Abstract
Objective
To measure the differential expression of microRNAs (miRNAs) in peripheral blood samples from patients with intracerebral haemorrhage (ICH) and to measure the levels of hsa-miR-21-5p in peripheral blood and haematoma samples from patients with ICH.
Methods
This case–control study enrolled individuals with ICH in the putamen treated by craniotomy and age- and sex-matched healthy control subjects. Serum miRNA expression profiles were determined in the patient and control groups using miRNA polymerase chain reaction (PCR) arrays. The ICH-related miRNA hsa-miR-21-5p was selected and its differential expression was assessed in peripheral blood and haematoma specimens from patients with ICH compared with peripheral blood samples controls using real-time PCR.
Results
Seven patients and five control subjects were included in the miRNA expression profile analysis; and 31 patients and 22 control subjects provided samples for the real-time PCR of hsa-miR-21-5p expression. A total of 59 miRNAs were significantly downregulated in patients with ICH. Relative hsa-miR-21-5p levels of 0.43 and 0.31 for peripheral blood and haematoma samples, respectively, were obtained in the patient group compared with the control subjects.
Conclusion
Hsa-miR-21-5p levels were significantly reduced in both peripheral blood and haematoma samples in patients with ICH.
Keywords: Intracerebral haemorrhage, peripheral blood, haematoma, microRNA, microRNA polymerase chain reaction array, has-miR-21-5p
Introduction
Intracerebral haemorrhage (ICH) is a common cerebrovascular disease accounting for approximately 10–15% of all stroke patients.1 ICH has an annual incidence of approximately 10–30 per 100 000 people, a morbidity rate of approximately 75%, and a mortality rate of 30–50%,2 which is associated with a heavy economic burden on individuals, families and society at large.3 Many studies have focused on finding ways to effectively predict ICH occurrence and improve its prognosis.4,5 To this end, researchers are evaluating molecular markers in order to improve the understanding of their potential roles in ICH pathogenesis, targeted therapy and prognosis.5,6
MicroRNAs (miRNAs) are endogenous small non-coding single-stranded RNA molecules (18–22 nucleotides long) that are encoded by endogenous genes and are involved in the regulation of post-transcriptional gene expression in plants and animals.7,8 As novel biomarkers and regulatory small molecules, miRNAs play important roles in the diagnosis and treatment of stroke.9–11 MiRNA expression and target gene regulation occurs mainly inside the cell, yet miRNAs are also found in extracellular fluids such as serum.12 A study that used rats as an animal model demonstrated that miRNAs can be used as peripheral blood biomarkers to reflect relevant changes in the brain.10 However, studies describing such a role for miRNAs in humans are scarce.5 As one of the earliest miRNAs to be discovered and one that has widespread distribution in human cells and tissues, miR-21 has been shown to have specific expression patterns in a variety of diseases, including tumours and cardiovascular disease.13,14 However, the involvement of miR-21 expression in ICH has not been reported to date. Therefore, this study aimed to determine the miRNA expression pattern in the peripheral blood of patients with ICH, and to investigate the differential expression patterns of hsa-miR-21-5p in peripheral blood and haematoma samples from patients with ICH.
Patients and methods
Study population
This case–control study enrolled consecutive patients with ICH treated with craniotomy by either the Department of Neurosurgery or the Department of Neurology, First Affiliated Hospital of China Medical University, Shenyang, Liaoning Province, China between August 2012 and December 2013. The diagnosis of ICH mainly relied on computed tomography (CT) scans undertaken on a 256-detector row spiral CT scanner (Brilliance CT Big Bore; Philips Healthcare, Best, the Netherlands) or magnetic resonance imaging (MRI) scans undertaken on a GE SIGNA™ Pioneer 3.0 T scanner (GE Healthcare, Piscataway, NJ, USA). According to the CT or MRI scan results, patients with bleeding in the putamen that had not broken into the ventricle were included in the study. Patients with secondary brain haemorrhage due to brain trauma, tumour, vascular malformation, arteritis, anticoagulant use and other causes, as well as those complicated with severe primary diseases of the heart, liver, kidney, blood and endocrine systems, were excluded from the study. Control subjects were healthy volunteers from a northeast Han Chinese population who were matched by sex and age to the patient group. The control subjects were attending a clinic at the Physical Examination Centre, First Affiliated Hospital of China Medical University for a routine medical examination.
Baseline parameters for the study population were recorded, including age, sex, body mass index, systolic blood pressure, diastolic blood pressure, smoking history, routine biochemistry data, serum fasting blood glucose levels, history of antiplatelet drug use and head CT or MRI scan results. A peripheral venous blood sample was collected after a 12-h overnight fast and serum levels of fasting blood glucose were determined immediately with standard laboratory techniques using a Roche Hitachi 912 chemistry analyser (Roche Diagnostics, Branchburg, NJ, USA). Serum miRNA expression profiles were determined in the patient and control groups using miRNA polymerase chain reaction (PCR) arrays as described below. Differential hsa-miR-21-5p expression was analysed in the serum from peripheral blood and haematoma specimens from the patient group and peripheral blood samples from the control group by real-time PCR.
This study was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University, Shenyang, Liaoning Province, China (no. [2012]38-1). All study participants provided written informed consent.
Sample collection, serum isolation and microRNA extraction
For the miRNA PCR array analysis, peripheral blood samples were collected from patients within 12 h of disease onset and from healthy control subjects when they attended the clinic at the start of the study. The real-time PCR validation used either peripheral blood specimens (patients and controls) or blood samples collected from the haematomas during craniotomy (patients only).
Serum was prepared within 2 h of blood collection by centrifugation at 1200 g for 10 min at 20℃ in a mini-41 C centrifuge (Heima Medical Apparatus Company, Zhuhai, China). Total RNA was extracted from serum samples (500 µl) using TRIzol® reagent (Life Technologies, Carlsbad, CA, USA) following the manufacturer’s instructions. The RNA concentration and purity were assessed using a Thermo Scientific™ NanoDrop™ One Microvolume UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA). All RNA samples had an A260/A280 ratio between 1.8 and 2.0, and concentrations of 16.31–33.58 ng/μl, which meant that they qualified for gene expression analysis.
MicroRNA PCR array technology and data analysis
Total RNA samples from the patient and control groups were assessed using a miRCURY LNA™ Universal RT miRNA PCR panel (Exiqon, Vedbaek, Denmark) according to the manufacturer’s instructions. This miRNA PCR panel examined 179 miRNAs. Raw Ct values were processed using the Exiqon GenEx qPCR analysis software.15 Target miRNAs were normalized with internal housekeeping control miRNAs, including hsa-miR-93-5p, hsa-miR-423-5p, and hsa-miR-191-5p, which are classic endogenous control miRNAs used for human serum samples.16–18 Ratios were calculated as described previously.19 All miRNAs with a ratio >2 or <0.5 were screened.
Real-time PCR
The miRNA target gene query page of the miRecords website was used to identify an miRNA target gene from the miRNA PCR microarray analysis results to analyse by real-time PCR.20 This research identified has-miR-21-5p because it is associated with a number of physiological and pathological mechanisms following ICH.21–26 To further investigate hsa-miR-21-5p expression, serum samples from peripheral blood and haematomas (patient group) and peripheral blood (control group) were assessed by real-time reverse transcriptase (RT) PCR. Total RNA extraction was carried out as described above. First-strand cDNA was synthesized using a PrimeScript RT reagent kit (Clontech Laboratories, Mountain View, CA, USA) according to the manufacturer’s instructions. Hsa-miR-191-5p was used as an internal reference. The following primers were used: hsa-miR-21-5p, forward 5′-GGGTAGCAGCACATAATGG-3′ and reverse 5′-CAGTGCGTGTCGTGGAGT-3′; hsa-miR-191-5p control, forward 5′-GGCAACGGAATC CCAAAAG-3′ and reverse 5′-GTGCGTGTCGTGGAGTCG-3′ (Kangcheng Company, Shanghai, China).
Each 25-μl RT reaction mixture used for real-time PCR contained the following: cDNA template (2 μl), forward and reverse primers (10 μM, 1 μl each), 2 × SYBR Green Premix Ex Taq™ (12.5 μl) (Exiqon) and nuclease free water (8.5 μl). Real-time PCR was performed on an ABI PRISM® 7900 real-time PCR instrument (Life Technologies). The cycling programme involved preliminary denaturation at 95℃ for 10 min, followed by 40 cycles of denaturation at 95℃ for 10 s, annealing at 60℃ for 60 s, and elongation at 95℃ for 10 s, followed by a final elongation step at 60℃ for 1 min. All samples were evaluated in triplicate with negative and positive controls on each plate. The relative expression levels were obtained as described previously.19
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 16.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Continuous data are presented as mean ± SD. Following a homogeneity of variance test, one-way analysis of variance was used to compare the patient and control groups. Categorical data are presented as n of patients (%) and Fisher’s exact test was used to compare the two groups. To demonstrate the relative expression of miRNA, Student’s t-test was used to compare the two groups. A P-value <0.05 was considered statistically significant.
Results
For the miRNA PCR microarray analysis, peripheral blood samples were collected from patients within 12 h of disease onset (patient group, n = 7) and from a group of healthy control subjects (n = 5). Real-time PCR validation was carried out with peripheral blood specimens or blood samples collected from haematomas from patients during craniotomy (n = 31) and from peripheral blood samples obtained from healthy controls (n = 22). The baseline clinical parameters of the two sets of patient and control groups were assessed (Tables 1 and 2). No statistically significant differences in age, sex distribution, body mass index, systolic and diastolic blood pressure at admission, routine blood examinations (white blood cell count and platelet count), fasting blood glucose level, smoking history and antiplatelet drug use history were observed between the two groups used in each analysis. Preoperative CT or MRI data showed a mean ± SD haematoma volume of 35.59 ± 7.11 cm3 for the seven patients with ICH who were assessed in the miRNA PCR microarray analysis; and the 31 patients with ICH whose samples were evaluated by real-time PCR analysis had a mean ± SD haematoma volume of 33.87 ± 6.47 cm3.
Table 1.
Demographic and clinical characteristics of the patient and control groups who were used for the microRNA polymerase chain reaction microarray analysis undertaken to determine the differential expression of microRNAs in patients with intracerebral haemorrhage.
| Patient group n = 7 | Control group n = 5 | |
|---|---|---|
| Age, years | 63.86 ± 5.34 | 64.60 ± 5.41 |
| Sex, male | 5 (71.4) | 3 (60.0) |
| Systolic blood pressure at admission, mmHg | 158.86 ± 16.83 | 143.80 ± 23.11 |
| Diastolic blood pressure at admission, mmHg | 103.43 ± 15.78 | 98.00 ± 22.36 |
| White blood cell count, ×109/l | 9.29 ± 2.12 | 8.31 ± 1.32 |
| Platelet count, ×109/l | 222.00 ± 46.79 | 196.40 ± 43.68 |
| Fasting blood glucose, mmol/l | 7.92 ± 2.31 | 7.49 ± 2.23 |
| Smoking history | 3 (42.9) | 3 (60.0) |
| History of antiplatelet drug use | 3 (42.9) | 2 (40.0) |
| Body mass index, kg/m2 | 26.50 ± 3.98 | 25.84 ± 2.84 |
Data presented as mean ± SD or n of patients (%).
No significant between-group differences (P ≥ 0.05).
Table 2.
Demographic and clinical characteristics of the patient and control groups who were used for the real-time polymerase chain reaction analysis undertaken to determine the differential expression of the microRNA hsa-miR-21-5p in patients with intracerebral haemorrhage.
| Patient group n = 31 | Control group n = 22 | |
|---|---|---|
| Age, years | 64.68 ± 6.04 | 64.45 ± 5.67 |
| Sex, male | 19 (61.3) | 12 (54.5) |
| Systolic blood pressure at admission, mmHg | 155.52 ± 21.18 | 144.27 ± 21.46 |
| Diastolic blood pressure at admission, mmHg | 107.61 ± 21.15 | 103.23 ± 21.81 |
| White blood cell count, ×109/l | 9.04 ± 2.95 | 7.58 ± 2.14 |
| Platelet count, ×109/l | 222.26 ± 64.90 | 210.27 ± 46.85 |
| Fasting blood glucose, mmol/l | 7.75 ± 2.70 | 7.74 ± 2.38 |
| Smoking history | 14 (45.2) | 9 (40.9) |
| History of antiplatelet drug use | 13 (41.9) | 7 (31.8) |
| Body mass index, kg/m2 | 24.86 ± 2.91 | 23.93 ± 2.83 |
Data presented as mean ± SD or n of patients (%).
No significant between-group differences (P ≥ 0.05).
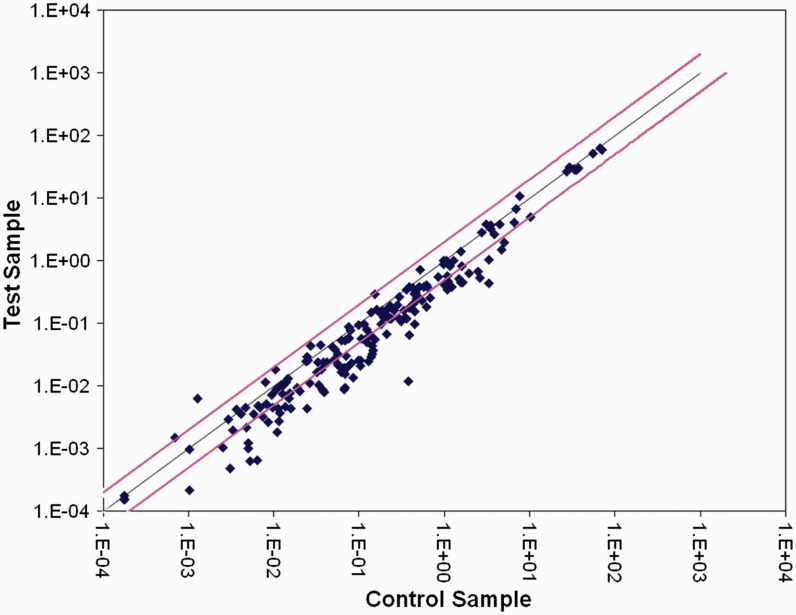
The miRNA profiling demonstrated no differences in the expression of the housekeeping genes between the patient and control groups. Fold changes of 1.07, 0.92 and 1.02 were consistently obtained for hsa-miR-93-5p, hsa-miR-191-5p, and hsa-miR-423-5p, respectively, which confirmed that these miRNAs were suitable to be used as internal controls. A total of 80 miRNAs that had a >2 fold change (FC) were recorded in this analysis. Of these, 78 were downregulated, with 59 showing statistically significant differences (P < 0.05) (Table 3). The other two miRNAs were upregulated (hsa-let-7i-3p and hsa-miR-296-5p), but there was no significant differences. As shown in Table 3 and Figure 1, many miRNAs showed a pronounced downregulation, with hsa-miR-27a-3p having the most notable reduction (FC 0.03); and among the upregulated miRNAs, hsa-miR-296-5p expression increased by approximately 5 fold.
Table 3.
Differentially expressed microRNAs in peripheral blood samples from patients with intracerebral haemorrhage (n = 7).
| Number | microRNA | Expression | Statistical significancea | Fold change |
|---|---|---|---|---|
| Fold change >2 (or <0.5) fold with P < 0.05 | ||||
| 1 | hsa-miR-27a-3p | Down | P = 0.009 | 0.03 |
| 2 | hsa-miR-205-5p | Down | P = 0.019 | 0.10 |
| 3 | hsa-miR-200a-3p | Down | P = 0.004 | 0.13 |
| 4 | hsa-miR-122-5p | Down | P = 0.034 | 0.13 |
| 5 | hsa-miR-885-5p | Down | P = 0.024 | 0.13 |
| 6 | hsa-miR-375 | Down | P = 0.001 | 0.14 |
| 7 | hsa-miR-10a-5p | Down | P = 0.007 | 0.16 |
| 8 | hsa-miR-151a-3p | Down | P = 0.023 | 0.16 |
| 9 | hsa-let-7f-5p | Down | P = 0.001 | 0.17 |
| 10 | hsa-miR-195-5p | Down | P < 0.001 | 0.17 |
| 11 | hsa-miR-146b-5p | Down | P = 0.042 | 0.18 |
| 12 | hsa-let-7c | Down | P < 0.001 | 0.19 |
| 13 | hsa-miR-92b-3p | Down | P = 0.012 | 0.21 |
| 14 | hsa-miR-142-3p | Down | P < 0.001 | 0.21 |
| 15 | hsa-miR-99a-5p | Down | P = 0.005 | 0.21 |
| 16 | hsa-let-7e-5p | Down | P = 0.001 | 0.21 |
| 17 | hsa-miR-331-3p | Down | P = 0.019 | 0.21 |
| 18 | hsa-miR-605 | Down | P = 0.016 | 0.22 |
| 19 | hsa-miR-125b-5p | Down | P = 0.027 | 0.22 |
| 20 | hsa-miR-335-5p | Down | P = 0.012 | 0.22 |
| 21 | hsa-miR-194-5p | Down | P = 0.013 | 0.23 |
| 22 | hsa-miR-424-5p | Down | P = 0.003 | 0.23 |
| 23 | hsa-miR-382-5p | Down | P = 0.016 | 0.24 |
| 24 | hsa-miR-376a-3p | Down | P = 0.040 | 0.25 |
| 25 | hsa-miR-30d-5p | Down | P = 0.003 | 0.25 |
| 26 | hsa-miR-152 | Down | P = 0.011 | 0.26 |
| 27 | hsa-miR-99b-5p | Down | P = 0.006 | 0.27 |
| 28 | hsa-miR-30a-5p | Down | P = 0.002 | 0.27 |
| 29 | hsa-miR-15a-5p | Down | P = 0.004 | 0.28 |
| 30 | hsa-miR-28-5p | Down | P = 0.004 | 0.28 |
| 31 | hsa-miR-23b-3p | Down | P = 0.004 | 0.28 |
| 32 | hsa-miR-584-5p | Down | P = 0.004 | 0.29 |
| 33 | hsa-miR-339-5p | Down | P = 0.027 | 0.30 |
| 34 | hsa-miR-27b-3p | Down | P = 0.003 | 0.30 |
| 35 | hsa-miR-145-5p | Down | P = 0.020 | 0.31 |
| 36 | hsa-let-7a-5p | Down | P < 0.001 | 0.31 |
| 37 | hsa-miR-30e-3p | Down | P = 0.012 | 0.32 |
| 38 | hsa-miR-766-3p | Down | P = 0.017 | 0.32 |
| 39 | hsa-miR-30c-5p | Down | P < 0.001 | 0.32 |
| 40 | hsa-miR-30e-5p | Down | P = 0.003 | 0.32 |
| 41 | hsa-miR-139-5p | Down | P = 0.029 | 0.33 |
| 42 | hsa-miR-126-3p | Down | P = 0.002 | 0.33 |
| 43 | hsa-miR-107 | Down | P = 0.025 | 0.33 |
| 44 | hsa-miR-26a-5p | Down | P = 0.004 | 0.34 |
| 45 | hsa-miR-151a-5p | Down | P = 0.001 | 0.35 |
| 46 | hsa-miR-146a-5p | Down | P = 0.003 | 0.35 |
| 47 | hsa-miR-125a-5p | Down | P = 0.049 | 0.35 |
| 48 | hsa-miR-103a-3p | Down | P = 0.004 | 0.36 |
| 49 | hsa-let-7d-5p | Down | P < 0.001 | 0.36 |
| 50 | hsa-miR-374b-5p | Down | P = 0.001 | 0.38 |
| 51 | hsa-miR-374a-5p | Down | P = 0.014 | 0.39 |
| 52 | hsa-miR-320a | Down | P = 0.046 | 0.39 |
| 53 | hsa-miR-21-5p | Down | P = 0.016 | 0.40 |
| 54 | hsa-miR-15b-5p | Down | P = 0.023 | 0.41 |
| 55 | hsa-miR-155-5p | Down | P = 0.003 | 0.41 |
| 56 | hsa-miR-28-3p | Down | P = 0.003 | 0.42 |
| 57 | hsa-miR-30b-5p | Down | P = 0.007 | 0.43 |
| 58 | hsa-let-7b-3p | Down | P = 0.031 | 0.43 |
| 59 | hsa-miR-23a-3p | Down | P = 0.019 | 0.49 |
| Fold change >2 (or <0.5) fold with P ≥ 0.05 | ||||
| 1 | hsa-miR-141-3p | Down | NS | 0.24 |
| 2 | hsa-miR-193b-3p | Down | NS | 0.31 |
| 3 | hsa-miR-485-3p | Down | NS | 0.32 |
| 4 | hsa-miR-20a-3p | Down | NS | 0.32 |
| 5 | hsa-miR-154-5p | Down | NS | 0.35 |
| 6 | hsa-miR-409-3p | Down | NS | 0.40 |
| 7 | hsa-miR-551b-3p | Down | NS | 0.40 |
| 8 | hsa-miR-215 | Down | NS | 0.41 |
| 9 | hsa-miR-95 | Down | NS | 0.42 |
| 10 | hsa-miR-127-3p | Down | NS | 0.43 |
| 11 | hsa-miR-185-5p | Down | NS | 0.43 |
| 12 | hsa-miR-133a | Down | NS | 0.43 |
| 13 | hsa-miR-1 | Down | NS | 0.45 |
| 14 | hsa-miR-199a-3p | Down | NS | 0.46 |
| 15 | hsa-miR-182-5p | Down | NS | 0.47 |
| 16 | hsa-miR-143-3p | Down | NS | 0.48 |
| 17 | hsa-miR-192-5p | Down | NS | 0.48 |
| 18 | hsa-miR-500a-5p | Down | NS | 0.48 |
| 19 | hsa-miR-590-5p | Down | NS | 0.49 |
| 20 | hsa-let-7i-3p | Up | NS | 2.20 |
| 21 | hsa-miR-296-5p | Up | NS | 5.00 |
Student’s t-test.
MicroRNAs were considered differentially expressed at a fold change >1.5.
NS, no significant difference (P ≥ 0.05).
Figure 1.
Scatter plots showing microRNAs expressed in peripheral blood samples from patients with intracerebral haemorrhage (n = 7). The black line represents a fold change (FC) of 1. Spots located above and below the pink lines represent upregulated and downregulated microRNAs, respectively (FC >2 or <0.5). The colour version of this figure is available at: http://imr.sagepub.com.
The following three databases, miRBase,27 MicroCosm Targets,28 and TargetScanHuman,29 were used to analyse the miRNA sequences and to predict the target genes. The function of each target gene and its related signal pathway were studied using the NCBI database.30,21–26,31–40 Haemorrhage-related miRNA was screened according to the target gene prediction, signal pathway and the significant expression of miRNA in this study (Table 4).21–26,31–40
Table 4.
Target gene prediction for the microRNAs identified in the microRNA polymerase chain reaction microarray analysis in peripheral blood samples from patients with intracerebral haemorrhage (n = 7).21–26,31–40
| MicroRNA ID | Target genes | Function |
|---|---|---|
| hsa-miR-21-5p21–26 | PTEN, PDCD4, RECK, HNRPK, JAG1, Bcl-2, PPARα | Apoptosis Integrity of vascular endothelium Inflammation Oxidative stress Jak-STAT signal transduction pathway |
| hsa-miR-15a-5p31–36 | EGFP, MEK1, MEK4, CCND1, CCNT2, IL-6, IL-4, IL-10 | Apoptosis Oxidative stress Inflammation |
| hsa-miR-12637,38 | PIK3R2, VCAM1, SPRED1 | Inflammation Integrity of vascular endothelium |
| hsa-miR-10739,40 | PTEN, p53, CDK6, FADD | Apoptosis Inflammation |
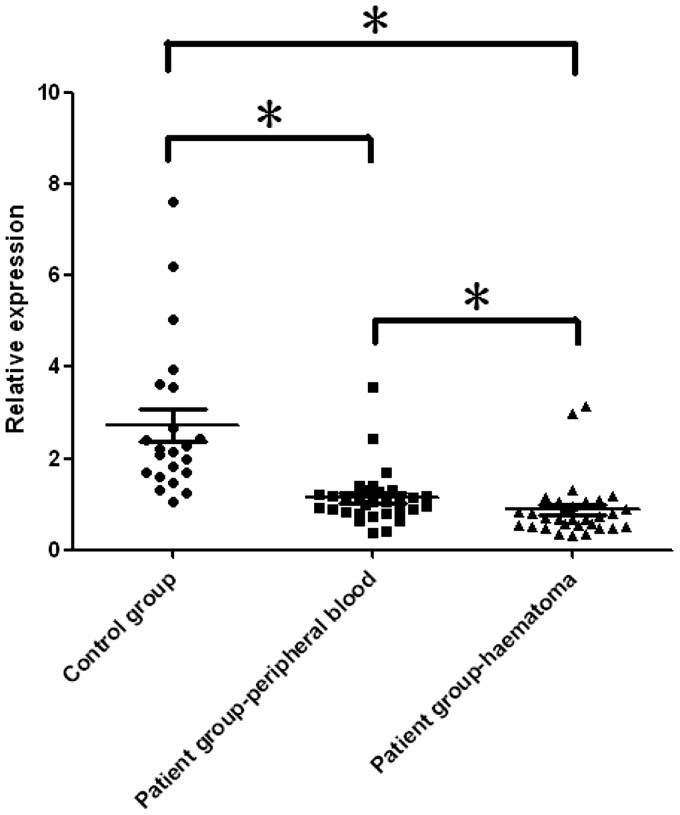
The expression of hsa-miR-21-5p was assessed by real-time PCR in peripheral blood and haematoma samples from patients with ICH (n = 31) and a group of healthy control subjects (n = 22). Both the target and reference genes had amplification curves presented as ‘S’ and the melting curves showed a single peak. The internal reference gene exhibited stable and constant expression among both groups without overt differences. Compared with control individuals, lower hsa-miR-21-5p levels were obtained in the patient group, both for peripheral blood and haematoma samples, with a more pronounced reduction in the latter, in agreement with the miRNA PCR microarray analysis. The relative expression levels of hsa-miR-21-5p were 0.43 and 0.31, in peripheral blood and haematoma samples, respectively (P = 0.006 for both comparisons versus the control group) (Table 5, Figure 2). The downregulation of hsa-miR-21-5p was more pronounced in haematoma specimens than peripheral blood samples from the patients with ICH (P = 0.002).
Table 5.
The relative expression of hsa-miR-21-5p in peripheral blood and haematoma samples from patients with intracerebral haemorrhage (n = 31) compared with a group of healthy control subjects (n = 22).
| Group | 2−ΔCT | Relative expression |
|---|---|---|
| Control group, n = 22 | 2.732 ± 1.680 | 1 |
| Patient group – peripheral blood, n = 31 | 1.140 ± 0.592 | 0.43* |
| Patient group – haematoma, n = 31 | 0.879 ± 0.638 | 0.31* |
Data presented as mean ± SD.
P < 0.05 compared with the control group; Student’s t-test.
Figure 2.
The relative expression of hsa-miR-21-5p in peripheral blood and haematoma samples from patients with intracerebral haemorrhage (n = 31) compared with a group of healthy control subjects (n = 22). *P < 0.05, Student’s t-test.
Discussion
A large number of cells possess unique miRNA expression profiles and certain miRNAs detected in body fluids can indicate the primary origin of the cell, suggesting that miRNAs might have diagnostic and prognostic values in certain diseases.40 Hypertension is the most common cause of cerebral haemorrhage in the putamen.41 To eliminate the influence of other causes of cerebral haemorrhage (e.g. amyloidosis-induced cerebral haemorrhage) on the experimental results, this current study only included patients who experienced ICH and had radiological evidence that the bleeding was restricted to the putamen and did not break into the ventricle. A total of 179 miRNAs were examined using a miRCURY LNA™ Universal RT miRNA PCR panel. Hsa-miR-93-5p, hsa-miR-423-5p and hsa-miR-191-5p were selected as housekeeping miRNAs,16–18 so that expression levels of the target miRNAs could be normalized and comparisons made between samples from the patient and control groups.
To date, miRNA research has focused on ischaemic stroke and reports assessing miRNA expression profiles in patients with ICH in humans are scarce.5 This present study identified a variety of miRNAs differentially expressed in peripheral blood samples from patients with ICH, which suggests that specific miRNAs might be potential biomarkers for ICH. Among the 179 miRNAs previously validated in serum/plasma samples, 80 showed a fold change >2, including 59 that were significantly downregulated. An analysis of 384 common miRNAs in a rat model of ICH found 36 and 19 miRNAs that were downregulated and upregulated, respectively, but only six showed significant changes.10
An increasing number of studies have shown the involvement of miRNA in multiple stages of brain damage. For example, miR-125b induces apoptosis via distinct pathways.42 In this present study, hsa-miR-125b-5p was significantly downregulated in peripheral blood samples from patients with ICH (FC 0.22; P = 0.027). In addition, MiR-126, miR-155 and miR-146a/b were shown to be involved in the inflammatory response following ICH.10,38,43–45 Likewise, significantly decreased expression levels of hsa-miR-126-3p (FC 0.33; P = 0.002), hsa-miR-155-5p (FC 0.41; P = 0.003), hsa-miR-146a-5p (FC 0.35; P = 0.003) and hsa-miR-146b-5p (FC 0.18; P = 0.042) were obtained in peripheral blood samples from patients with ICH in the present study. Furthermore, miR-143 was found to regulate brain oedema after ICH;46 and similarly, this present study found decreased hsa-miR-143-3p levels in the peripheral blood of patients with ICH (FC 0.48), but it was not significant. The present study showed that the expression of hsa-miR-27a-3p was significantly reduced in the peripheral blood of patients with ICH (FC 0.03; P = 0.009). However, analysis using miRNA target gene prediction software and relevant databases suggested that hsa-miR-21-5p (FC 0.40; P = 0.016) is associated with a number of physiological and pathological mechanisms that occur following ICH, so this miRNA was selected for the real-time PCR analysis in a larger group of patients and control subjects.
The gene encoding hsa-miR-21-5p is found on chromosome 17q23.2 and is located within intron 10 of the TMEM49 gene.47 Hsa-miR-21-5p is widely distributed in human cells and tissues. For example, miR-21 levels are elevated in lung, breast, colon and other cancers.48 In addition, hsa-miR-21 plays a pivotal role in cardiovascular disease.49 Increased hsa-miR-21-5p expression was found around the infarct area in the later stage of acute myocardial infarction.50 Furthermore, hsa-miR-21-5p has a protective role against ischaemia-induced apoptosis.50 Hsa-miR-21-5p is also highly expressed in vascular tissues in patients with pulmonary hypertension.51,52 In contrast, reduced miR-21-expression was found in plasma samples from patients with acute cerebral infarction.53
The present study examined miR-21-5p expression in peripheral blood and haematoma samples from patients with ICH and healthy control subjects using real-time PCR. To our knowledge, this is the first study to describe miR-21-5p expression in haematoma samples from patients with ICH. The present study identified identical changes in miR-21-5p expression (i.e. downregulation) in both peripheral blood and haematoma samples from patients with ICH, consistent with previous findings in a rat model of ICH.10 In addition, the present study found a more pronounced reduction of miR-21-5p expression in haematoma samples compared with peripheral blood (relative expression 0.31 versus 0.43, respectively; P < 0.05). Previous studies have demonstrated the involvement of miR-21 in multiple pathophysiological responses, including apoptosis and inflammation. For example, miR-21 reduces apoptosis via Fas ligand regulation, which is associated with its protective effects on neurons as shown in a recent intracerebral ischaemia study.23 In addition, miR-21 can repress the expression of the ‘phosphatase and tensin homolog deleted on chromosome 10’ (PTEN) gene, whose product negatively regulates the PI3K/Akt pathway to decrease endothelial nitric oxide synthase amounts and activity, thereby reducing inflammatory responses in endothelial cells.24 MiR-21 has a regulatory effect on the matrix metalloproteinase inhibitors reversion-inducing-cysteine-rich protein with kazal motifs and tissue inhibitor of metalloproteinase-3, enhancing the synthesis of cerebral matrix metalloproteinase (MMP).21 MMP-2 and MMP- 9 have been suggested to be closely related to post-stroke vasogenic oedema.25 MMP-9 contributes to the development of cerebral vasogenic oedema during the early stage of brain haemorrhage through destruction of the blood–brain barrier,54 whilst further deteriorating the oedema along with synergistic effects of thrombin.55 The evidence suggests, in our opinion, that miRNA-21 might be engaged in multiple regulatory pathways following cerebral haemorrhage, namely neuronal apoptosis, inflammatory response and brain oedema. Therefore, miRNA-21 might have an important role to play in the prognosis of patients with ICH. Downregulation of miR-21 expression in the peripheral blood of patients with ICH might induce pathological changes to promote neuron apoptosis, possibly reducing inflammation and the occurrence of cerebral oedema.
This study had a number of limitations. First, perihaematoma tissues from patients with ICH were not available for ethical reasons, but alterations of miRNA expression in perihaematoma tissues can be inferred based on those occurring in the haematoma. A number of issues remain to be clarified by future studies. For example, it is not known why different miR-21-5p expression levels were found in haematoma and peripheral blood samples. In addition, miR-21 participation in regulatory pathways in ICH and the exact mechanisms of its effects need to be investigated.
In conclusion, miRNAs are engaged in the pathophysiological mechanisms of multiple processes following ICH, which provides a theoretical basis for the further clinical evaluation of miRNAs to predict ICH occurrence and improve patient prognosis. Considering the large number of miRNAs that exist, and that a single gene can be regulated by multiple miRNAs, a comprehensive understanding of the mechanisms by which miRNAs might be involved in ICH remain to be validated in follow-up studies. More in-depth research should result in novel therapeutic strategies based on miRNAs to mitigate brain haemorrhage after injury and improve patient prognosis.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (no. 81271291).
References
- 1.Qureshi Al, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009; 373: 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010; 9: 167–176. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi Al, Tuhrim S, Broderick JP, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001; 344: 1450–1460. [DOI] [PubMed] [Google Scholar]
- 4.Yaghi S, Dibu J, Achi E, et al. Hematoma expansion in spontaneous intracerebral hemorrhage: predictors and outcome. Int J Neurosci 2014; 124: 890–893. [DOI] [PubMed] [Google Scholar]
- 5.Zheng HW, Wang YL, Lin JX, et al. Circulating MicroRNAs as potential risk biomarkers for hematoma enlargement after intracerebral hemorrhage. CNS Neurosci Ther 2012; 18: 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JM, Lee ST, Chu K, et al. Inhibition of Let7c microRNA is neuroprotective in a rat intracerebral hemorrhage model. PLoS One 2014; 24: e97946–e97946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagos-Quintana M, Rauhut R, Lendeckel W, et al. Identification of novel genes coding for small expressed RNAs. Science 2001; 294: 853–858. [DOI] [PubMed] [Google Scholar]
- 8.Kim VN, Nam JW. Genomics of microRNA. Trends Genet 2006; 22: 165–173. [DOI] [PubMed] [Google Scholar]
- 9.Tan JR, Koo YX, Kaur P, et al. microRNAs in stroke pathogenesis. Curr Mol Med 2011; 11: 76–92. [DOI] [PubMed] [Google Scholar]
- 10.Liu DZ, Tian Y, Ander BP, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab 2010; 30: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saugstad JA. MicroRNAs as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab 2010; 30: 1564–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winter J, Jung S, Keller S, et al. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 2009; 11: 228–234. [DOI] [PubMed] [Google Scholar]
- 13.Jazbutyte V, Thum T. MicroRNA-21: from cancer to cardiovascular disease. Curr Drug Targets 2010; 11: 926–935. [DOI] [PubMed] [Google Scholar]
- 14.Ribas J, Lupold SE. The transcriptional regulation of miR-21, its multiple transcripts, and their implication in prostate cancer. Cell Cycle 2010; 9: 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Exiqon GenEx Software for qPCR Data Analysis. www.exiqon.com/mirna-pcr-analysis (2009, accessed 15 September 2014).
- 16.Mahdipour M, van Tol HT, Stout TA, et al. Validating reference microRNAs for normalizing qRT-PCR data in bovine oocytes and preimplantation embryos. BMC Devl Biol 2015; 15: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 2002; 3 research0034.1–research0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gharbi S, Shamsara M, Khateri S, et al. Identification of reliable reference genes for quantification of microRNAs in serum samples of sulfur mustard-exposed veterans. Cell J 2015; 17: 494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 20.miRecords website. Subramanian's Lab, Department of Surgery, University of Minnesota, Minneapolis, MN, USA, http://www.tc.umn.edu/∼subree/Links Page.html (2010, accessed 3 June 2015).
- 21.Gabriely G, Wurdinger T, Kesari S, et al. MicroRNA-21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol 2008; 28: 5369–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayed D, He M, Hong C, et al. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem 2010; 285: 20281–20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buller B, Liu X, Wang X, et al. MicroRNA-21 protects neurons from ischemic death. FEBS J 2010; 277: 4299–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rippe C, Blimline M, Magerko KA, et al. MicroRNA changes in human arterial endothelial cells with senescence: relation to apoptosis, eNOS and inflammation. Exp Gerontol 2012; 47: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CZ, Xu B, Hashimoto T, et al. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke 2004; 35: 1715–1719. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, Wang KC, Wu W, et al. microRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci USA 2011; 108: 10355–10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.miRBase: the microRNA database, http://mirbase.org/index.shtml (2010, accessed 1 August 2015).
- 28.MicroCosm Targets Version 5, http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/ (2010, accessed 3 August 2015).
- 29.TargetScanHuman, http://www.targetscan.org/vert_70/ (2009, accessed 5 August 2015).
- 30.National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/ (2001, accessed 10 August 2015).
- 31.Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Diffe 2010; 17: 215–220. [DOI] [PubMed] [Google Scholar]
- 32.Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA 2005; 102: 13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonci D, Coppola V, Musumeci M, et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 2008; 14: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 34.Calin GA, Cimmino A, Fabbri M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci USA 2008; 105: 5166–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katsuno M, Yokota H, Yamamoto Y, et al. Increased regional interleukin-4 during the acute stage of severe intracranial disorders. Neurol Med Chir (Tokyo) 2006; 46: 471–475. [DOI] [PubMed] [Google Scholar]
- 36.Dziedzic T, Bartus S, Klimkowicz A, et al. Intracerebral hemorrhage triggers interleukin-6 and interleukin-10 release in blood. Stroke 2002; 33: 2334–2335. [DOI] [PubMed] [Google Scholar]
- 37.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 2008; 15: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris TA, Yamakuchi M, Ferlito M, et al. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule. Proc Natl Acad Sci U S A 2008; 105: 1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohlig L, Friedrich M, Engeland K. p53 activates the PANK1/miRNA-107 gene leading to downregulation of CDK6 and p130 cell cycle proteins. Nucleic Acids Res 2011; 39: 440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hergenreider E, Heydt S, Treguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 2012; 14: 249–256. [DOI] [PubMed] [Google Scholar]
- 41.Mizutani T, Kojima H, Miki Y. Arterial dissections of penetrating cerebral arteries causing hypertension-induced cerebral hemorrhage. J Neurosurg 2000; 93: 859–862. [DOI] [PubMed] [Google Scholar]
- 42.Le MT, Teh C, Shyh-Chang N, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev 2009; 23: 862–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Nunez RT, Louafi F, Friedmann PS, et al. MicroRNA-155 modulates the pathogen binding ability of dendritic cells (DCs) by down-regulation of DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN). J Biol Chem 2009; 284: 16334–16342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taganov KD, Boldin MP, Chang KJ, et al. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006; 103: 12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhaumik D, Scott GK, Schokrpur S, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY) 2009; 1: 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Hu G, Zhou J. Repression of versican expression by microRNA-143. J Biol Chem 2010; 285: 23241–23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol 2011; 8: 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 2006; 103: 2257–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res 2010; 3: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A 2008; 105: 13027–13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piepoli MF, Guazzi M, Boriani G, et al. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part II. Eur J Cardiovasc Prev Rehabil 2010; 17: 643–648. [DOI] [PubMed] [Google Scholar]
- 52.Sabatel C, Malvaux L, Bovy N, et al. MicroRNA-21 exhibits antiangiogenic function by targeting RhoB expression in endothelial cells. PLoS One 2011; 6: e16979–e16979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou J, Zhang J. Identification of miRNA-21 and miRNA-24 in plasma as potential early stage markers of acute cerebral infarction. Mol Med Rep 2014; 10: 971–976. [DOI] [PubMed] [Google Scholar]
- 54.Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood–brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis 2010; 38: 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue M, Hollenberg MD, Yong VW. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J Neurosci 2006; 26: 10281–10291. [DOI] [PMC free article] [PubMed] [Google Scholar]