Abstract
Objective
To evaluate oxidative damage in leukocytes from patients with type 2 diabetes by examining 8-hydroxy-2’-deoxyguanosine (8-OHdG) levels.
Methods
Patients with type 2 diabetes and healthy controls were assessed for demographic, clinical and biochemical characteristics. Levels of 8-OHdG in extracted leukocyte DNA were determined by enzyme linked immunosorbent assay.
Results
Of 108 patients with type 2 diabetes (56 with microangiopathy, 52 without) and 65 healthy controls, leukocyte 8-OHdG levels were higher in patients with type 2 diabetes versus controls (median ± interquartile range [IQR], 3.19 ± 2.17 versus 0.38 ± 1.00 ng/ml), and higher in patients with type 2 diabetes and microangiopathy versus those without microangiopathy (median ± IQR, 3.34 ± 1.87 versus 2.71 ± 2.26 ng/ml). Patients with type 2 diabetes and microangiopathy had higher serum creatinine and urinary albumin levels versus those without microangiopathy. Leukocyte 8-OHdG levels, duration of type 2 diabetes, albuminuria, use of insulin and use of angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) were independently associated with microangiopathy in patients with type 2 diabetes after adjustment for smoking.
Conclusions
Leukocyte oxidative DNA damage was high in patients with type 2 diabetes and microangiopathy. Presence of microangiopathy was associated with leukocyte 8-OHdG levels, duration of type 2 diabetes, albuminuria and use of ACE inhibitors/ARBs or insulin.
Keywords: Type 2 diabetes, DNA damage, oxidative stress, diabetic microangiopathy, 8-hydroxy-2’-deoxyguanosine
Introduction
Type 2 diabetes is an endocrine disorder characterized by insulin resistance and deficiency, resulting in chronic hyperglycaemia that may lead to renal, neurological and cardiovascular complications.1 Obesity and age are risk factors for type 2 diabetes.1 Long-term vascular complications are the main cause of type 2 diabetes-associated morbidity and mortality.1 In 2008, worldwide prevalence of type 2 diabetes was 9.8% in males and 9.2% in females;2 in 2010, prevalence of type 2 diabetes in the Chinese population was reported to be 11.6%.3
Metabolic dysfunctions associated with type 2 diabetes include dyslipidaemia,4 oxidative stress5 and activated leukocytes.6 Oxidative stress and leukocyte activation have been associated with the development of type 2 diabetes complications.5–7 Oxidative stress may lead to cell damage and dysfunction through the free-radical-mediated decomposition of vital molecules (particularly DNA, which lacks chemical stability);8 oxidative stress has been shown to activate nuclear transcription factor (NF)-κB, leading to DNA damage.9 Studies have shown increased oxidative DNA damage10–12 and accumulation of 8-hydroxy-2’-deoxyguanosine (8-OHdG)11–13 in patients with type 2 diabetes, thus suggesting the involvement of hyperglycaemia in oxidative DNA damage. A product of oxidative DNA damage following specific enzymatic cleavage after 8-hydroxylation of the guanine base, 8-OHdG is considered to be a good marker of oxidative DNA damage that can easily be measured using an enzyme linked immunosorbent assay (ELISA).14
Microangiopathy in patients with type 2 diabetes is associated with increased platelet and leukocyte activation;15 however, specific leukocyte DNA damage and factors involved in this damage are poorly understood. The authors of the present study hypothesized that leukocyte DNA is damaged in type 2 diabetes, and that an advanced diabetic state (i.e. with microangiopathy) is associated with further DNA damage. Therefore, the aim of the study was to evaluate the extent of oxidative DNA damage in leukocytes from patients with type 2 diabetes and microangiopathy, by examining peripheral blood leukocyte 8-OHdG levels, in an attempt to provide further insights into the pathogenesis of microangiopathy and indications for treatment.
Patients and methods
Study population
This case-control study included sequentially enrolled patients with type 2 diabetes recruited at the Department of Endocrinology, Jinling Hospital, Nanjing, China, between October 2007 and April 2008. All patients had been diagnosed according to World Health Organization criteria.16 Inclusion criteria were: (i) aged 40–70 years; (ii) no history of coronary artery disease, cerebral infarction or peripheral vascular diseases, as shown by Holter monitoring, Doppler ultrasound, magnetic resonance imaging, computed tomography and/or coronary angiography; (iii) no antioxidant therapy; (iv) no lipid-lowering therapy in the 6 months prior to study entry; (v) no history of angina or myocardial infarction (normal 12-lead resting electrocardiograms and normal peripheral Doppler studies); (vi) no confirmed atherosclerosis, vascular stenosis or occlusion in the lower extremities as shown by Doppler ultrasound.
Healthy controls were recruited from individuals who consulted for routine physical examinations at the Health Examination Centre, Jinling Hospital. Inclusion criteria for controls were: (i) aged 40–70 years; (ii) no personal or family history of hypertension, diabetes, coronary diseases or kidney diseases; (iii) no drug use in the 6 months prior to study entry (including pain medications, e.g. over-the-counter acetaminophen or non-steroidal anti-inflammatory drugs, and dietary supplements, e.g. vitamins).
Microangiopathy was diagnosed if nephropathy or retinopathy, or both, were present. Nephropathy was diagnosed if patients had either persistent proteinuria (≥500 mg/day) or persistent microalbuminuria (albuminuria estimated by the albumin:creatinine ratio >30 µg/mg) in the absence of urinary tract infection.17 Retinopathy was assessed using stereoscopic colour retinal photography as previously described.18 Hypertension was diagnosed if patients were being treated with antihypertensive drugs or had a systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg. Patients with diabetes without microangiopathy were diagnosed based on the absence of retinopathy (assessed by retinal photography) or nephropathy (24 h protein excretion <100 mg/day and albumin : creatinine ratio <30 µg/mg).
The study was approved by the ethics committee of Nanjing Jinling Hospital. Written informed consent was obtained from all study participants.
Clinical parameters and laboratory measurements
All participants underwent a clinical and physical (including height, weight and body mass index [BMI]) examination, and demographic and clinical data were recorded.
Blood pressure was recorded in the right arm while participants were seated. Two readings were taken 5 min apart and the mean was used for analysis. Blood samples (5 ml) were taken following an overnight fast. Serum was separated by leaving samples to clot for 1 h at room temperature. For plasma collection, blood samples were drawn into ethylenediaminetetra-acetic acid (EDTA) anti-coagulation tubes and plasma was separated by centrifugation at 400 g for 5 min at room temperature. Samples were stored at −70℃ prior to use. Biochemical analyses were performed on a Hitachi 7600 Autoanalyser (Hitachi, Tokyo, Japan) and included fasting plasma glucose (Glucose Kit, TCI, Japan), serum total cholesterol (Total Cholesterol test kit, Randox Laboratories Ltd, County Antrim, UK), serum triglycerides (Triglycerides test kit, Randox Laboratories Ltd) and high-density lipoprotein cholesterol (HDL-C; HDL Cholesterol test kit, Randox Laboratories Ltd). Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula ([LDL-C] = [Total cholesterol] - [HDL-C] - ([TG]/2.2). Glycosylated haemoglobin (HbA1c) was estimated by high-pressure liquid chromatography (HLC-723G8; TOSOH, Tokyo, Japan), according to the manufacturer’s instructions. Samples were processed with the hospital’s usual clinical samples; laboratory staff were thus blinded to the study groups.
Leukocyte DNA 8-OHdG measurements
Venous blood samples (10 ml drawn into EDTA anti-coagulation tubes) were obtained from participants following an overnight fast. Leukocyte DNA was extracted from peripheral blood samples within 1 h of blood collection, using PureGene™ reagents (SBS Genetech Co. Ltd, Shanghai, China) according to the manufacturer’s protocol, which included separation of the nucleated cells from whole blood and the salting-out method.19 DNA purity was determined using absorbance at 260/280 nm and absorbance at 260/230 nm. Isolated DNA was stored at −80℃ prior to use. For each sample, 200 µg DNA was dissolved in 135 µl of nuclease free water, then 15 µl (200 mM) sodium acetate and 15 µl (6 units) nuclease P1 (Sigma, St Louis, MO, USA) were added to the DNA solution and incubated for 30 min at 37℃. Following incubation, 15 µl Tris-HCl buffer (1 M, pH 7.4) and 7 µl (2 units) alkaline phosphatase (Takara, Tokyo, Japan) were added and incubated for another 30 min at 37℃. The hydrolysate was filtered through a Millipore Microcon® Centrifugal Filter (Merck Millipore, Darmstadt, Germany) at 1000g centrifugation for 10 min, and then 50 µl of each digested DNA sample was assessed using a Highly Sensitive 8-OHdG Check ELISA kit (JaICA, Fukuroi, Shizuoka, Japan), according to the manufacturer’s instructions. Absorbance was measured at 450 nm using a microtitre plate reader (JaICA, Fukuroi, Shizuoka, Japan). Results were expressed in ng/ml, then 1 ng/ml was converted to 4.8 8-OHdG/106 dG, based on a previously described method.20 Samples were assayed in a blind manner.
Statistical analyses
All statistical analyses were performed using SPSS® version 11.0 (SPSS Inc., Chicago, IL, USA). Normally distributed continuous data are presented as mean ± SD, and were compared using Student’s t-test. Not normally distributed continuous data are presented as median ± interquartile range (IQR), and were compared using the Mann–Whitney U-test. Categorical variables are presented as proportions and were compared using Fisher’s exact test or χ2-test, as appropriate. Multivariate logistic regression was used to analyse independent factors associated with microangiopathy in patients with type 2 diabetes. Variables with P-values < 0.05 by univariate analyses were included in the multivariate model. P-values < 0.05 were considered statistically significant.
A post-hoc power analysis was performed. For comparison of 8-OHdG levels between controls and patients with type 2 diabetes, the power was 100% (n = 65 and n = 108, respectively; α = 5%). For the comparison of 8-OHdG levels between patients with type 2 diabetes with and without microangiopathy, the power was 84% (n = 56 and n = 52, respectively; α = 5%).
Results
Demographic and clinical characteristics
A total of 108 patients with type 2 diabetes (56 with microangiopathy, 52 without) and 65 healthy controls were included (Table 1). Age, sex, BMI, SBP, DBP, triglycerides, HDL-C, serum creatinine and smoking were comparable between patients with type 2 diabetes and controls (all P > 0.05; Table 1). Fasting plasma glucose (P < 0.001), HbA1c (P < 0.001), total cholesterol (P = 0.030), LDL-C (P = 0.012) and albuminuria (P < 0.001) were higher in patients with type 2 diabetes compared with controls (Table 1).
Table 1.
Demographic and clinical characteristics of Chinese patients with type 2 diabetes and healthy controls.
| Study group |
|||
|---|---|---|---|
| Variable | Healthy controls n = 65 | Type 2 diabetes n = 108 | Statistical significance |
| Age, years | 55 ± 11 | 60 ± 17 | NS |
| Sex, male | 36 (55.4) | 67 (62.0) | NS |
| Duration of diabetes, months | 0 | 98.02 ± 77.68 | – |
| Fasting plasma glucose, mmol/l | 4.6 ± 1.1 | 10.4 ± 3.3 | P < 0.001 |
| HbA1c, % | 5.5 ± 0.7 | 9.0 ± 2.9 | P < 0.001 |
| BMI, kg/m2 | 22.5 ± 3.2 | 23.5 ± 5.4 | NS |
| SBP, mmHg | 114 ± 28 | 126 ± 39 | NS |
| DBP, mmHg | 70 ± 14 | 79 ± 27 | NS |
| Total cholesterol, mmol/l | 3.22 ± 0.88 | 4.74 ± 1.67 | P = 0.030 |
| Triglycerides, mmol/l | 1.44 ± 0.76 | 1.67 ± 1.20 | NS |
| LDL cholesterol, mmol/l | 1.77 ± 0.58 | 2.83 ± 0.85 | P = 0.012 |
| HDL cholesterol, mmol/l | 1.13 ± 0.24 | 1.24 ± 0.21 | NS |
| Serum creatinine, mg/dl | 0.82 ± 0.19 | 0.91 ± 0.31 | NS |
| Albuminuria, mg/24 h | 9.22 ± 3.44 | 141.92 ± 116.36 | P < 0.001 |
| 8-OHdG, ng/ml | 0.38 ± 1.00 | 3.19 ± 2.17 | P < 0.001a |
| Smoker, yes | 19 (29.2) | 27 (25.0) | NS |
Data presented as mean ± SD, median ± interquartile range (IQR) or n (%) prevalence, as appropriate.
Leukocyte 8-OHdG levels are shown as median ± IQR (Mann–Whitney U-test).
HbA1c, glycosylated haemoglobin; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; 8-OHdG, 8-hydroxy-2’-deoxyguanosine.
NS, no statistically significant between-group differences (P > 0.05, Student’s t-test).
In patients with type 2 diabetes grouped according to the presence or absence of microangiopathy, patient age, sex, fasting plasma glucose, HbA1c, BMI, SBP, DBP, total cholesterol, triglycerides, HDL-C, use of metformin, use of sulphonylureas, use of α-glycosidase inhibitor and use of antiplatelet drugs were comparable between the two groups (all P > 0.05; Table 2). Patients with type 2 diabetes and microangiopathy showed higher LDL-C (P = 0.042), serum creatinine (P = 0.032) and albuminuria (P = 0.005) levels versus patients with type 2 diabetes without microangiopathy. Patients with type 2 diabetes and microangiopathy also had a higher prevalence of smokers (P = 0.010), use of insulin (P = 0.019), use of Ca2+channel blockers (P = 0.014), use of angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) (P = 0.035) and longer duration of diabetes (P = 0.033; Table 2).
Table 2.
Demographic and clinical characteristics of Chinese patients with type 2 diabetes with or without microangiopathy.
| Type 2 diabetes subgroup |
|||
|---|---|---|---|
| Variable | Without microangiopathy n = 52 | With microangiopathy n = 56 | Statistical significance |
| Age, years | 59 ± 8 | 61 ± 11 | NS |
| Sex, male | 32 (61.5) | 38 (67.9) | NS |
| Duration of diabetes, months | 80.93 ± 59.90 | 126.04 ± 84.45 | P = 0.033 |
| Fasting plasma glucose, mmol/l | 10.2 ± 2.3 | 11.8 ± 3.7 | NS |
| HbA1c, % | 8.9 ± 2.8 | 9.4 ± 3.1 | NS |
| BMI, kg/m2 | 23.8 ± 4.6 | 23.4 ± 5.8 | NS |
| SBP, mmHg | 125 ± 38 | 132 ± 35 | NS |
| DBP, mmHg | 78 ± 24 | 82 ± 22 | NS |
| Total cholesterol, mmol/l | 4.66 ± 1.04 | 4.77 ± 0.99 | NS |
| Triglycerides, mmol/l | 1.58 ± 1.58 | 1.59 ± 0.53 | NS |
| LDL cholesterol, mmol/l | 2.53 ± 0.84 | 3.09 ± 0.74 | P = 0.042 |
| HDL cholesterol, mmol/l | 1.24 ± 0.21 | 1.17 ± 0.21 | NS |
| Serum creatinine, mg/dl | 0.79 ± 0.23 | 1.12 ± 0.38 | P = 0.032 |
| Albuminuria, mg/24 h | 11.72 ± 4.53 | 234.85 ± 27.2 | P = 0.005 |
| 8-OHdG, ng/ml | 2.71 ± 2.26 | 3.34 ± 1.87 | P = 0.044a |
| Smoker | 13 (25.0) | 22 (39.3) | P = 0.010 |
| Insulin | 21 (40.4) | 34 (60.7) | P = 0.019 |
| Metformin | 38 (73.1) | 33 (58.9) | NS |
| Sulphonylureas | 26 (50.0) | 22 (39.3) | NS |
| α-glycosidase inhibitor | 16 (30.8) | 20 (35.7) | NS |
| Ca2+ channel blocker | 17 (32.7) | 32 (57.1) | P = 0.014 |
| ACE inhibitor/ARB | 28 (53.8) | 41 (73.2) | P = 0.035 |
| Antiplatelet drugs (aspirin/clopidogrel) | 30 (57.7) | 36 (64.3) | NS |
Data presented as mean ± SD, median ± interquartile range (IQR) or n (%) prevalence, as appropriate.
Leukocyte 8-OHdG levels are shown as median ± IQR (Mann–Whitney U-test).
HbA1c, glycosylated haemoglobin; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein; 8-OHdG, 8-hydroxy-2’-deoxyguanosine; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
NS, no statistically significant between-group differences (P > 0.05, Student’s t-test).
Leukocyte DNA damage detectable by 8-OHdG
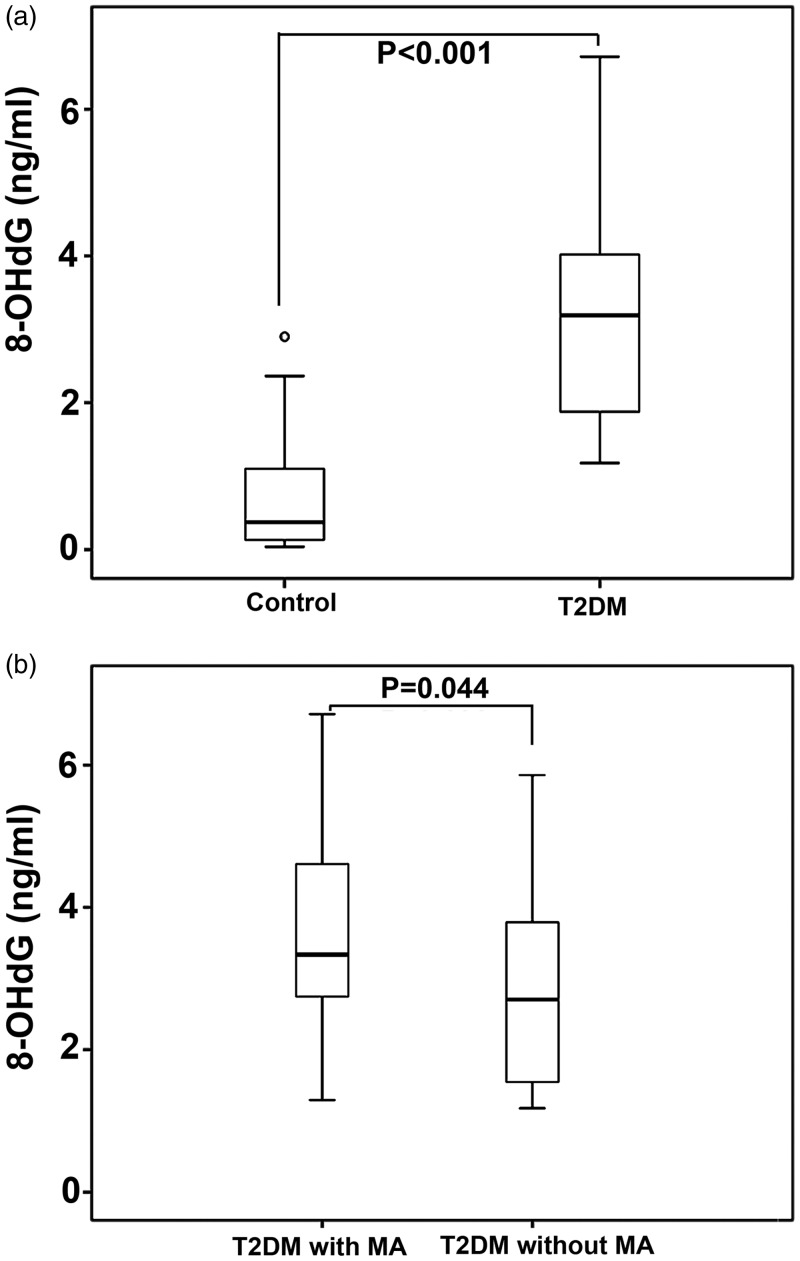
Patients with type 2 diabetes were found to have higher leukocyte levels of 8-OHdG compared with controls (median ± IQR, 3.19± 2.17 versus 0.38 ± 1.00 ng/ml, P < 0.001; Table 1 and Figure 1a). Leukocyte levels of 8-OHdG were higher in patients with type 2 diabetes and microangiopathy versus patients with type 2 diabetes without microangiopathy (median ± IQR, 3.34 ± 1.87 versus 2.71 ±2.26 ng/ml, P = 0.044; Table 2 and Figure 1b).
Figure 1.
8-hydroxy-2’-deoxyguanosine (8-OHdG) levels in Chinese patients with type 2 diabetes and healthy controls. (a) 8-OHdG levels in patients with type 2 diabetes and controls; (b) 8-OHdG levels in patients with type 2 diabetes and microangiopathy and in patients with type 2 diabetes without microangiopathy. The central (heavy) black lines within the boxes represent the medians, the extremities of the boxes are the 25th and 75th percentiles, the error bars represent minimum and maximum outliers, the circle above the ‘control’ bar represents an extreme outlier. T2DM, type 2 diabetes; MA, microangiopathy. (P < 0.05, Mann–Whitney U-test).
Univariate analyses
Univariate analyses revealed that leukocyte 8-OHdG (P < 0.001), duration of diabetes (P = 0.003), albuminuria (P = 0.009), insulin use (P = 0.028) and ACE inhibitor/ARB use (P = 0.01) were associated with microangiopathy in patients with type 2 diabetes (Table 3).
Table 3.
Univariate and multivariate logistic regression analyses of the association between 8-OHdG in leukocyte DNA and microangiopathy in Chinese patients with type 2 diabetes (n = 108).
| Statistical analysis |
||||||
|---|---|---|---|---|---|---|
| Variable | Univariate |
Multivariate (adjusted for smokers) |
||||
| OR | 95%CI | Statistical significance | OR | 95% CI | Statistical significance | |
| 8-OHdG | 3.15 | 1.83, 8.19 | P < 0.001 | 3.62 | 2.83, 8.71 | P = 0.002 |
| Duration of diabetes | 1.17 | 1.03, 4.52 | P = 0.003 | 1.84 | 1.16, 3.89 | P = 0.01 |
| Smoker | 1.56 | 0.48, 3.15 | NS | / | / | / |
| Age | 1.34 | 0.39, 2.36 | NS | / | / | / |
| BMI | 2.76 | 0.81, 11.46 | NS | / | / | / |
| Fasting plasma glucose | 2.27 | 0.91, 5.74 | NS | / | / | / |
| HbA1c | 1.01 | 0.38, 2.66 | NS | / | / | / |
| Serum creatinine | 2.32 | 0.78, 6.88 | NS | / | / | / |
| Albuminuria | 4.75 | 1.48, 15.35 | P = 0.009 | 5.02 | 3.99, 8.08 | P < 0.001 |
| Triglycerides | 1.16 | 0.34, 2.08 | NS | / | / | / |
| Total cholesterol | 2.28 | 0.91, 5.74 | NS | / | / | / |
| LDL cholesterol | 3.36 | 0.79, 14.37 | NS | / | / | |
| Insulin | 0.99 | 0.91, 3.07 | P = 0.028 | 0.58 | 0.38, 3.29 | P = 0.04 |
| Metformin | 0.03 | 0.01, 1.25 | NS | / | / | / |
| Sulphonylureas | 1.48 | 0.91, 5.75 | NS | / | / | / |
| α-glycosidase inhibitor | 1.52 | 0.99, 9.63 | NS | / | / | |
| Ca2+ channel blocker | 1.03 | 0.07, 1.15 | NS | / | / | / |
| ACE inhibitor/ARB | 0.79 | 0.37, 0.95 | P = 0.01 | 0.57 | 0.36, 0.98 | P = 0.03 |
| Antiplatelet drugs (aspirin/clopidogrel) | 0.78 | 0.32, 8.93 | NS | / | / | / |
OR, odds ratio; CI, confidence interval; 8-OHdG, 8-hydroxy-2’-deoxyguanosine; BMI, body mass index; HbA1c, glycosylated haemoglobin; LDL, low-density lipoprotein; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker.
NS, no statistically significant correlation (P > 0.05).
Multivariate analyses
Multivariate analyses revealed that leukocyte 8-OHdG levels (P = 0.002), duration of type 2 diabetes (P = 0.01), albuminuria (P < 0.001), use of insulin (P = 0.04) and use of ACE inhibitors/ARBs (P = 0.03) were independently associated with microangiopathy in patients with type 2 diabetes after adjustment for smoking (Table 3).
Discussion
Leukocyte 8-OHdG levels were higher in patients with type 2 diabetes compared with healthy controls. Patients with type 2 diabetes and microangiopathy were found to have higher leukocyte 8-OHdG, serum creatinine and urine albumin levels versus patients with type 2 diabetes without microangiopathy. Multivariate analyses revealed that leukocyte 8-OHdG levels, duration of type 2 diabetes, albuminuria, use of insulin and use of ACE inhibitors/ARBs were independently associated with microangiopathy in patients with type 2 diabetes after adjustment for smoking.
The results of the present study are consistent with those already obtained in patients with type 1 diabetes21 and patients with type 2 diabetes.13 In addition, a study in patients with Leber’s hereditary optic neuropathy revealed increased levels of leukocyte 8-OHdG compared with controls, due to increased oxidative stress.22 Although some evidence suggests an association between hyperglycaemia and DNA damage, few clinical studies have been performed to examine their relationship in humans with diabetes. In hyperglycaemic states, increased DNA damage might be due to impaired DNA repair following augmentation of poly(ADP) ribosylation and NF-κB,23 as well as impaired reduction-oxidation balance24 and increased NADPH oxidase expression coupled with decreased HO-1 expression.25 In the present study, increased DNA damage in leukocytes of patients with type 2 diabetes compared with control subjects, as well as increased leukocyte DNA damage in patients with type 2 diabetes and microangiopathies versus patients with type 2 diabetes without microangiopathies, emphasizes the role of oxidative stress in the development and progression of microvascular complications.
Urinary 8-OHdG has been reported to be a biomarker of oxidative DNA damage in patients with diabetic nephropathy26,27 and higher serum 8-OHdG levels have been observed in patients with diabetes and advanced microvascular complications.13 Increased 8-OHdG levels haven been reported in the eyes of patients with retinopathy28 and in the serum of patients with psoriasis;29 both conditions are associated with increased oxidative stress. Results of the present study concur with these published studies and suggest that 8-OHdG levels could be a predictor of diabetic microvascular complications.
Negative correlations between markers of oxidative stress and levels of antioxidant vitamins C and E have been reported, further supporting the role of oxidative stress in microangiopathy in patients with type 2 diabetes.30 In addition, using a vitamin E-coated membrane during haemodialysis has been shown to reduce leukocyte 8-OHdG levels.31 These studies suggest that the use of antioxidant therapy might help prevent and/or alleviate microangiopathy in patients with type 2 diabetes.
In the present study, oxidative DNA damage was significantly correlated to albuminuria, but negatively correlated to serum creatinine; this suggests that albuminuria may be a more reliable and early predictor of renal dysfunction than serum creatinine, which is supported by a previous study.32 Levels of oxidative DNA damage have been reported to be significantly higher in patients with creatinine clearance <70 ml/min versus those with creatinine clearance >70 ml/min.23 Further studies are required to clarify the causative mechanism of DNA damage in diabetic microvascular complications.
Angiotensin II is known to be involved in oxidative stress,33,34 and previous studies have shown that ACE inhibitors/ARBs decrease the oxidative stress in different organs.35–37 Consistent with these published studies, use of ACE inhibitors/ARBs in the present study was associated with a decreased risk of developing microangiopathy in patients with type 2 diabetes, and decreased oxidative stress is a likely mechanism. Intensive insulin treatment has been associated with decreased oxidative stress in patients with type 2 diabetes,38 which could explain the association between insulin treatment and levels of 8-OHdG observed in the present study.
The present study has a number of limitations. First, the sample size was relatively small and from a single population. Multicentre studies should be performed to increase the sample size and to represent the entire population. Secondly, the present results were obtained from a Chinese population, whose genetics, environmental factors and lifestyles differ from western populations. Thirdly, the proportion of smokers was different between the groups; however, multivariate analysis was adjusted for this variable and the association between 8-OHdG levels and microangiopathy remained significant. Finally, a larger number of markers of oxidative stress measured within the same study would provide a more complete picture of oxidative stress and microangiopathy in type 2 diabetes.39,40
In conclusion, leukocyte oxidative DNA damage was high in patients with type 2 diabetes and microangiopathy. In patients with type 2 diabetes, microangiopathy was associated with leukocyte 8-OHdG levels, duration of type 2 diabetes, albuminuria and use of ACE inhibitors/ARBs. These results imply that treatments aiming to prevent oxidative DNA damage might lead to a reduction in microangiopathy, but further studies are necessary to clarify this. In the authors’ opinions, the present results may also be applicable to other conditions with oxidative stress-associated microangiopathy.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This study was supported by the Natural Science Foundation of China (81173622) and the Natural Science Foundation of Jiangsu Province (BK2011664), China.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37(Suppl 1): S81–S90. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378: 31–40. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948–959. [DOI] [PubMed] [Google Scholar]
- 4.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 2009; 5: 150–159. [DOI] [PubMed] [Google Scholar]
- 5.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010; 107: 1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung FM, Tsai JC, Chang DM, et al. Peripheral total and differential leukocyte count in diabetic nephropathy: the relationship of plasma leptin to leukocytosis. Diabetes Care 2005; 28: 1710–1717. [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 8.Blasiak J, Arabski M, Krupa R, et al. DNA damage and repair in type 2 diabetes mellitus. Mutat Res 2004; 554: 297–304. [DOI] [PubMed] [Google Scholar]
- 9.Niu J, Wang K, Kolattukudy PE. Cerium oxide nanoparticles inhibit oxidative stress and nuclear factor-kB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J Pharmacol Exp Ther 2011; 338: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi SW, Benzie IF, Lam CS, et al. Inter-relationships between DNA damage, ascorbic acid and glycaemic control in Type 2 diabetes mellitus. Diabet Med 2005; 22: 1347–1353. [DOI] [PubMed] [Google Scholar]
- 11.Sampson MJ, Winterbone MS, Hughes JC, et al. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006; 29: 283–289. [DOI] [PubMed] [Google Scholar]
- 12.Nishikawa T, Sasahara T, Kiritoshi S, et al. Evaluation of urinary 8-hydroxydeoxy-guanosine as a novel biomarker of macrovascular complications in type 2 diabetes. Diabetes Care 2003; 26: 1507–1512. [DOI] [PubMed] [Google Scholar]
- 13.Shin CS, Moon BS, Park KS, et al. Serum 8-hydroxy-guanine levels are increased in diabetic patients. Diabetes Care 2001; 24: 733–737. [DOI] [PubMed] [Google Scholar]
- 14.Shimoi K, Kasai H, Yokota N, et al. Comparison between high-performance liquid chromatography and enzyme-linked immunosorbent assay for the determination of 8-hydroxy-2’-deoxyguanosine in human urine. Cancer Epidemiol Biomarkers Prev 2002; 11: 767–770. [PubMed] [Google Scholar]
- 15.Miyamoto K, Ogura Y, Kenmochi S, et al. Role of leukocytes in diabetic microcirculatory disturbances. Microvasc Res 1997; 54: 43–48. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia, Geneva: World Health Organization, 2006. [Google Scholar]
- 17.Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 2005; 28: 164–176. [DOI] [PubMed] [Google Scholar]
- 18.Maker MP, Noble J, Silva PS, et al. Automated Retinal Imaging System (ARIS) compared with ETDRS protocol color stereoscopic retinal photography to assess level of diabetic retinopathy. Diabetes Technol Ther 2012; 14: 515–522. [DOI] [PubMed] [Google Scholar]
- 19.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988; 16: 1215–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliwell B. Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: measurement, mechanism and the effects of nutrition. Mutat Res 1999; 443: 37–52. [DOI] [PubMed] [Google Scholar]
- 21.Pacal L, Varvarovska J, Rusavy Z, et al. Parameters of oxidative stress, DNA damage and DNA repair in type 1 and type 2 diabetes mellitus. Arch Physiol Biochem 2011; 117: 222–230. [DOI] [PubMed] [Google Scholar]
- 22.Yen MY, Kao SH, Wang AG, et al. Increased 8-hydroxy-2’-deoxyguanosine in leukocyte DNA in Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci 2004; 45: 1688–1691. [DOI] [PubMed] [Google Scholar]
- 23.Adaikalakoteswari A, Rema M, Mohan V, et al. Oxidative DNA damage and augmentation of poly(ADP-ribose) polymerase/nuclear factor-kappa B signaling in patients with type 2 diabetes and microangiopathy. Int J Biochem Cell Biol 2007; 39: 1673–1684. [DOI] [PubMed] [Google Scholar]
- 24.Sampathkumar R, Balasubramanyam M, Sudarslal S, et al. Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin Biochem 2005; 38: 892–899. [DOI] [PubMed] [Google Scholar]
- 25.Adaikalakoteswari A, Balasubramanyam M, Rema M, et al. Differential gene expression of NADPH oxidase (p22phox) and hemoxygenase-1 in patients with type 2 diabetes and microangiopathy. Diabet Med 2006; 23: 666–674. [DOI] [PubMed] [Google Scholar]
- 26.Xu GW, Yao QH, Weng QF, et al. Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J Pharm Biomed Anal 2004; 36: 101–104. [DOI] [PubMed] [Google Scholar]
- 27.Dong QY, Cui Y, Chen L, et al. Urinary 8-hydroxydeoxyguanosine levels in diabetic retinopathy patients. Eur J Ophthalmol 2008; 18: 94–98. [DOI] [PubMed] [Google Scholar]
- 28.Wakabayashi Y, Usui Y, Shibauchi Y, et al. Increased levels of 8-hydroxydeoxyguanosine in the vitreous of patients with diabetic retinopathy. Diabetes Res Clin Pract 2010; 89: e59–e61. [DOI] [PubMed] [Google Scholar]
- 29.Basavaraj KH, Vasu Devaraju P, Rao KS. Studies on serum 8-hydroxy guanosine (8-OHdG) as reliable biomarker for psoriasis. J Eur Acad Dermatol Venereol 2013; 27: 655–657. [DOI] [PubMed] [Google Scholar]
- 30.Kumari S, Panda S, Mangaraj M, et al. Plasma MDA and antioxidant vitamins in diabetic retinopathy. Indian J Clin Biochem 2008; 23: 158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarng DC, Huang TP, Liu TY, et al. Effect of vitamin E-bonded membrane on the 8-hydroxy 2’-deoxyguanosine level in leukocyte DNA of hemodialysis patients. Kidney Int 2000; 58: 790–799. [DOI] [PubMed] [Google Scholar]
- 32.Asakawa H, Tokunaga K, Kawakami F. Elevation of fibrinogen and thrombin-antithrombin III complex levels of type 2 diabetes mellitus patients with retinopathy and nephropathy. J Diabetes Complications 2000; 14: 121–126. [DOI] [PubMed] [Google Scholar]
- 33.Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol 2007; 22: 311–315. [DOI] [PubMed] [Google Scholar]
- 34.Usui M, Egashira K. Angiotensin II receptor and oxidative stress. Nihon Rinsho 2002; 60: 1893–1897. [in Japanese, English abstract]. [PubMed] [Google Scholar]
- 35.Takao T, Horino T, Kagawa T, et al. Possible involvement of intracellular angiotensin II receptor in high-glucose-induced damage in renal proximal tubular cells. J Nephrol 2011; 24: 218–224. [DOI] [PubMed] [Google Scholar]
- 36.Fiordaliso F, Cuccovillo I, Bianchi R, et al. Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetes. Life Sci 2006; 79: 121–129. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Horke S, Forstermann U. Oxidative stress in vascular disease and its pharmacological prevention. Trends Pharmacol Sci 2013; 34: 313–319. [DOI] [PubMed] [Google Scholar]
- 38.Kocic R, Pavlovic D, Kocic G. Impact of intensive insulin treatment on the development and consequences of oxidative stress in insulin-dependent diabetes mellitus. Vojnosanit Pregl 2007; 64: 623–628. [DOI] [PubMed] [Google Scholar]
- 39.Julius U, Drel VR, Grassler J, et al. Nitrosylated proteins in monocytes as a new marker of oxidative-nitrosative stress in diabetic subjects with macroangiopathy. Exp Clin Endocrinol Diabetes 2009; 117: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang ZS, Song ZC, Bai JH, et al. Red blood cell count as an indicator of microvascular complications in Chinese patients with type 2 diabetes mellitus. Vasc Health Risk Manag 2013; 9: 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]