Abstract
Objective
To evaluate the clinical efficacy of intravenous (IV) fluid warming in patients undergoing laparoscopic colorectal surgery.
Methods
Adult patients undergoing laparoscopic colorectal surgery were randomly assigned to receive either IV fluids at room temperature (control group) or warmed IV fluids (warm fluids group). Each patient received a standardized goal-directed fluid regimen based on stroke volume variances. Oesophageal temperature was measured at 15 min intervals for 2 h after induction of anaesthesia.
Results
A total of 52 patients were enrolled in the study. The drop in core temperature in the warm fluids group was significantly less than in the control group 2 h after the induction of anaesthesia. This significant difference was seen from 30 min after induction.
Conclusion
IV fluid warming was associated with a smaller drop in core temperature than room temperature IV fluids in laparoscopic colorectal surgery incorporating goal-directed fluid therapy.
Keywords: Colorectal surgery, enhanced recovery after surgery, fluid warmer, goal-directed fluid therapy, hypothermia
Introduction
Over recent decades, major developments in colorectal surgery include the introduction of laparoscopy and the implementation of enhanced recovery after surgery (ERAS) programmes. Within ERAS programmes, the prevention of intraoperative hypothermia and the use of goal-directed fluid therapy (GDFT) are important elements.
The most recent ERAS Society guidelines strongly recommend the routine use of intravenous (IV) fluid warming during surgery.1 However, to date, no studies demonstrating the usefulness of IV fluid warming in the setting of ERAS programmes including laparoscopic surgery have been reported. In view of the decreased heat loss due to minimal bowel exposure,2,3 the advantages of IV fluid warming may be reduced in laparoscopic colorectal surgery. In addition, as patients receive a smaller volume of intraoperative fluids in GDFT than in a liberal fluid regimen,4 the usefulness of IV fluid warming may be further reduced in laparoscopic colorectal surgery incorporating GDFT. This prospective, randomized, controlled study compared the effects on core temperature of either warmed IV fluids or IV fluids at room temperature in patients undergoing laparoscopic colorectal surgery incorporating GDFT.
Patients and methods
Patients aged 18–65 years classified as American Society of Anesthesiologists physical status I or II and scheduled for elective laparoscopic resection for colorectal cancer under general anaesthesia with an expected operating time of at least 2 h at the Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Seoul, Republic of Korea, from December 2014 to May 2015 were enrolled in the study. Patients with a preoperative tympanic temperature ≥ 38.0℃ or ≤ 35.5℃ or preoperative use of vasodilators or medications likely to alter thermoregulation were excluded. Patients were randomized using computer-generated random allocation to receive warmed IV fluids (warm fluids group) or room-temperature fluids (control group).
The study period was defined as the first 2 h after induction of anaesthesia, derived from the median operating time for laparoscopic colorectal surgery at our institution. Patients who were randomized but whose surgery duration was shorter than the 2 h study period were excluded from the analysis.
Written informed consent was obtained from all study participants and the study protocol was approved by the Institutional Review Board of the Samsung Medical Center, Seoul, Republic of Korea. This study was registered with the Clinical Research Information Service (KCT0001295).
Anaesthetic and operative procedures
Without premedication, anaesthesia was induced with propofol (1.5–2 mg/kg) and vecuronium (0.15 mg/kg) and then maintained with sevoflurane (1.5–3.5 vol%) and 50% oxygen in air. During induction, an arterial cannula was placed into a radial artery and connected to a FloTrac/Vigileo monitoring system (FloTrac/Vigileo version 3.02, Edwards Lifesciences, Irvine, CA, USA), an autocalibrated device that analyses the arterial pressure waveform to calculate cardiac output, stroke volume and stroke volume variance (SVV). SVV was used as a predictor of fluid responsiveness for the GDFT.
In all patients, a standard laparoscopic procedure was performed using one 10 mm and two 5 mm disposable trocars. A pneumoperitoneum was established with heated and humidified CO2 gas using a Stryker 40 l Highflow Insufflator (Stryker Endoscopy, San Jose, CA, USA). Intra-abdominal pressure was maintained at 12 mmHg with an insufflation rate of 20 l/min.
Fluid management and outcome measures
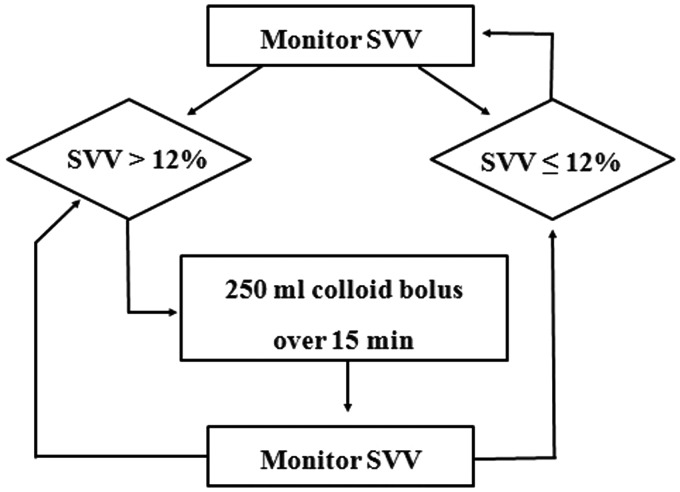
In the warmed fluid group, all intraoperative fluids were administered via the Hotline® fluid warmer (Level 1 Technologies, Rockland, MA, USA) using a setpoint of 42℃. The Hotline® tube was attached to the patient’s IV catheter through a 52 cm control pressure line to minimize heat loss distal to the fluid warmer. This fluid warming system can deliver fluids at 38–39℃ over a wide range of clinically relevant flow rates.5 In the control group, patients received intraoperative IV fluids at room temperature. In both groups, intraoperative basal fluid replacement was achieved by continuous infusion of the crystalloid lactated Ringer’s solution at a rate of 5 ml/kg per h. Additional boluses of 250 ml colloid solution (Volulyte® 6%, Fresenius Kabi, Bad Homburg, Germany) were administered based on the algorithm given in Figure 1.
Figure 1.
Intraoperative fluid management protocol for goal-directed therapy. SVV, stroke volume variance.
Core temperatures were monitored continuously intraoperatively and recorded at 15 min intervals following tracheal intubation until the end of surgery using an oesophageal temperature probe (Mon-a-therm Esophageal, YSI 400 series, Mallinckrodt Medical, St Louis, MO, USA). The presence of intraoperative hypothermia was defined as a core temperature < 36.0℃ at any time during the study period.
The presence and severity of shivering on arrival in the postanaesthesia care unit (PACU) was recorded using a 4-point scale: 0, no shivering; 1, mild fasciculations of the jaw or neck; 2, moderate tremors involving the head, neck, shoulders and/or extremities; 3, severe generalized shaking.6 Postoperative recovery profiles (length of time in PACU, time to pass flatus and length of hospital stay) were also documented.
Statistical analyses
The primary outcome was the magnitude of the core temperature drop 2 h after the induction of anaesthesia. Based on a previous study,7 the standard deviation was projected to be 0.3℃ in the control group. A difference of 0.3℃ in this primary outcome between the groups was considered to be clinically significant.8 Using these parameters, 23 patients per group would be required to ensure a power of 90% and a 0.05 significance level (on an unpaired t-test).
Data were presented as the mean ± SD. Time-dependent data were analysed using repeated measures analysis of variance (between periods in each group and between groups), and data at corresponding time points were additionally analysed between the two groups using Fisher’s exact or Pearson χ2 tests for categorical variables and unpaired t- or Mann–Whitney U-tests for continuous variables. All statistical analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL, USA). A P-value < 0.05 was considered to be statistically significant.
Results
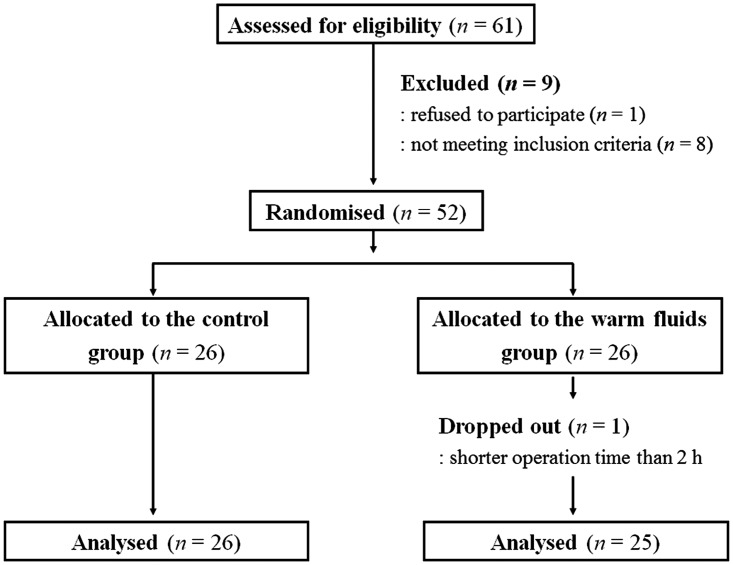
A total of 52 patients were enrolled in the study and randomized to receive warmed IV fluids (warm fluids group; n = 26) or room-temperature fluids (control group; n = 26). Of these, one patient in the warm fluids group was excluded from the study because of an operation time < 2 h. Thus, 26 patients in the control group and 25 in the warm fluids group were included in the final analysis (Figure 2).
Figure 2.
Flow diagram of attrition numbers in patients undergoing laparoscopic colorectal surgery who received warmed IV fluids (warm fluids group) or IV fluids at room temperature (control group).
Demographic, anaesthetic and operative characteristics were comparable between the groups (Table 1). The median (interquartile range) for the total delivered volume in the warm fluids group was 750.0 ml (620.0–995.0 ml).
Table 1.
Demographic, anaesthetic and operative data in patients undergoing laparoscopic colorectal surgery who received warmed IV fluids (warm fluids group) or IV fluids at room temperature (control group).
| Control group n = 26 | Warm fluids group n = 25 | |
|---|---|---|
| Gender | ||
| Female | 13 | 12 |
| Male | 13 | 13 |
| Age, years | 50.5 ± 8.7 | 51.8 ± 10.3 |
| Body mass index, kg/m2 | 23.8 ± 2.8 | 23.2 ± 3.1 |
| Preoperative tympanic temperature, ℃ | 36.8 ± 0.3 | 36.8 ± 0.4 |
| Type of laparoscopic procedure | ||
| Hemicolectomy | 5 | 11 |
| Anterior resection | 8 | 5 |
| Low anterior resection | 9 | 6 |
| Extended colectomy | 4 | 3 |
| Duration of surgery, min | 140.3 ± 34.7 | 134.0 ± 31.0 |
| Duration of anaesthesia, min | 185.3 ± 35.4 | 178.7 ± 35.0 |
| Colloid administered in 2 h study period, ml | 288.5 ± 241.8 | 320.0 ± 284.3 |
| Crystalloid administered in 2 h study period, ml | 437.1 ± 176.8 | 486.8 ± 134.2 |
| Estimated blood loss in 2 h study period, ml | 60.2 ± 46.7 | 67.0 ± 45.0 |
| Urine output in 2 h study period, ml | 196.2 ± 82.0 | 153.0 ± 89.7 |
Data are presented as number of patients or mean ± SD.
No statistically significant between-group differences (P ≥ 0.05) using Fisher’s exact or Pearson χ2-tests for categorical variables and unpaired t- or Mann–Whitney U-tests for continuous variables.
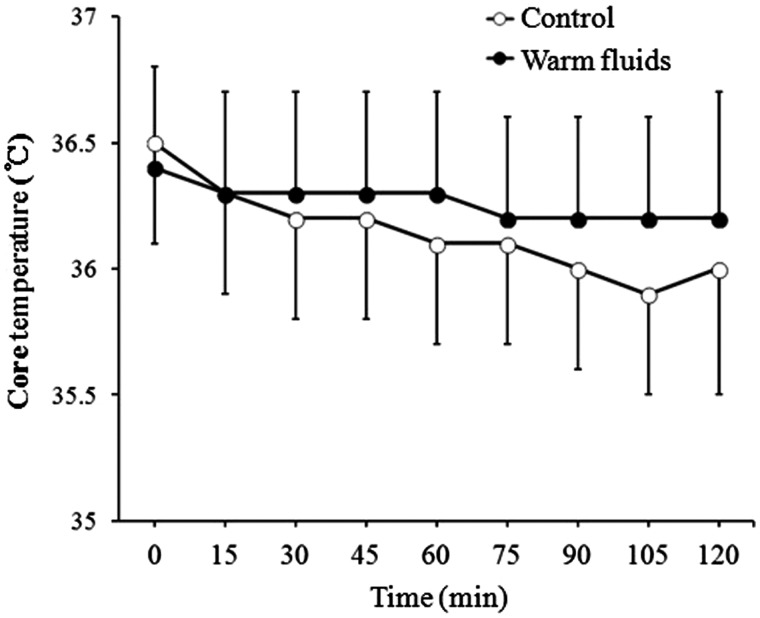
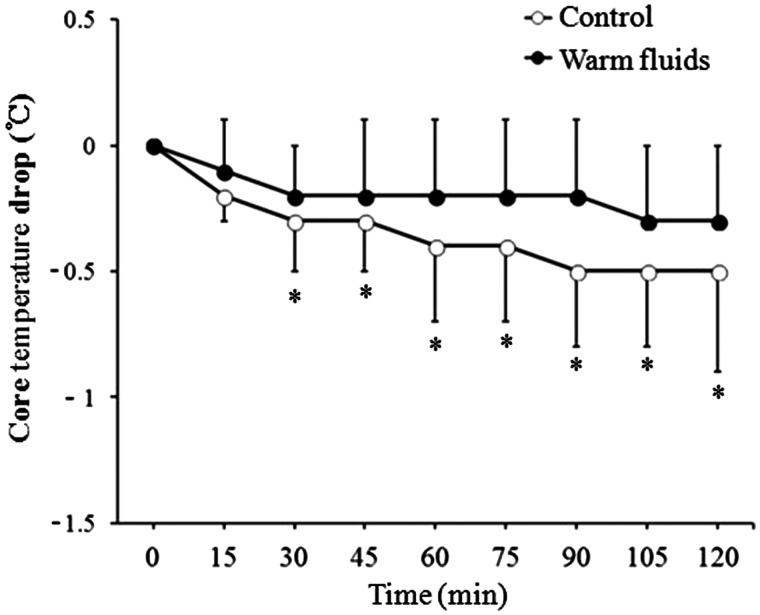
Changes in core temperature during the 2 h study period were significantly different between the groups (P = 0.033; Figure 3). The core temperature drop in the warm fluids group was significantly less than that in the control group from 30 min after induction of anaesthesia (P < 0.05; Figure 4). The temperature drop at the 2 h time point (the primary outcome) was therefore significantly less in the warm fluids group than in the control group (0.3 ± 0.3℃ compared with 0.5 ± 0.4℃; P = 0.013). A total of 14 patients (53.8%) in the control group and seven patients (28.0%) in the warm fluids group developed intraoperative hypothermia. However, this difference was not statistically significant.
Figure 3.
Changes in core temperature over a 2 h period after induction of anaesthesia in patients undergoing laparoscopic colorectal surgery who received warmed IV fluids (warm fluids) or IV fluids at room temperature (control). Data are presented as the mean ± SD.
Figure 4.
Drop in core temperature over a 2 h period after induction of anaesthesia in patients undergoing laparoscopic colorectal surgery who received warmed IV fluids (warm fluids) or IV fluids at room temperature (control). Data are presented as the mean ± SD. *P < 0.05 using Fisher’s exact test or unpaired t-test.
Postoperative shivering did not occur in any patients in the warm fluids group, but occurred in five patients in the control group. The severity of shivering was grade 1 in one patient and grade 4 requiring meperidine in four patients. However, the differences in the incidence and severity of postoperative shivering did not reach statistical significance. Postoperative recovery profiles were similar in both groups (Table 2).
Table 2.
Postoperative data in patients undergoing laparoscopic colorectal surgery who received warmed IV fluids (warm fluids group) or IV fluids at room temperature (control group).
| Control group n = 26 | Warm fluids group n = 25 | |
|---|---|---|
| Postoperative shivering | ||
| Yes | 5 | 0 |
| No | 21 | 25 |
| PACU stay, min | 67.2 ± 13.7 | 67.2 ± 11.0 |
| Time to pass flatus, days | 3.0 ± 1.0 | 3.2 ± 1.0 |
| Length of hospital stay, days | 7.0 ± 3.2 | 6.6 ± 2.1 |
Data are presented as number of patients or mean ± SD.
PACU, postanaesthesia care unit.
No statistically significant between-group differences (P ≥ 0.05) using Fisher’s exact or Pearson χ2-tests for categorical variables and unpaired t- or Mann–Whitney U-tests for continuous variables.
Discussion
Intraoperative maintenance of normothermia and its impact on patients has been consistently highlighted by several versions of the ERAS guidelines as one of the major elements of perioperative care in colorectal surgery.1,9 Thermodynamically, the use of thermally neutral fluids is advantageous over fluids at room temperature when administering IV fluids. In addition, the clinical efficacy of IV fluid warming via commercial devices is well documented in open surgical procedures with a duration of 30–120 min.10 The latest version of the ERAS Society guidelines specifically recommends the routine use of IV fluid warming during elective colonic surgery.1 However, the value of its routine use in laparoscopic colorectal surgery is not clear as this type of surgery is associated with reduced heat loss, surgical blood loss and intraoperative fluid requirements compared with open surgery. In addition, its usefulness may be further limited in the setting of laparoscopic surgery incorporating GDFT.4
In the present study, contrary to this hypothesis, the total core temperature drop was significantly less in the warm fluids group than in the control group, even within a 2 h study period. Though the median (interquartile range) total infused volume was only 750.0 ml (620.0–995.0 ml), IV fluid warming exerted a beneficial effect on temperature control in the warm fluids group. These results are consistent with theoretical calculations that predict that administration of IV fluids would decrease the mean body temperature by 0.25℃ for each litre of crystalloid given at ambient temperature in a 70 kg patient.11
However, the difference in the total temperature drop between the warm fluids group and the control group was small. Although it reached statistical significance, such a small difference may have only limited clinical significance. It is noteworthy that the proportion of patients who developed intraoperative hypothermia (a lowest core temperature < 36.0℃) was not significantly different between the two groups.
One reason for the limited efficacy of intraoperative IV fluid warming in the present study is that the ability of fluid warming to prevent intraoperative hypothermia is mainly dependent on the volume infused.12 This is consistent with a previous study that showed no preventive effect of intraoperative IV fluid warming on intraoperative hypothermia in patients receiving a fixed IV fluid volume of 1 l.13
Another possible reason for its limited efficacy may be related to the surgical conditions. In colorectal surgery, the use of laparoscopic techniques reduces the risk of perioperative hypothermia by reducing heat loss associated with bowel exteriorization and/or exposure of the peritoneal cavity to air during open surgery.2,3 In addition, the use of heated and humidified CO2 gas in the present study may have affected the outcome. A meta-analysis reported that, compared with cold and dry CO2 gas, pneumoperitoneum using heated and humidified CO2 gas reduced the risk of perioperative hypothermia.14 However, to date, the only randomized controlled study in laparoscopic colon surgery failed to demonstrate any benefit of heated and humidified CO2 in preventing intraoperative hypothermia.15 Further investigation is therefore needed to clarify whether warming and humidification of insufflated CO2 gas could have contributed to the limited efficacy of intraoperative IV fluid warming in the present study.
The major limitation of this study is that the study period was limited to 2 h after induction of anaesthesia. Therefore the results cannot be extrapolated to other ERAS cases such as lengthy operations or those with greater intraoperative blood loss. The other major concern may be an absence of blinding of the attending anaesthesiologist. Because of the typical presence of air bubbles in the tubing of a Hotline® fluid warmer, complete double blinding could not be achieved. However, since standardized thermal management protocols were applied to all patients, this should not affect the results.
In conclusion, the present study showed that intraoperative application of IV fluid warming caused the core temperature to drop less than room-temperature IV fluids in laparoscopic colorectal surgery incorporating GDFT. However, as the magnitude of the core temperature difference was small during the first 2 h after induction of anaesthesia, warming IV fluids cannot provide a protective effect on intraoperative hypothermia in laparoscopic colorectal surgery lasting for a relatively short duration and incorporating GDFT. Therefore routine use of IV fluid warming, as suggested by the ERAS guidelines, cannot be justified for an ERAS programme in the setting of laparoscopic colorectal surgery.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not for-profit sectors.
References
- 1.Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg 2013; 37: 259–284. [DOI] [PubMed] [Google Scholar]
- 2.Luck AJ, Moyes D, Maddern GJ, et al. Core temperature changes during open and laparoscopic colorectal surgery. Surg Endosc 1999; 13: 480–483. [DOI] [PubMed] [Google Scholar]
- 3.Stewart BT, Stitz RW, Tuch MM, et al. Hypothermia in open and laparoscopic colorectal surgery. Dis Colon Rectum 1999; 42: 1292–1295. [DOI] [PubMed] [Google Scholar]
- 4.Doherty M, Buggy DJ. Intraoperative fluids: how much is too much? Br J Anaesth 2012; 109: 69–79. [DOI] [PubMed] [Google Scholar]
- 5.Patel N, Knapke DM, Smith CE, et al. Simulated clinical evaluation of conventional and newer fluid-warming devices. Anesth Analg 1996; 82: 517–524. [DOI] [PubMed] [Google Scholar]
- 6.Guffin A, Girard D, Kaplan JA. Shivering following cardiac surgery: hemodynamic changes and reversal. J Cardiothorac Anesth 1987; 1: 24–28. [DOI] [PubMed] [Google Scholar]
- 7.Huh J, Cho YB, Yang MK, et al. What influence does intermittent pneumatic compression of the lower limbs intraoperatively have on core hypothermia? Surg Endosc 2013; 27: 2087–2093. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence. Hypothermia: prevention and management in adults having surgery. www.nice.org.uk/guidance/cg65 (2008, accessed 29 October 2015).
- 9.Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg 2009; 144: 961–969. [DOI] [PubMed] [Google Scholar]
- 10.Campbell G, Alderson P, Smith AF, et al. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev 2015; 4: CD009891–CD009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentilello LM, Cortes V, Moujaes S, et al. Continuous arteriovenous rewarming: experimental results and thermodynamic model simulation of treatment for hypothermia. J Trauma 1990; 30: 1436–1449. [PubMed] [Google Scholar]
- 12.Barthel ER, Pierce JR. Steady-state and time-dependent thermodynamic modeling of the effect of intravenous infusion of warm and cold fluids. J Trauma Acute Care Surg 2012; 72: 1590–1600. [DOI] [PubMed] [Google Scholar]
- 13.Andrzejowski JC, Turnbull D, Nandakumar A, et al. A randomised single blinded study of the administration of pre-warmed fluid vs active fluid warming on the incidence of peri-operative hypothermia in short surgical procedures. Anaesthesia 2010; 65: 942–945. [DOI] [PubMed] [Google Scholar]
- 14.Sajid MS, Mallick AS, Rimpel J, et al. Effect of heated and humidified carbon dioxide on patients after laparoscopic procedures: a meta-analysis. Surg Laparosc Endosc Percutan Tech 2008; 18: 539–546. [DOI] [PubMed] [Google Scholar]
- 15.Sammour T, Kahokehr A, Hayes J, et al. Warming and humidification of insufflation carbon dioxide in laparoscopic colonic surgery: a double-blinded randomized controlled trial. Ann Surg 2010; 251: 1024–1033. [DOI] [PubMed] [Google Scholar]