was no significant between-group difference for negative emotions. Consistent with previous studies of major depressive disorder, first-onset and previously untreated mild or moderate depression showed a mood-congruent memory effect.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This work is supported by the National Basic Research Program of China (2014CB744600), the International Science & Technology Cooperation Program of China (2013DFA32180), the National Natural Science Foundation of China (61420106005), the Beijing Outstanding Talent Training Foundation (2014000020124G039), the Beijing Natural Science Foundation (4164080), the Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science (26350994), the Beijing Municipal Science and Technology Project (D12100005012003), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (ZY201403), and the Beijing Municipal Science and technology achievement transformation and industrialization projects funds (Z121100006112057).
References
- 1.MacLeod AK, Byrne A. Anxiety, depression, and the anticipation of future positive and negative experiences. J Abnorm Psychol 1996; 105: 286–289. [DOI] [PubMed] [Google Scholar]
- 2.Suslow T, Junghanns K, Arolt V. Detection of facial expressions of emotions in depression. Percept Mot Skills 2001; 92(3 Pt 1): 857–868. [DOI] [PubMed] [Google Scholar]
- 3.Surguladze SA, Young AW, Senior C, et al. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology 2004; 18: 212–218. [DOI] [PubMed] [Google Scholar]
- 4.Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry 2006; 19: 34–39. [DOI] [PubMed] [Google Scholar]
- 5.Bywaters M, Andrade J, Turpin G. Determinants of the vividness of visual imagery: the effects of delayed recall, stimulus affect and individual differences. Memory 2004; 12: 479–488. [DOI] [PubMed] [Google Scholar]
- 6.Weiland-Fiedler P, Erickson K, Waldeck T, et al. Evidence for continuing neuropsychological impairments in depression. J Affect Disord 2004; 82: 253–258. [DOI] [PubMed] [Google Scholar]
- 7.Nakano Y, Baba H, Maeshima H, et al. Executive dysfunction in medicated, remitted state of major depression. J Affect Disord 2008; 111: 46–51. [DOI] [PubMed] [Google Scholar]
- 8.Marazziti D, Consoli G, Picchetti M, et al. Cognitive impairment in major depression. Eur J Pharmacol 2010; 626: 83–86. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg PB, Mielke MM, Xue QL, et al. Depressive symptoms predict incident cognitive impairment in cognitive healthy older women. Am J Geriatr Psychiatry 2010; 18: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elderkin-Thompson V, Moody T, Knowlton B, et al. Explicit and implicit memory in late-life depression. Am J Geriatr Psychiatry 2011; 19: 249–255. [DOI] [PubMed] [Google Scholar]
- 11.Doumas M, Smolders C, Brunfaut E, et al. Dual task performance of working memory and postural control in major depressive disorder. Neuropsychology 2012; 26: 110–118. [DOI] [PubMed] [Google Scholar]
- 12.Medalia A, Lim R. Treatment of cognitive dysfunction in psychiatric disorders. J Psychiatr Pract 2004; 10: 17–25. [DOI] [PubMed] [Google Scholar]
- 13.Schatzberg AF, Posener JA, DeBattista C, et al. Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am J Psychiatry 2000; 157: 1095–1100. [DOI] [PubMed] [Google Scholar]
- 14.Fossati P, Coyette F, Ergis AM, et al. Influence of age and executive functioning on verbal memory of inpatients with depression. J Affect Disord 2002; 68: 261–271. [DOI] [PubMed] [Google Scholar]
- 15.Sternberg S. High speed scanning in human memory. Science 1966; 111: 652–654. [DOI] [PubMed] [Google Scholar]
- 16.Pelosi L, Slade T, Blumhardt LD, et al. Working memory dysfunction in major depression: an event-related potential study. Clin Neurophysiol 2000; 111: 1531–1543. [DOI] [PubMed] [Google Scholar]
- 17.Braver TS, Cohen JD, Nystrom LE, et al. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 1997; 5: 49–62. [DOI] [PubMed] [Google Scholar]
- 18.Harvey PO, Le Bastard G, Pochon JB, et al. Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res 2004; 38: 567–576. [DOI] [PubMed] [Google Scholar]
- 19.Barch DM, Sheline YI, Csernansky JG, et al. Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry 2003; 53: 376–384. [DOI] [PubMed] [Google Scholar]
- 20.Rose EJ, Ebmeier KP. Pattern of impaired working memory during major depression. J Affect Disord 2006; 90: 149–161. [DOI] [PubMed] [Google Scholar]
- 21.Squire LR, Knowlton BJ. The medial temporal lobe, the hippocampus, and memory systems of the brain. In: MS Gazzaniga. (eds). The New Cognitive Neurosciences, 2nd ed Massachusetts: MIT Press, 2000, pp. 765–780. [Google Scholar]
- 22.Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol 2004; 14: 198–202. [DOI] [PubMed] [Google Scholar]
- 23.Marvel CL, Paradiso S. Cognitive and neurological impairment in mood disorders. Psychiatr Clin North Am 2004; 27: 19–36, vii–viii–19–36, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schloesser RG, Wagner G, Koch K, et al. Fronto-cingulate effective connectivity in major depression: a study with fMRI and dynamic causal modeling. Neuroimage 2008; 43: 645–655. [DOI] [PubMed] [Google Scholar]
- 25.Goldin PR, McRae K, Ramel W, et al. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 2008; 63: 577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen AR, Rohwer WD. The Stroop color-word test: a review. Acta Psychol (Amst) 1966; 25: 36–93. [DOI] [PubMed] [Google Scholar]
- 27.Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia 2008; 46: 2904–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnstone T, van Reekum CM, Urry HL, et al. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 2007; 27: 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegle GJ, Thompson W, Carter CS, et al. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry 2007; 61: 198–209. [DOI] [PubMed] [Google Scholar]
- 30.Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry 2008; 63: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banich MT, Mackiewicz KL, Depue BE, et al. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev 2009; 33: 613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavric A, Rippon G, Gray JR. Threat-evoked anxiety disrupts spatial working memory performance: An attentional account. Cognitive Ther Res 2003; 27: 489–504. [Google Scholar]
- 33.Biringer E, Lundervold A, Stordal K, et al. Executive function improvement upon remission of recurrent unipolar depression. Eur Arch Psychiatry Clin Neurosci 2005; 255: 373–380. [DOI] [PubMed] [Google Scholar]
- 34.Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord 2005; 89: 125–135. [DOI] [PubMed] [Google Scholar]
- 35.Smith DJ, Muir WJ, Blackwood D. Neurocognitive impairment in euthymic young adults with bipolar spectrum disorder and recurrent major depressive disorder. Bipolar Disord 2006; 8: 40–46. [DOI] [PubMed] [Google Scholar]
- 36.Reppermund S, Ising M, Lucae S, et al. Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med 2009; 39: 603–614. [DOI] [PubMed] [Google Scholar]
- 37.Bearden CE, Glahn DC, Monkul ES, et al. Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry Res 2006; 142: 139–150. [DOI] [PubMed] [Google Scholar]
- 38.Wang CE, Halvorsen M, Sundet K, et al. Verbal memory performance of mildly to moderately depressed outpatient younger adults. J Affect Disord 2006; 92: 283–286. [DOI] [PubMed] [Google Scholar]
- 39.Castaneda AE, Marttunen M, Suvisaari J, et al. The effect of psychiatric co-morbidity on cognitive functioning in a population-based sample of depressed young adults. Psychol Med 2010; 40: 29–39. [DOI] [PubMed] [Google Scholar]
- 40.Liao C, Feng Z, Zhou D, et al. Dysfunction of fronto-limbic brain circuity in depression. Neuroscience 2012; 201: 231–238. [DOI] [PubMed] [Google Scholar]
- 41.Oberauer K. Removing Irrelevant Information from Working Memory: A Cognitive Aging Study with the Modified Sternberg Task. J Exp Psychol Learn Mem Cogn 2001; 27: 948–957. [PubMed] [Google Scholar]
- 42.Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science 1960; 132: 349–350. [DOI] [PubMed] [Google Scholar]
- 43.Hess EH. Attitude and pupil size. Sci Am 1965; 212: 46–54. [DOI] [PubMed] [Google Scholar]
- 44.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59: 22–33. [PubMed] [Google Scholar]
- 45.APA. Diagnostic and statistical manual of mental disorders: DSM-IV, 4th ed Washington DC: American Psychiatric Association, 1994. [Google Scholar]
- 46.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rush AJ, Trivedia MH, Ibrahim HM, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54: 573–583. [DOI] [PubMed] [Google Scholar]
- 48.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 49.Lang PJ, Bradley MM and Cuthbert BN. International affective picture System (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8, 2008. Gainesville: University of Florida.
- 50.Rouder JN, Speckman PL, Sun D, et al. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev 2009; 16: 225–237. [DOI] [PubMed] [Google Scholar]
- 51.Shur E, Checkley S. Pupil studies in depressed patients: an investigation of the mechanism of action of desipramine. Br J Psychiatry 1982; 140: 181–184. [DOI] [PubMed] [Google Scholar]
- 52.Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry 2006; 19: 34–39. [DOI] [PubMed] [Google Scholar]
- 53.Kaviani H, Gray JA, Checkley SA, et al. Affective modulation of the startle response in depression: influence of the severity of depression, anhedonia, and anxiety. J Affect Disord 2004; 83: 21–31. [DOI] [PubMed] [Google Scholar]
- 54.Heller AS, Johnstone T, Shackman AJ, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci USA 2009; 106: 22445–22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasler G, Drevets WC, Manji HK, et al. Discovering endophenotypes for major depression. Neuropsychopharmacology 2004; 29: 1765–1781. [DOI] [PubMed] [Google Scholar]
- 56.Matt GE, Vazquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clin Psychol Rev 1992; 12: 227–255. [Google Scholar]
- 57.Joormann J, Siemer M. Memory accessibility, mood regulation, and dysphoria: difficulties in re-pairing sad mood with happy memories? J Abno Psychol 2004; 113: 179–188. [DOI] [PubMed] [Google Scholar]
- 58.Joormann J, Siemer M, Gotlib IH. Mood regulation in depression: differential effects of distraction and recall of happy memories on sad mood. J Abnorm Psychol 2007; 116: 484–490. [DOI] [PubMed] [Google Scholar]
- 59.Duque A, Vazquez C. Double attention bias for positive and negative emotional faces in clinical depression: evidence from an eye-tracking study. J Behav Ther Exp Psychiatry 2015; 46: 107–114. [DOI] [PubMed] [Google Scholar]