Abstract
Objective
We investigated the relationship between diabetes and telomere length by meta-analysis.
Methods
We searched five popular databases for articles published between 1990 and 2015 using “diabetes” and “telomere” as search terms. Data were processed with RevMan5, and random- or fixed-effects meta-analysis was applied. The effects of geographical region, diabetes type, body mass index (BMI), age and sex were examined. Funnel plots were applied to evaluate publication bias.
Results
Seventeen articles were obtained from 571 references. We identified a significant association between telomere length and diabetes mellitus (standardized mean difference [SMD]: −3.41; 95% confidence interval [CI]: −4.01, −2.80; heterogeneity, I2 = 99%) by comparing 5575 patients with diabetes and 6349 healthy individuals. The pooled SMD by geographic region indicated a significant association between shortened telomere length and diabetes mellitus (SMD: −3.41; 95% CI: −4.01, −2.80; heterogeneity, I2 = 99%). In addition, telomere length was significantly associated with age (SMD: −3.41; 95% CI: −4.01, −2.80), diabetes type (SMD: −3.41; 95% CI: −4.01, −2.80), BMI (SMD: −1.61; 95% CI: −1.98, −1.23) and sex (SMD: −4.94; 95% CI: −9.47, −0.40).
Conclusions
The study demonstrated a close relationship between diabetes mellitus and telomere length, which was influenced by region, age, diabetes type, BMI and sex.
Keywords: Telomere length, diabetes mellitus, meta-analysis
Introduction
Diabetes incidence continues to increase globally at alarming rates. In 2013, 382 million people were diagnosed with diabetes, with more than 100 million of those in China alone.1 Diabetes mellitus is a metabolic disease characterized by hyperglycaemia and impaired biological functions leading to severe complications, such as cardiovascular, kidney and eye diseases. Treatment of diabetes and its co-morbidities imposes a high burden on the world economy, amounting to $548 billion USD in 2013 and an estimated $627 billion USD by 2035.1 Therefore, understanding the molecular mechanisms underlying diabetes as well as finding and developing efficient treatment strategies are more pressing than ever.
The concept of “telomeres” was first proposed by McClintock and Muller.2,3 They determined that the stability and integrity of the chromosome was retained within the ends of the chromosome, which Muller named the “telomere”.3 Thus, the telomere is located at the end of chromosomal DNA and is composed of thousands of tandem repeats of the TTAGGG nucleotide sequence and a number of associated proteins, which confer protection against chromosome degradation.4 The telomere is necessary for DNA replication. It undergoes shortening during each DNA replication cycle until it reaches a certain length, at which time cell apoptosis is signaled. Therefore, telomere length is often used as a biological marker for cell aging.5
Accumulating evidence indicates that diabetes may affect changes in telomere length. Jeanclos6 was the first to demonstrate an association between type 2 diabetes mellitus (T2DM) and shortened telomere length. Similarly, Li7 determined that the leukocyte telomere length in patients with diabetes was shorter than that in healthy individuals. They suggested that with the development of diabetes, islet β cells undergo senescence or apoptosis, because the telomere length gradually becomes shorter, which in turn can lead to a cascade of damaging events characteristic of diabetes complications. Therefore, to further examine the association between diabetes mellitus and telomere length, this study employed a meta-analysis model to assess the contribution of geographical region, diabetes type, body mass index (BMI), age and sex on telomere length in patients with diabetes.
Methods
Study eligibility and identification
We performed systematic computerized searches of PubMed (MEDLINE), EMBASE, China National Knowledge Infrastructure, Wanfang data and the VIP database of China. We used “telomere” and “diabetes” as search terms to examine articles that were published between January 1st, 1990 and December 31st, 2015.
Study selection and exclusion criteria
We used the referenced QUADAS literature evaluation standard established by Whiting.8 Two authors (JW and LZ) reviewed studies for inclusion independently. Accordingly, the literature included in the meta-analysis was selected based on the following criteria: 1) it assessed the association between diabetes mellitus and telomere length; 2) it included case-control groups and a complete set of data; 3) the human subjects analyzed included adults only; and 4) it provided standardized mean differences (SMD) and 95% confidence intervals (CIs) or presented sufficient information to allow their computational calculations. The following exclusion criteria were used: 1) the studies contained incomplete data; 2) the studies were conducted using children or animal models; 3) the studies included patients with gestational diabetes; and 4) the subjects were diagnosed with other pathologies, such as heart or kidney diseases.
Quality assessment and data extraction
Study quality was independently assessed by two authors (XD and LC) using the Cochrane Risk of Bias Tool (Newcastle-Ottawa scale9). Two authors (JW and XD) extracted data from selected articles independently. The following information was recorded from the selected literature: title, first author, year of publication, country, case-control basic information (subjects, age, sex and BMI), diabetes mellitus type, telomere length and the SMD and 95% CIs. The mean and SD (range, SE and CI for BMI) and other tests were tabulated along with the main findings reported in each study.
Meta-analysis
Meta-analyses were performed using RevMan5 software (The Cochrane Collaboration, Copenhagen, Denmark). Homogeneity was assessed using the χ2 test with Cochran Q and I2 statistics. Heterogeneity was defined as low, medium and high when I2 < 25%, 25% < I2 < 50% and I2 > 50%, respectively. The fixed-effect model was used when there was no significant heterogeneity among the included studies (P > 0.05 and I2 < 50%). In all other cases, the random-effects model was applied.10
The SMD and 95% CIs were used to evaluate differences between groups.11 We used a funnel plot to evaluate publication bias. Subgroup analyses were performed to evaluate the effect of region (Asia, Europe and the USA), age (below and above 60 years old), diabetes type (T1DM and T2DM), BMI (normal, overweight and obese) and sex (male and female). P values for all comparisons were obtained using a two-tailed model, and statistical significance was set at α < 0.05.
Results
Literature search
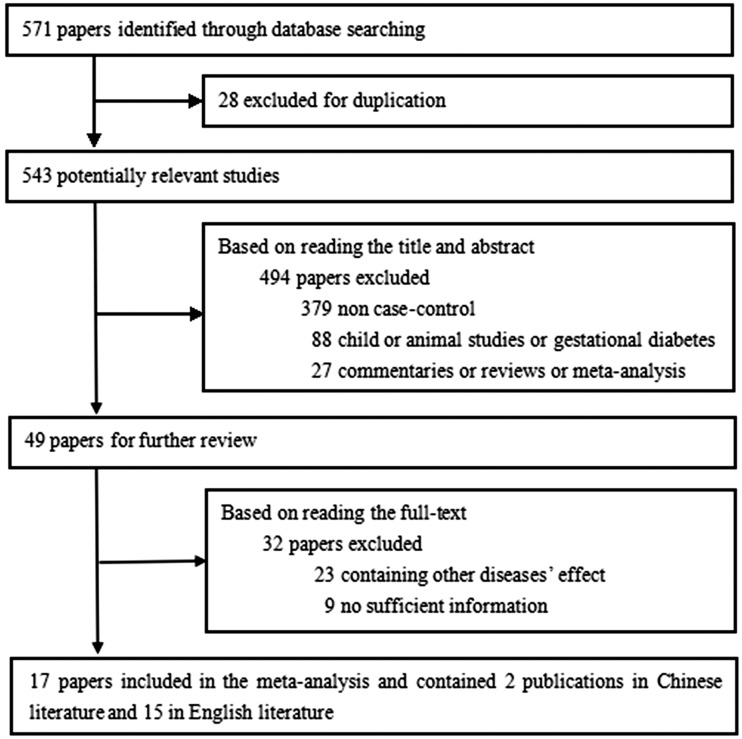
Using the search terms “diabetes” and “telomere,” our initial search yielded 571 studies. After applying the inclusion/exclusion criteria, 522 papers were excluded. Of the 49 papers selected, only 17 were included in the meta-analysis,12–28 including 2 publications in Chinese12,13 and 15 in English.14–28 The article selection process is summarized in Figure 1, and the primary parameters of the study are presented in Table 1.
Figure 1.
Flow chart of the literature search strategy and the process of manuscript selection.
Table 1.
Characteristics of studies included in the meta-analysis.
| case |
control |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author | Year | Country | Quantity | Type | Male | Mean Age | Mean BMI | Telomere Length | Quantity | Male | Mean Age | Mean BMI | Telomere Length |
| Lu et al.12 | 2007 | China | 20 | 1 | 11 | 23.85 | − | 8.29 | 20 | 11 | 28.1 | − | 8.94 |
| Liu et al.13 | 2012 | China | 21 | 2 | 11 | 44.9 | 23.73 | 1.34 | 47 | 24 | 42.5 | 22.48 | 3.83 |
| Ma D et al.14§ | 2013 | China | 34 | 1 | 21 | 26.32 | 20.38 | 1.77 | 40 | 21 | 32.25 | 21.82 | 2.39 |
| Ma D et al.14§ | 2013 | China | 62 | 2 | 35 | 50.15 | 23.39 | 1.67 | |||||
| Dudinskaya et al.15 | 2014 | Russia | 50 | 2 | − | 56 | − | 9.51 | 49 | − | 53.47 | − | 9.8 |
| Fyhrquist et al.16 | 2010 | Finland | 48 | 1 | 22 | 39 | 25 | 8.4 | 44 | 24 | 39.4 | 23.7 | 8.5 |
| Ma et al.17 | 2015 | China | 38 | 2 | 17 | 45.68 | 24.4 | 1.58 | 31 | 15 | 41.63 | 24 | 3.98 |
| Adaikalakoteswari et al.18 | 2005 | India | 40 | 2 | 20 | 49 | 25.2 | 6.01 | 40 | 20 | 49 | 23.5 | 9.11 |
| Murillo et al.19 | 2012 | Mexico | 93 | 2 | 93 | 54.5 | 25.5 | 5.4 | 98 | 98 | 52.8 | 27.1 | 9.5 |
| Liu et al.20 | 2014 | China | 71 | 2 | 40 | 54.55 | 25.21 | 2.01 | 52 | 30 | 51.27 | 23.86 | 2.28 |
| Sampson et al.21 | 2006 | USA | 21 | 2 | 21 | 62 | 29.5 | 4 | 28 | 28 | 61.2 | 17.3 | 5.5 |
| Zee et al.22 | 2010 | USA | 432 | 2 | 256 | 60 | 33.3 | 2.4 | 424 | 187 | 51 | 25.4 | 2.46 |
| Olivieri et al.23 | 2009 | Italy | 103 | 2 | 61 | 70 | 29 | 0.44 | 104 | 52 | 69 | 27 | 0.53 |
| Testa et al.24 | 2011 | Italy | 217 | 2 | 121 | 65.9 | 29.3 | 0.46 | 400 | 220 | 65.1 | 26.9 | 0.45 |
| Salpea et al.25 | 2010 | UK | 569 | 2 | 338 | 68 | − | 6.94 | 367 | 367 | 53 | − | 7.85 |
| Monickaraj et al.26 | 2012 | India | 145 | 2 | − | 43.6 | 25.9 | 0.97 | 145 | − | 41.4 | 24.5 | 1.2 |
| You et al.27 | 2012 | USA | 1675 | 2 | 0 | 62.11 | 31 | 3.97 | 2380 | 0 | 62.12 | 27 | 4.12 |
| Shen et al.28 | 2012 | China | 1936 | 2 | 1140 | 64 | 25.1 | 0.98 | 2080 | 1452 | 58 | 24.5 | 1.04 |
Ma Da included both type 1 and type 2 diabetes with one control group.
BMI, body mass index.
Association between telomere length and diabetes
From 17 studies, we extracted 5575 experimental cases and 6389 controls. The results using RevMan5 software29 are presented in Figure 2. There was a significant effect of heterogeneity (χ2 = 2753.47, I2 = 99%, P < 0.00001) among the studies included as well as a significant random-effect (P < 0.05). The pooled SMD (−3.41; 95% CI: −4.01, −2.80) and the diamond were located on the left side of the vertical line of the forest graph. These results indicated that telomere length in patients with diabetes was shorter than that in healthy individuals.
Figure 2.
Forest plot depicting meta-analysis of telomere length comparison between patients with diabetes and healthy individuals. Results are presented using a random effects model. CI, confidence interval; IV, inverse variance method.
The shape of the funnel plots did not appear symmetrical, suggesting that there was a publication bias in the meta-analysis (Figure 3).
Figure 3.
Funnel diagram analysis of telomere length comparison between patients with diabetes and healthy individuals.
Subgroup analyses
The results of subgroup analyses and the respective sample sizes in each subgroup (region, age, type, BMI and sex) are summarized in Table 2.
Table 2.
Results from the subgroup analysis of the meta-analysis.
| Characteristic | Studies | Case | Control | SMD (95% CI) | Heterogeneity |
P-Value | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P-Value | ||||||
| All Studies | 17 | 5575 | 6389 | −3.41 (−4.01, −2.80) | 99 | <0.00001 | <0.00001 |
| Region | |||||||
| Asia | 8 | 2367 | 2495 | −4.73 (−6.29, −3.17) | 99 | <0.00001 | <0.00001 |
| Europe | 5 | 987 | 964 | −2.34 (−4.65, −0.04) | 100 | <0.00001 | 0.05 |
| USA | 4 | 2221 | 2930 | −2.94 (−3.97, −1.91) | 99 | <0.00001 | <0.00001 |
| Age | |||||||
| > 60 years | 7 | 4953 | 5783 | −1.47 (−2.19, −0.76) | 100 | <0.00001 | <0.0001 |
| < 60 years | 10 | 622 | 606 | −5.45 (−7.33, −3.57) | 99 | <0.00001 | <0.00001 |
| Type | |||||||
| T1DM | 3 | 102 | 104 | −0.74 (−1.46, −0.03) | 83 | 0.003 | 0.04 |
| T2DM¶ | 15 | 5473 | 6285 | −3.98 (−4.65, −3.31) | 99 | <0.00001 | <0.00001 |
| BMI | |||||||
| Normal | 3 | 155 | 158 | −3.28 (−5.06, −1.50) | 97 | <0.00001 | 0.0003 |
| Overweight | 4 | 2095 | 2216 | −1.69 (−2.82, −0.56) | 98 | <0.00001 | 0.003 |
| Obese | 5 | 2448 | 3336 | −1.12 (−1.75, −0.49) | 99 | <0.00001 | 0.0005 |
| Gender | |||||||
| Male | 2 | 114 | 126 | −7.46 (−19.49, 4.56) | 100 | <0.00001 | 0.22 |
| Female | 1 | 1675 | 2380 | −0.11 (−0.17, −0.05) | − | − | 0.0007 |
Data originate from the same paper (Ref. 14). The same control group was used for each comparison (n = 80).
SMD, standardized mean difference; CI, confidence interval; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; BMI, body mass index.
The effect of geographical region on diabetes and telomere length
There were eight, five and four articles that included studies from Asia, Europe and the USA, respectively (Figure 4). The SMD in European studies (−2.34; 95% CI: −4.65, −0.04; P = 0.05) was significantly lower than that in Asian (−4.73; 95% CI: −6.29, −3.17; P < 0.00001) and US (−2.94; 95% CI: −3.97, −1.91; P < 0.00001) studies. Additionally, the SMD in Asian studies was higher than that in US studies (P < 0.00001). Therefore, the telomere length SMD in European patients with diabetes versus healthy individuals was lower than that in Asian and US patients (P < 0.00001). Similarly, the telomere length SMD in Asian patients with diabetes versus healthy individuals was significantly higher than that in US patients.
Figure 4.
Forest plot depicting meta-analysis of telomere length comparison between patients with diabetes and healthy individuals from different regions (Asia, Europe and the Americas). Results are presented using a random effects model. CI, confidence interval; IV, inverse variance method.
Additionally, we wanted to identify the difference in telomere length between patients with diabetes and healthy individuals from China (Figure 5). Analysis of the eight papers that included Chinese subjects revealed that the SMD was lower in Chinese populations (−2.00; 95% CI: −2.91, −1.08; P < 0.0001) compared with that of other Asian countries (−14.88; 95% CI: −30.64, 0.87). This finding indicates that the telomere length SMD between patients with diabetes and healthy individuals in China was significantly lower than that in other Asian countries (P < 0.00001); however, the difference was not significant in a non-Chinese population.
Figure 5.
Forest plot depicting meta-analysis of telomere length comparison between patients with diabetes and healthy individuals from China and other countries in Asia. Results are presented using a random effects model. CI, confidence interval; IV, inverse variance method.
The effect of age on diabetes and telomere length
Telomere shortening is considered the molecular clock that triggers cell senescence and can be used as a biological marker of aging.30 We grouped the studies into two age categories: below and above 60 years of age. We determined that the pooled SMD for individuals older than 60 years of age (−1.47; 95% CI: −2.19, −0.76; P < 0.0001) was lower than that for younger individuals (−5.45; 95% CI: −7.33, −3.57; P < 0.00001). This indicates that the telomere length SMD between patients with diabetes and healthy individuals in older cohorts (>60 years of age) was significantly lower than that in younger cohorts (<60 years of age) (P < 0.00001) (Figure 6).
Figure 6.
Forest plot depicting meta-analysis of telomere length comparison between patients with diabetes and healthy individuals of different ages (below and above 60 years of age). Results are presented using a random effects model. CI, confidence interval; IV, inverse variance method.
The effect of diabetes type on telomere length
We performed a subgroup analysis between patients with T1DM and patients with T2DM. The pooled SMD for patients with T1DM (−0.74; 95% CI: −1.46, −0.03; P = 0.04) was significantly lower than that for patients with T2DM (−3.98; 95% CI: −4.65, −3.31; P < 0.00001), demonstrating that the telomere length SMD between patients with T1DM and healthy individuals was significantly lower than that of patients with T2DM (Figure 7).
Figure 7.
Forest plot depicting meta-analysis of telomere length comparison between patients with T1DM and T2DM and healthy individuals. Results are presented using a random effects model. CI, confidence interval; IV, inverse variance method.
The effect of BMI on diabetes and telomere length
The pooled SMD for normal weight individuals (−3.28; 95% CI: −5.06, −1.50; P = 0.0003) was higher than that for overweight individuals (−1.69; 95% CI: −2.82, −0.56; P = 0.003). Furthermore, as expected, the pooled SMD for obese individuals (−1.12; 95% CI: −1.75, −0.49; P = 0.0005) was lower than that of normal weight (P < 0.00001) and overweight (P < 0.00001) individuals. Thus, in obese individuals, the telomere length SMD between patients with diabetes and healthy individuals was lower than that in normal weight and overweight individuals (Figure 8).
Figure 8.
Forest plot depicting meta-analysis of telomere length comparison between patients with diabetes and healthy individuals with different BMIs (normal, overweight and obese). Results are presented using a random effects model. BMI, body mass index; CI, confidence interval; IV, inverse variance method.
The effect of sex on diabetes and telomere length
The pooled SMD for females (−0.11, 95% CI: −0.17, −0.05; P = 0.0007) was lower than that for males (−7.46; 95% CI: −19.49, 4.56). These results suggest that the telomere length SMD between patients with diabetes and healthy individuals in female cohorts was lower than that in male cohorts (P = 0.03); however, the difference was not significant in males (Figure 9).
Figure 9.
Forest plot depicting meta-analysis of telomere length comparison between patients with diabetes and healthy individuals based on sex (male and female). Results are presented using a random effects model. CI, confidence interval; IV, inverse variance method.
Discussion
This meta-analysis study demonstrates that diabetes affects telomere length. Specifically, telomere length in patients with diabetes is reduced compared with that of healthy individuals. We reviewed 17 papers that met the inclusion criteria and conducted a five-subgroup meta-analysis that included geographical region, age, diabetes type, BMI and sex. We determined that telomere length in patients with diabetes varied based on geographical region. Specifically, European patients with diabetes displayed a reduced telomere length compared with that of Asian and US patients. Additionally, we demonstrated that telomere length was affected by diabetes type, BMI, age and sex. As such, patients with T1DM, obese patients, patients over 60 years of age and female patients all exhibited a shorter telomere length compared with that of patients with T2DM, lean patients, patients below 60 years of age and male patients, respectively.
Our results are in agreement with recent meta-analysis reports demonstrating a significant association between diabetes and telomere length that was influenced by geographical region and diabetes type.31,32 Our study builds upon previous work that indicated that there was a significant difference in telomere length SMD between Chinese patients with and without diabetes compared with that in patients from other Asian countries. However, it should be noted that our analyses included only two reports that studied Asian patients with diabetes and telomere length. Furthermore, we demonstrated that BMI and sex are stronger predictors of the association between diabetes and telomere length.
Telomere length is often used as a biological marker for cell aging, because with increased age, telomere length is shortened.33 However, how diabetes influences telomere length as a function of age is still unclear. Some studies have identified no age-related decline in telomere length between patients with T1DM and non-diabetic patients,16,34 while others have demonstrated an age effect.6,35 You27 compared Caucasian, African and Asian Americans and demonstrated that telomere length in Caucasian Americans was shorter than that of African and Asian Americans. Several contributing factors have been proposed that may account for these differences. For example, living at high altitudes could affect telomere length. Low oxygen levels at high altitudes can induce hypoxia-inducible factor-1α, which can increase telomerase activity and preserve telomere length.36 However, low oxygen levels can increase oxidative stress, which may accelerate telomere shortening.37,38 Furthermore, poor socioeconomic status and adverse living environments have also been associated with shorter telomeres because of increased oxidative stress, poor nutrition, unhealthy behaviours and physical, emotional and psychological pressure.39–43
T1DM and T2DM differ in their pathogenicity. Therefore, the mechanisms by which the two diseases might affect telomere length are also different. T1DM is a T-cell-mediated autoimmune disease, resulting from autoimmune destruction of pancreatic β-cells and typically leading to insulin deficiency.44,45 Markers of β-cell immune destruction include autoantibodies to insulin, islet cell autoantibodies, autoantibodies to glutamic acid decarboxylase and autoantibodies to the tyrosine phosphatases IA-2 and IA-2β.46 T1DM is primarily first diagnosed in infants and children, and its diagnosis generally relies on autoantibody examination, with ketoacidosis often present as a first manifestation.47 Magalhase48 demonstrated a link between autoimmunity and telomere length. However, short telomeres may be secondary to T1DM autoimmune changes, although both may together contribute to T1DM aetiology. Two polymorphisms (BsmI, FokI) in the Vitamin D Receptor (VDR) gene have been suggested as potential genetic factors underlying T1DM.45 However, the specific cause(s) of T1DM and/or how T1DM influences telomere length remain unclear. It is worth pointing out that in a recent cross-sectional study of patients with T1DM, relative telomere length did not correlate with HbA1c, oxidation or smoking but inversely correlated with age, T1DM duration, inflammation and vascular function.34 This speaks to the well-known complicated T1DM mechanisms that are affected by both genetic as well as environmental factors.49–51 Indeed, telomere length is determined early in life,52 with genetic factors53 and intrauterine environment playing an important role.54
In contrast to T1DM, T2DM is characterized by insulin resistance and typically relative (rather than absolute) insulin deficiency.47 Almost 90% of patients with T2DM are adults, although T2DM prevalence in children is on the rise because of increasing rates of childhood obesity.55,56 As such, a recent large study of 301 twin pairs demonstrated that individuals with short telomere lengths are more likely to develop insulin resistance later in life.57 Previous studies have demonstrated that oxidative stress is a major contributor to T2DM development.21 The telomere sequence is rich in guanine residues, and guanine is particularly prone to oxidative stress, which is markedly increased in uncontrolled diabetes.14,58,59 Therefore, it is likely that, at least in the case of T2DM, telomere length is influenced by oxidative stress. Once the telomere undergoes shortening, it increases the risk of β-cell injury and apoptosis, leading to a decline in islet cell functioning and diabetes development and progression.60,61 This is consistent with a recent report demonstrating that antioxidant defences are critical in maintaining telomere integrity, thereby reducing the progression of cardiovascular complications associated with T2DM.62 Several studies have identified a positive association between telomere length and T2DM time of onset and duration;19 however, this could be population-specific, as such a correlation was not identified in a study using Chinese patients.21,28
Obesity is one of the most important contributors to diabetes development,63 with increasing obesity rates paralleling those of diabetes.64 It is well known that obesity causes and aggravates insulin resistance and increases oxidative stress, leading to β-cell apoptosis.65 Thus, obesity development may shorten telomere length, although this relationship has been questioned.66 Indeed, our meta-analysis demonstrated that increased BMI resulted in reduced telomere length. Among the multitude of factors contributing to obesity and diabetes development, overconsumption of high-fat, high-energy foods plays a major role.67 Consumption of high-calorie, high-fat diets as well as the associated obesity have been shown to cause an increase in endoplasmic reticulum (ER) cellular stress. The ER is the organelle responsible for folding, maturation, quality control, trafficking and processing of secretory and membrane proteins.68 There is increasing evidence demonstrating that certain pathological stress conditions present in obese individuals disrupt ER homeostasis, leading to impaired control in the unfolded protein response, a complex process involved in the cellular stress response.69 Furthermore, obesity is characterized by a chronic low-grade inflammatory state, a condition characterized by enhanced adipocyte hypertrophy and hyperplasia, increased inflammatory cell infiltration, inflammatory cytokine activation and increased markers of fatty acid-induced oxidative and ER stress in various tissues.70,71 As such, mitochondrial oxidative stress injury has been linked with shortening telomere length in patients with T2DM,70 which may explain our results. In mice, short telomeres have been implicated in metabolic dysfunction via mitochondrial functional disruptions.72 Furthermore, disruption of Rap1, a telomere-binding protein, results in increased abdominal fat and insulin resistance.73 Finally, complications of diabetes, such as cardiovascular events,74 can also influence telomere shortening.75 However, such a correlation was not identified in patients with T1DM, but this effect might have been masked by survivor bias and vasoprotective drug treatment.34 Our study could not assess whether the effect of obesity on telomere length is more pronounced in individuals with both diabetes and obesity. Given the fact that both obesity and diabetes are associated with shorter telomere length, we speculate that the combination of obesity and diabetes may accentuate this effect; however, this remains to be elucidated.
In our meta-analysis study, we also determined that telomere length in females was shorter than that in males; however, the published results to date have been inconclusive. For example, Benetos et al.76 determined that telomere length in females was longer than that in males. Furthermore, the authors demonstrated that telomere length in leukocytes, which is heritable, was also longer in women than in men. Oestrogen can diminish oxidative stress and stimulate telomerase production, which protects telomere lengthening and attenuates telomere attrition, an effect that may be lost during menopause.77 Indeed, this is supported by studies investigating the effect of hormone therapy on telomerase activity that have demonstrated an upregulation of telomerase reverse transcriptase expression. This effect is mediated by the oestrogen-responsive element in the gene promoter, thus explaining longer telomeres in postmenopausal women undergoing hormone-replacement therapy.78 Oestrogen stimulates telomerase via the phosphoinositol 3-Kinase/Akt pathway79 or NO stimulation.80 Therefore, low oestrogen levels and/or diabetes-induced changes in telomere length might have accounted for the short telomeres observed in our analysis. Furthermore, women display increased diabetes-induced systemic inflammation compared with men and are at higher risk of cardiovascular events and developing diabetes complications, all of which can result in shorter telomeres.81,82. In contrast, shorter telomeres have also been reported in male patients with diabetes, which has been attributed to poor control of diabetes in males compared with that in women, independent of smoking and drinking habits.28 Therefore, additional larger studies are required to conclusively determine whether sex plays a role in influencing telomere length in patients with diabetes.
A meta-analysis is a useful statistical technique that applies quantitative methods to systematically evaluate and summarize multiple research results.83 Based on heterogeneity, the pooled SMD and 95% CI resulting from the 17 referenced articles analysed, we demonstrated that diabetes accelerates the telomere shortening process. However, several limitations should be acknowledged when interpreting these results. These include a relatively low number of published studies, un-accounting variables in some studies (e.g., age of patients with diabetes differed from that of healthy individuals) as well as inconsistencies in calculating the telomere length (T/S ratio (T stands for telomere, S stands for single-copy gene) or KB (kilo base pairs)). Nevertheless, our findings are consistent with previous data indicating that diabetes shortens telomere length, aggravates cell apoptosis and ultimately impacts quality of life.84,85
In summary, we have demonstrated that diabetes can significantly affect telomere length, an effect that is influenced by geographical region, diabetes type, obesity, age and sex. Understanding the mechanisms by which diabetes affects telomere length is important for diabetes mellitus prevention and treatment.
Declaration of conflicting interest
The Authors declare that there is no conflict of interest.
Funding
MC is supported by the Romanian National Program PN-II-ID-PCE-2012-4-0608 no. 48/02.09.2013, “Analysis of novel risk factors influencing control of food intake and regulation of body weight”. This work was also supported by the National Natural Science Foundation of China (No. 81272444 and 81472744 to LZ).
References
- 1.International Diabetes Federation. IDF diabetes ATLAS. Sixth Edition, 2013, 12–15.
- 2.McClintock B. The stability of broken end of chromosome in Zea mays. Genetics 1941; 26: 234–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller HJ. The remaking of chromosomes. The collecting net-Woods Hole 1938; 13: 181–198. [Google Scholar]
- 4.Blackburn EH. Structure and function of telomeres. Nature 1991; 350: 569–573. [DOI] [PubMed] [Google Scholar]
- 5.Zakian VA. Structure, function, and replication of Saccharomyces cerevisiae telomeres. Annu Rev Genet 1996; 30: 141–172. [DOI] [PubMed] [Google Scholar]
- 6.Jeanclos E, Krolewski A, Skurnick J, et al. Shortened telomere length in white blood cells of patients with IDDM. Diabetes 1998; 47: 482–486. [DOI] [PubMed] [Google Scholar]
- 7.Li FZ, Chen YX, He JJ. Telomere and diabetes (in Chinese). Chinese Chronic Disease Prevention and Control 2014; 22: 604–606. [Google Scholar]
- 8.Whiting P, Rutjes AW, Reitsma JB. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003; 3: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603–605. [DOI] [PubMed] [Google Scholar]
- 10.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 11.Zhou X, Qin B, Del Giovane C, et al. Efficacy and tolerability of antidepressants in the treatment of adolescents and young adults with depression and substance use disorders: a systematic review and meta-analysis. Addiction 2015; 110: 38–48. [DOI] [PubMed] [Google Scholar]
- 12.Lu Y, Lu Y, Cheng X, et al. Alteration and significance of telomere length of peripheral white blood cells in patients with type 1 diabetes mellitus (in Chinese). Jiangsu Medicine 2007; 33: 127–128. [Google Scholar]
- 13.Liu ZL, Yang Y, Hu P, et al. The effect of glycemic control to the leukocyte telomere length in patients with Type 2 diabetes (in Chinese). Journal of Clinical Department of Medicine 2012; 29: 237–239. [Google Scholar]
- 14.Ma D, Zhu W, Hu S, et al. Association between oxidative stress and telomere length in type 1and type 2 diabetic patients. J Endocrinol Invest 2013; 36: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 15.Dudinskaya E, Brailova NV, Strazhesko ID, et al. The role of telomere biology and diabetes mellitus in vascular aging. J Am Coll Cardiol 2014; 63: 2136–2136. [Google Scholar]
- 16.Fyhrquist F, Tiitu A, Saijonmaa O, et al. Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. J Intern Med 2010; 267: 278–286. [DOI] [PubMed] [Google Scholar]
- 17.Ma D, Yu Y, Yu X, et al. The changes of leukocyte telomere length and telomerase activity after sitagliptin intervention in newly diagnosed type 2 diabetes. Diabetes Metab Res Rev 2015; 31: 256–261. [DOI] [PubMed] [Google Scholar]
- 18.Adaikalakoteswari A, Balasubramanyam M, Mohan V. Telomere shortening occurs in Asian Indian type 2 diabetic patients. Diabet Med 2005; 22: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 19.Murillo-Ortiz B, Albarran-Tamayo F, Arenas-Aranda D, et al. Telomere length and type 2 diabetes in males, a premature aging syndrome. Aging Male 2012; 15: 54–58. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Zhang J, Yan J, et al. Leucocyte telomere shortening in relation to newly diagnosed type 2 diabetic patients with depression. Oxid Med Cell Longev 2014; 2014: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sampson MJ, Winterbone MS, Hughes JC, et al. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006; 29: 283–289. [DOI] [PubMed] [Google Scholar]
- 22.Zee RY, Castonguay AJ, Barton NS, et al. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl Res 2010; 155: 166–169. [DOI] [PubMed] [Google Scholar]
- 23.Olivieri F, Lorenzi M, Antonicelli R, et al. Leukocyte telomere shortening in elderly Type2DM patients with previous myocardial infarction. Atherosclerosis 2009; 206: 588–593. [DOI] [PubMed] [Google Scholar]
- 24.Testa R, Olivieri F, Sirolla C, et al. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet Med 2011; 28: 1388–1394. [DOI] [PubMed] [Google Scholar]
- 25.Salpea KD, Talmud PJ, Cooper JA, et al. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 2010; 209: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monickaraj F, Aravind S, Gokulakrishnan K, et al. Accelerated aging as evidenced by increased telomere shortening and mitochondrial DNA depletion in patients with type 2 diabetes. Mol Cell Biochem 2012; 365: 343–350. [DOI] [PubMed] [Google Scholar]
- 27.You NY, Chen BH, Song Y, et al. A prospective study of leukocyte telomere length and risk of type 2 diabetes in postmenopausal women. Diabetes 2012; 61: 2998–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Q, Zhao X, Yu L, et al. Association of Leukocyte telomere length with type 2 diabetes in mainland Chinese populations. J Clin Endocrinol Metab 2012; 97: 1371–1374. [DOI] [PubMed] [Google Scholar]
- 29.Review Manager 5 Tutorial, Available from the Rev Man Help menu, Review Manager (RevMan) [Computer program], Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration 2008.
- 30.Bekaert S, De Meyer T, Van Oostveldt P. Telomere attrition as aging biomarker. Anticancer Res 2005; 25: 3011–3021. [PubMed] [Google Scholar]
- 31.D’Mello MJ, Ross SA, Briel M, et al. The association between shortened leukocyte telomere length and cardio-metabolic outcomes: a systematic review and meta-analysis. Circ Cardiovasc Genet 2015; 8: 82–90. [DOI] [PubMed] [Google Scholar]
- 32.Zhao J, Miao K, Wang H, et al. Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PLoS One 2013; 8: e79993–e79993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cawthon RM, Smith KR, O’Brien E, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003; 361: 393–395. [DOI] [PubMed] [Google Scholar]
- 34.Januszewski AS, Sutanto SS, McLennan S, et al. Shorter telomeres in adults with type 1 diabetes correlate with diabetes duration, but only weakly with vascular function and risk factors. Diabetes Res Clin Pract 2016; 117: 4–11. [DOI] [PubMed] [Google Scholar]
- 35.Uziel O, Singer JA, Danicek V, et al. Telomere dynamics in arteries and mononuclear cells of diabetic patients: effect of diabetes and of glycemic control. Exp Gerontol 2007; 42: 971–978. [DOI] [PubMed] [Google Scholar]
- 36.Nishi H, Nakada T, Kyo S, et al. Hypoxia-inducible factor 1 mediates up-regulation of telomerase (hTERT). Mol Cell Biol 2004; 24: 6076–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong YC, Luo PL, Jin GE, et al. The dynamic changing profiles of peripheral white blood cell telomere length in populations of different ages living at different altitude areas. Zhonghua Yu Fang Yi Xue Za Zhi 2008; 42: 502–505. [in Chinese, Abstract in English]. [PubMed] [Google Scholar]
- 38.Satoh M, Ishikawa Y, Takahashi Y, et al. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis 2008; 198: 347–353. [DOI] [PubMed] [Google Scholar]
- 39.Robertson T, Batty GD, Der G, et al. Is telomere length socially patterned? Evidence from the West of Scotland twenty-07 study. PLoS One 2012; 7: e41805–e41805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deroo LA, Parks CG, Kim S, et al. Childhood socioeconomic factors and telomere length. Am J Epidemiol 2009; 169: S88–S88. [Google Scholar]
- 41.Sies H. Oxidative stress: oxidants and antioxidants, London: San Diego: Academic Press, 1991. [Google Scholar]
- 42.Adams JM, White M. Biological ageing: a fundamental, biological link between socio-economic status and health? Eur J Public Health 2004; 14: 331–334. [DOI] [PubMed] [Google Scholar]
- 43.Blaze J, Asok A, Roth TL. The long-term impact of adverse caregiving environments on epigenetic modifications and telomeres. Front Behav Neurosci 2015; 9: 79–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kolb H, Kolb-Bachofen V, Roep BO. Autoimmune versus inflammatory type 1 diabetes: a controversy? Immunol Today 1995; 16: 170–172. [DOI] [PubMed] [Google Scholar]
- 45.Wang G, Zhang Q, Xu N, et al. Associations between two polymorphisms (FokI and BsmI) of Vitamin D receptor gene and type 1 diabetes mellitus in Asian population: a meta-analysis. PLoS One 2014; 9: 89325–89325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gavin JR, Alberti KGMM, Davidson MB, et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1998; 1: S5–S19. [DOI] [PubMed] [Google Scholar]
- 47.American Diabetes Association Classification and diagnosis of diabetes. Sec 2. In Standards of Medical Care in Diabetes 2015. Diabetes Care 2015; 38: S8-S16. [DOI] [PubMed]
- 48.de Magalhaes JP. From cells to ageing: a review of models and mechanisms of cellular senescence and their impact on human ageing. Exp Cell Res 2004; 300: 1–10. [DOI] [PubMed] [Google Scholar]
- 49.Baxter AG, Jordan MA. From markers to molecular mechanisms: type 1 diabetes in the post-GWAS Era. Rev Diabet Stud 2012; 9: 201–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graham KL, Sutherland RM, Mannering SI, et al. Pathogenic mechanisms in type 1 diabetes: the islet is both target and driver of disease. Rev Diabet Stud 2012; 9: 148–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider DA, von Herrath MG. Potential viral pathogenic mechanism in human type 1 diabetes. Diabetologia 2014; 57: 2009–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun 2013; 4: 1597–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res 2012; 730: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Entringer S, Epel ES, Kumsta R, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A 2011; 108: E513–E518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peters A, Iverson E, Ruelas V, et al. Adult and childhood obesity and diabetes in underserved communities. Diabetes 2007; 56(Supplement 1): A241–A241. [Google Scholar]
- 56.Eckers A, Altschmied J, Haendeler J. Oxidative stress in endothelial cells and in diabetes type 2. Z Gerontol Geriatr 2012; 45: 90–94. [in German, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 57.Verhulst S, Dalgård C, Labat C, et al. A short leucocyte telomere length is associated with development of insulin resistance. Diabetologia 2016; 59: 1258–1265. [DOI] [PubMed] [Google Scholar]
- 58.Dabelea D, Harrod CS. Role of developmental overnutrition in pediatric obesity and type 2 diabetes. Nutr Rev 2013; 71(Suppl 1): S62–S67. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Liu JL, Hu P, et al. Association of shortened telomere length with oxidative stress in leukocytes of T1DM and T2DM patients. Chin J Diabetes 2013; 21: 252–255. [Google Scholar]
- 60.Tentolouris N, Nzietchueng R, Cattan V, et al. White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care 2007; 30: 2909–2915. [DOI] [PubMed] [Google Scholar]
- 61.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- 62.Masi S, D’Aiuto F, Cooper J, et al. Telomere length, antioxidant status and incidence of ischaemic heart disease in type 2 diabetes. Int J Cardiol 2016; 216: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponlirolid AE, Galfi L. Duration of obesity is a risk factor for non-insulin-dependent diabetes mellitus, not for arterial hypertension or for hyperlipidaemia. Acta Diabetol 1998; 35: 130–136. [DOI] [PubMed] [Google Scholar]
- 64.Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes 2014; 7: 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jonas JC, Bensellam M, Duprez J, et al. Glucose regulation of islet stress responses and beta-cell failure in type 2 diabetes. Diabetes Obes Metab 2009; 11(Suppl 4): 65–81. [DOI] [PubMed] [Google Scholar]
- 66.Hardikar S, Song X, Risques RA, et al. Obesity and inflammation markers in relation to leukocyte telomere length in a cross-sectional study of persons with Barrett’s esophagus. BMC obes 2015; 2: 32–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mutowo M, Gowda U, Mangwiro JC, et al. Prevalence of diabetes in Zimbabwe: a systematic review with meta-analysis. Int J Public Health 2015; 60: 1–11. [DOI] [PubMed] [Google Scholar]
- 68.Halperin L, Jung J, Michalak M. The many functions of the endoplasmic reticulum chaperones and folding enzymes. IUBMB Life 2014; 66: 318–326. [DOI] [PubMed] [Google Scholar]
- 69.Kaufman RJ, Back SH, Song B, et al. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in β-cells. Diabetes Obes Metab 2010; 12(Suppl 2): 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ametov AS, Kulidzhanian NK. Diabetes mellitus is an independent risk factor for cardiovascular disease. Ter Arkh 2012; 84: 91–94. [in Russian, English Abstract]. [PubMed] [Google Scholar]
- 71.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997; 20: 1183–1197. [DOI] [PubMed]
- 72.Sahin E, Colla S, Liesa M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011; 470: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martínez P, Gómez-López G, García F, et al. RAP1 protects from obesity through its extratelomeric role regulating gene expression. Cell rep 2013; 3: 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Savarese G, Perrone-Filardi P, D’Amore C, et al. Cardiovascular effects of dipeptidyl peptidase-4 inhibitors in diabetic patients: a meta-analysis. Int J Cardiol 2015; 181: 239–244. [DOI] [PubMed] [Google Scholar]
- 75.Saliques S, Zeller M, Lorin J, et al. Telomere length and cardiovascular disease. Arch Cardiovasc Dis 2010; 103: 454–459. [DOI] [PubMed] [Google Scholar]
- 76.Benetos A, Dalgard C, Labat C, et al. Sex difference in leukocyte telomere length is ablated in opposite-sex co-twins. Int J Epidemiol 2014; 43: 799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Needham BL, Diez Roux AV, Bradley R, et al. A Test of Biological and Behavioral Explanations for Gender Differences in Telomere Length: The Multi-Ethnic Study of Atherosclerosis. Biodemography Soc Biol 2014; 60: 156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee DC, Im JA, Kim JH, et al. Effect of long-term hormone therapy on telomere length in postmenopausal women. Yonsei Med J 2005; 46: 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simoncini T, Hafezi-Moghadam A, Brazil DP, et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 2000; 407: 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vasa M, Breitschopf K, Zeiher AM, et al. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ Res 2000; 87: 540–542. [DOI] [PubMed] [Google Scholar]
- 81.Saltevo J, Kautiainen H, Vanhala M. Gender differences in adiponectin and low-grade inflammation among individuals with normal glucose tolerance, prediabetes, and type 2 diabetes. Gend Med 2009; 6: 463–470. [DOI] [PubMed] [Google Scholar]
- 82.Halm MA, Penque S. Heart failure in woman. Prog Cardiovasc Nurs 2000; 15: 121–133. [DOI] [PubMed] [Google Scholar]
- 83.Sackett DL. Evidence based medicine: How to practice and teach EMB, 2nd ed Toronto: Churchill Livingstone Publish House, 2000. [Google Scholar]
- 84.Elks CE, Scott RA. The long and short of telomere length and diabetes. Diabetes 2014; 63: 65–67. [DOI] [PubMed] [Google Scholar]
- 85.Salpea KD, Humphries SE. Telomere length in atherosclerosis and diabetes. Atherosclerosis 2010; 209: 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]