Abstract
Objective
This systematic review aimed to define the relationship between diabetes mellitus (DM) and the risk of aneurysmal subarachnoid haemorrhage (aSAH).
Methods
Studies associated with DM and aSAH published until March 2016 were retrieved from Pubmed, Embase, Web of Science, and Cochrane Library databases. A random-effects model was used to calculate the relative risks (RRs) with 95% confidence intervals (CIs).
Results
Eighteen observational studies were retrieved. The overall RRs for DM and aSAH were RRs = 0.59 (0.44, 0.79), with moderate heterogeneity (I2 = 55.7%, Pheterogeneity = 0.000). Subgroup analysis by study quality revealed a reduced association between DM and aSAH risk in high quality studies only (RRs = 0.40, 95% CI: 0.29, 0.56; I2 = 0.0%, Pheterogeneity = 0.549), therefore study quality may be a source of heterogeneity.
Conclusion
A potential decreased risk of aSAH in DM patients was found in high quality studies. Further studies are required to confirm this causal relationship and to investigate the biological mechanisms.
Keywords: Diabetes, intracranial aneurysm, meta-analysis, subarachnoid hemorrhage, systematic review
Introduction
Aneurysmal subarachnoid haemorrhage (aSAH) comprises 85% of all SAH cases.1,2 Approximately 17,000 people in the United States are affected by aSAH each year.3 It has been deemed a serious subtype of stroke with high fatality rate and poses a significant threat to public health.1,4–8 Despite the recent advance in treatments and management, the poor prognosis has not been improved.9 Effective prevention based on a better understanding of the etiology of aSAH is vital. Some researchers have reported that race, female sex, age, cigarette smoking, hypertension, heavy alcohol intake, and family history are predisposing risk factors for aSAH.10–14
Diabetes mellitus (DM) is the most common endocrine disorder and a major cause of disability worldwide.15 It is calculated that the number of DM patients will increase from 246 million to 380 million over the next twenty years16 due to burgeoning population, changed lifestyles, and population ageing.17 DM influences the arterial wall by different mechanisms18–20 and is a well-known trigger for cerebral infarction.21,22
A recent study suggested that DM was associated with a decreased risk of saccular intracranial aneurysm rupture, which may cause aSAH.23 However, multiple epidemiologic studies have produced inconsistent and inconclusive results regarding the relationship between DM and aSAH.10,24–40 Hence, we conducted a systematic review and meta-analysis to evaluate the current evidence from published observational studies relating to the association between DM and the risk of aSAH.
Materials and methods
We conducted this systematic review and meta-analysis in adherence to the guidelines of the Meta-analyses of Observational Studies in Epidemiology (MOOSE) group.41
Data sources and searches
Two investigators (X.Y. and C.J.) independently performed a systematic search of literature published until March 28, 2016 in Pubmed, Embase, Web of Science, and Cochrane Library databases. The following search terms were used: (diabetes OR diabetes mellitus) AND (intracranial aneurysm OR intracerebral aneurysm OR cerebral aneurysm OR subarachnoid haemorrhage) AND risk. No restrictions on language or types of publications were imposed. The reference lists of retrieved publications were also searched for additional relevant studies.
Study selection
Studies were included in our meta-analysis if they met the following criteria: 1) The study design was a cohort or case-control study; 2) the study described the impact of DM on the risk of aSAH; 3) the study presented odds ratios (ORs), hazard ratios (HRs), or relative risks (RRs) with corresponding 95% confidence intervals (CIs) (or sufficient data to calculate them). In instances where multiple reports were based on the same study population, we included the most informative and comprehensive studies in our systematic review.
Data extraction and methodological quality assessment
Study selection and data extraction were conducted respectively by two investigators (X.Y. and C.J.). We extracted the following information using a standardized collection form: the first author’s last name, year of publication, study period, study design, number of aSAH patients and participants, source of controls, outcome data, and adjustment for major confounding factors.
The study quality of each included case-control or cohort study was assessed using the Critical Appraisal Skills Program (CASP).42 These questions appraised the study validity, risk of bias in recruitment, exposure and outcome measurement, confounders, the reported results, generalizability, and transferability. Each single included study was independently assessed by two reviewers (X.Y. and C.J.). Any disagreements were judged by a third investigator (G.J.) and settled by consensus.
Statistical analysis
If the prevalence of aSAH was rare, ORs and HRs were considered equal to RRs.43 So, a RR with 95% CI was used to measure the relationship between DM and aSAH. If outcomes for males and females were provided, then risk estimates for males and females were regarded as two separate results.44 The Cochran’s Q test was used to assess the statistical heterogeneity (significant if P < 0.10).We employed I2 statistics to evaluate the degree of heterogeneity among included studies.45 We applied the random-effects model, which combined both within- and between-study heterogeneity to summarize the risk estimates.46 Subgroup analyses were made for study design and study quality assessed by CASP. Sensitivity was analysed by omitting one study at a time to assess the stability of the pooled consequences. We examined the potential evidence of publication bias by Begg funnel plots and Egger’s test.47,48 A P-value of < 0.05 was regarded as statistically significant. All statistical tests were performed using STATA 12.0 software (Stata Corporation, College Station, TX, USA).
Results
Literature search
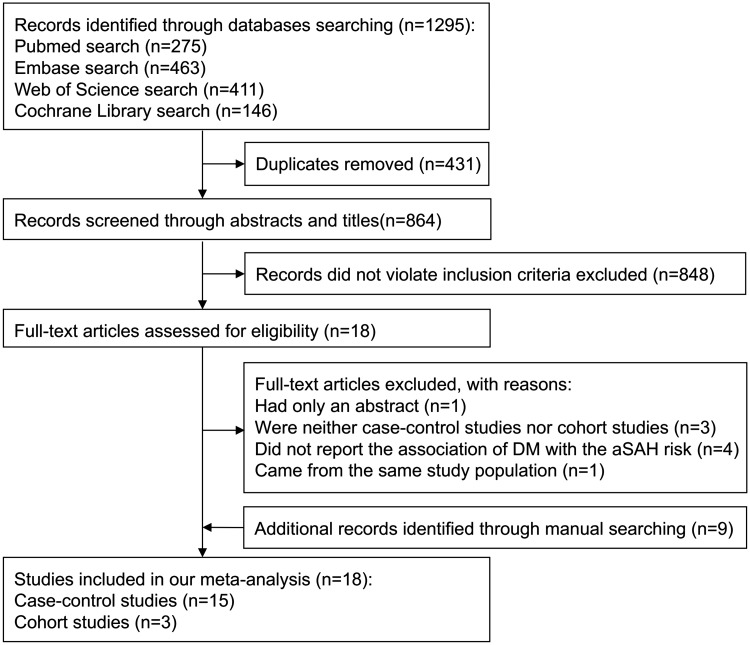
The electronic search yielded 1,295 potential articles. Duplicates and studies that did not meet the inclusion criteria were excluded. After this, 18 studies were chosen for detailed appraisal. Among them, one study49 only contained an abstract; three studies23,50,51 were neither case-control studies nor cohort studies; four studies52–55 did not report the association of DM with the risk of aSAH; one study56 came from the same study population. The remaining nine studies30–38 of interest were found by manual searching. Thus, 18 studies10,24–40 were included in our meta-analysis. The flow chart of our searching process is shown in Figure 1.
Figure 1.
Flow chart of the search procedure.
Description of included studies
Characteristics of the included studies are presented in Table 1. Studies were published between 1991 and 2015. Six of the 18 included studies25,26,29,30,35,39 were conducted in Japan, three31,33,34 in the United States, two32,36 in Australia and New Zealand, and one each in Finland,10 Germany,38 England,40 the Netherlands,37 Portugal,24 Mexico,27 and India.28Fifteen24–38 studies were case-control studies and three10,39,40 were cohort studies that reported the correlation between DM and the risk of aSAH. Except for one32 study that only included female subjects, males and females were included. Various covariates were adjusted in most studies, although these differed between studies.
Table 1.
Main characteristics of the included studies.
| Author, year | Study period/ years of follow-up | Country | Study design | Sex | No. of cases/ No. of controls | RR and 95% CI (DM versus non-DM) | Adjustment |
|---|---|---|---|---|---|---|---|
| Knekt 1991 | 1966–1972/22 | Finland | Cohort | M + F | 187/42862 | M: 0.7 (0.1, 4.7) F: 0 (0, 2.9) | Age |
| Canhao 1994 | 1985–1990 | Portugal | Case-control | M + F | 134/134 | 2.7 (0.9, 8.3) | None |
| Kunze 2000 | 1997–1998 | Germany | Case-control | M + F | 44/44 | 0.06 (0, 1.02) | None |
| Kubota 2001 | NS | Japan | Case-control | M + F | 127/127 | 0.49 (0.09, 2.74) | None |
| Qureshi 2001 | 1990–1997 | United States | Case-control | M + F | 323/969 | 0.7 (0.4, 1.2) | Age, sex, race |
| Mhurchu 2001 | 1995–1998 | Australia/ New Zealand | Case-control | F | 268/286 | 0.47(0.22,1.00) | Age, sex, city of residence |
| Kissela 2002 | 1997–2000 | United States | Case-control | M + F | 107/197 | 0.6 (0.2, 1.6) | Age, sex, race |
| Ohkuma 2003 | 1989–1998 and 2000–2001 | Japan | Case-control | M + F | 390/390 | 0.73 (0.42,1.27) | Age, sex |
| Broderick 2003 | 1994–1999 | United States | Case-control | M + F | 312/618 | 0.75 (0.41,1.38) | Age, sex, race |
| Inagawa 2005 | 1980–1998 | Japan | Case-control | M + F | 247/247 | 0.33 (0.13, 0.84) | Age, sex, hypertension, smoking, drinking, total cholesterol level, heart disease, liver disease |
| Okamoto 2005 | 1992–1997 | Japan | Case-control | M + F | 201/402 | 2.6 (1.2, 6.7) | Age, sex, family history, smoking |
| Sandoval 2009 | 2002–2004 | Mexico | Case-control | M + F | 231/231 | 0.34 (0.17, 0.68) | Age, sex, hypertension, smoking, drinking |
| Koshy 2010 | 2003–2008 | India | Case-control | M + F | 163/150 | 0.34 (0.15, 0.76) | None |
| Inagawa 2010 | 1981–2005 | Japan | Case-control | M + F | 798/798 | 0.41 (0.26, 0.64) | Age, sex, hypertension, smoking, drinking, hypercholesterolemia, heart disease |
| Cui 2011 | NS/median 12.0 | Japan | Cohort | M + F | 122/35657 | M: 0.15 (0.01, 2.47) F: 0.97 (0.3, 3.15) | Age, systolic blood pressure, smoking, drinking, BMI, HDL cholesterol, total cholesterol, triglycerides, antihypertensive medication, fasting status, residential areas. |
| Shiue 2012 | 1995–1998 | Australia/ New Zealand | Case-control | M + F | 432/473 | 0.61 (0.35, 1.06) | None |
| Vlak 2013 | 2006–2009 | Netherlands | Case-control | M + F | 250/574 | 0.5 (0.2, 1.2) | Age, sex |
| Shah 2015 | 1998–2010/NS | England | Cohort | M + F | 11/1921260 | 0.48 (0.26, 0.89) | Age, sex, systolic blood pressure, smoking, BMI, HDL cholesterol, total cholesterol, deprivation, statin and antihypertensive drugs |
Abbreviations: NS, not stated; RR, risk ratio; CI, confidence interval; F, female; BMI, body mass index; HDL, high-density lipoprotein.
Only one study distinguished between type 1 and type 2 DM; this study described the association between type 2 DM and the risk of aSAH.40 Most studies did not define DM and confirmed DM through the patient’s medical history. Nevertheless, three10,24,39 studies defined DM according to the World Health Organization’s criteria, and one27 used the definition established by the standard guidelines of the American Diabetes Association. In most studies, aSAH was diagnosed using computed tomography and/or cerebral angiography. Some studies used surgery or necropsy and cerebrospinal fluid analysis to confirm the diagnosis. A few studies30,37,40 confirmed aSAH from the medical history. Most studies reported participant satisfaction; two studies10,38 showed < 90% satisfaction and participant satisfaction was not reported in two studies.24,32
Methodological quality assessment
The study quality assessment is presented in Tables 2 and 3. In total, five 26,27,29,39,40 studies were high quality and the others were poor quality. Seven25,26,28,30,34,35,38 case-control studies recruited controls from hospitals, which was not representative of the participants. Results from two24,35 studies contradicted other available evidence. Most studies only adjusted for age and sex. Adjustment for other important confounders such as cigarette smoking, hypotension, alcohol consumption, body mass index (BMI), and cholesterol varied among the studies. Two39,40 studies adjusted for the use of antihypertensive drugs. Very few studies investigating the risk of DM and aSAH adjusted for other chronic diseases, such as heart26,29 and liver disease.26
Table 2.
Summary of critical appraisal of case-control studies.
| Criteria | Canhao 1994 | Kunze 2000 | Kubota 2001 | Qureshi 2001 | Mhurchu 2001 | Kissela 2002 | Ohkuma 2003 | Broderick 2003 |
|---|---|---|---|---|---|---|---|---|
| Clearly focused issue? | Y | Y | Y | Y | Y | Y | Y | Y |
| Appropriate method? | Y | Y | Y | Y | Y | Y | Y | Y |
| Acceptable case recruitment? | Y | Y | Y | Y | Y | Y | Y | Y |
| Acceptable control recruitment? | Y | C/T | C/T | Y | Y | Y | C/T | C/T |
| Exposure accurately measured? | Y | Y | Y | Y | Y | Y | Y | Y |
| Confounders accounted for? | Y | Y | Y | Y | Y | Y | Y | Y |
| Confounding factors in the design and/or analysis taken account of? | N | N | N | N | N | N | N | N |
| What are the results? | C/T | C/T | C/T | C/T | C/T | C/T | C/T | C/T |
| How precise are the results? | C/T | C/T | C/T | C/T | C/T | C/T | C/T | C/T |
| Do you believe the results? | Y | Y | Y | Y | Y | Y | Y | Y |
| Applicable to the local population? | Y | Y | Y | Y | Y | Y | Y | Y |
| Fits with other available evidence? | N | Y | Y | Y | Y | Y | Y | Y |
| Do you believe the results? | Y | Y | Y | Y | Y | Y | Y | Y |
| Total methodological quality | L | M | M | M | M | M | M | M |
Y-Yes; N-No; H-High; L-Low; M-Moderate; C/T-Cannot tell.
Table 3.
Summary of critical appraisal of cohort studies.
| Criteria | Knekt 1991 | Cui 2011 | Shah 2015 |
|---|---|---|---|
| Clearly focused issue? | Y | Y | Y |
| Acceptable cohort recruitment? | Y | Y | Y |
| Exposure accurately measured? | Y | Y | Y |
| Outcome accurately measured? | Y | Y | Y |
| Important confounding factors identified? | Y | Y | Y |
| Confounding factors in the design and/or analysis taken into account? | N | Y | Y |
| Was the follow-up complete? | Y | Y | Y |
| Was the follow-up long enough? | Y | Y | Y |
| What are the results of the study? | C/T | Y | Y |
| How precise are the results? | C/T | C/T | Y |
| Do you believe the results? | Y | Y | Y |
| Applicable to the local population? | Y | Y | Y |
| Agrees with other evidence? | C/T | Y | Y |
| Total methodological quality | M | H | H |
Y-Yes; N-No; H-High; L-Low; M-Moderate; C/T-Cannot tell.
Qualitative association of DM and aSAH risk
Data were available on 2,005,419 participants, including 4,327 aSAH patients. All included studies except two24,35 reported reduced RRs for aSAH patients with DM versus non-DM. Knekt et.al.10 and another two39,40 cohort studies did not show a significant reduction in the association between DM and aSAH risk with regard to sex. However, Inagawa reported a statistically inverse correlation between DM and aSAH risk in female participants, especially those who were ≥ 60 years of age.26,29 The largest cohort study40 from England revealed a significantly decreased association [HR, 0.48 (0.26,0.89)] between DM and aSAH risk, while other European studies gave non-significant results. Most high quality studies were from Asia26,29,39 and these authors reported conflicting outcomes.
Synthetic association of DM and aSAH risk
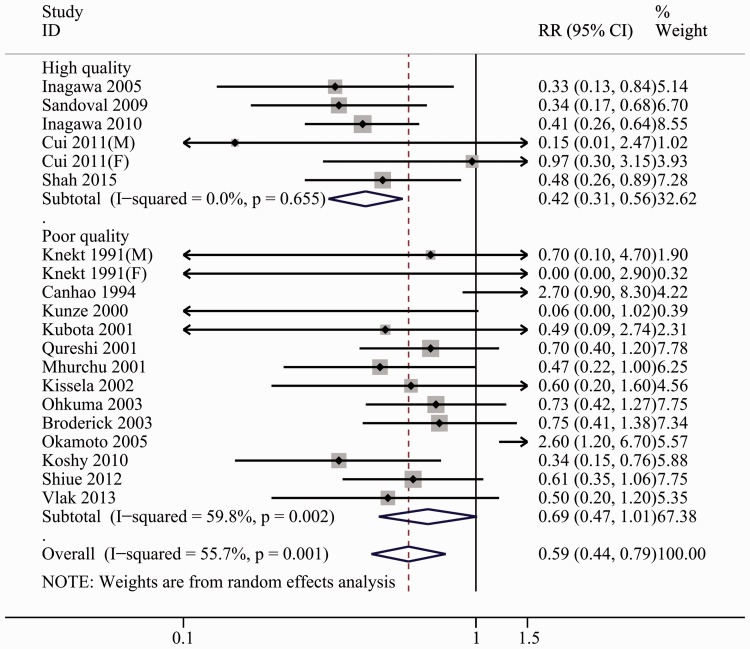
RRs for the association of DM and aSAH risk were calculated in 18 studies and ranged from 0 – 2.7.10,24–40 Overall, DM significantly reduced the risk of aSAH [0.59 (0.44, 0.79)], with substantial heterogeneity (I2 = 55.7%, Pheterogeneity = 0.000). Forest plots of the association between DM and aSAH risk are presented in Figure 2.
Figure 2.
Forest plots for the risk of diabetes mellitus and aneurysmal subarachnoid hemorrhage.
Subgroup analyses
Study design
The correlation between DM and aSAH was evaluated in three cohort studies and 15 case-control studies (Table 4). The summarized RR was 0.34 (95% CI: 0.09, 2.14; I2 = 68.4%, Pheterogeneity = 0.013) in the cohort studies,10,39,40 and 0.61 (95% CI: 0.46, 0.82; I2 = 53.1%, Pheterogeneity = 0.001) in case-control studies.24–38
Table 4.
Synthetic RRs for diabetes mellitus and aneurysmal subarachnoid hemorrhage.
| Synthetic estimation |
Heterogeneity |
||||
|---|---|---|---|---|---|
| Subgroup | No. of studies | RR (95% CI) | P-value | I2 (%) | P-value |
| Total studies | 18 | 0.59 (0.44, 0.79) | 0 | 55.7 | 0.001 |
| Study design | |||||
| Cohort | 3 | 0.34 (0.09, 2.14) | 0.103 | 68.4 | 0.013 |
| Case-control | 15 | 0.61 (0.46, 0.82) | 0.001 | 53.1 | 0.008 |
| Study quality | |||||
| High | 5 | 0.38 (0.27,0.54) | 0 | 0 | 0.655 |
| Moderate and Low | 13 | 0.69 (0.47,1.01) | 0.056 | 59.8 | 0.002 |
Abbreviations: RR, relative risk; CI, confidence interval; BMI, body mass index.
Study quality assessed by CASP
No significant connection between DM and aSAH was discovered in low and moderate quality studies10,24,25,28,30–38 (RRs = 0.69, 95% CI: 0.47, 1.01; I2 = 58.3%, Pheterogeneity = 0.002), whereas a statistically significant correlation was found in high quality studies26,27,29,39,40 (RRs = 0.40, 95% CI: 0.29, 0.56; I2 = 0.0%, Pheterogeneity = 0.549) (Figure 2, Table 4).
Sensitivity analysis and publication bias
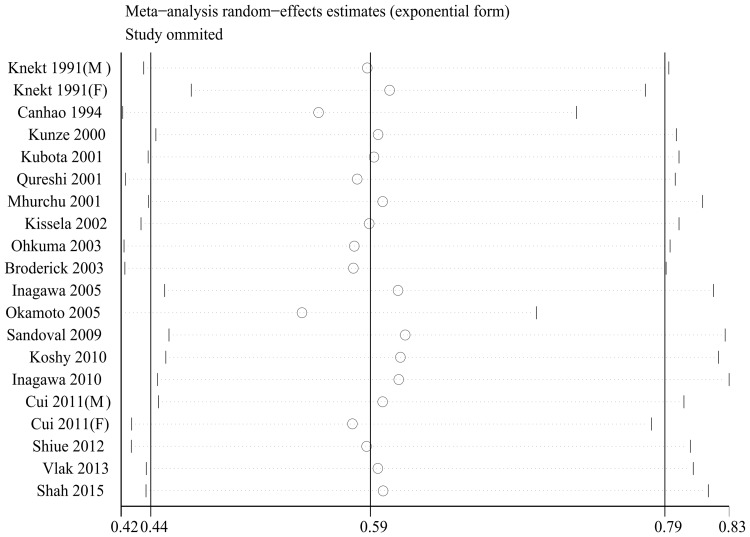
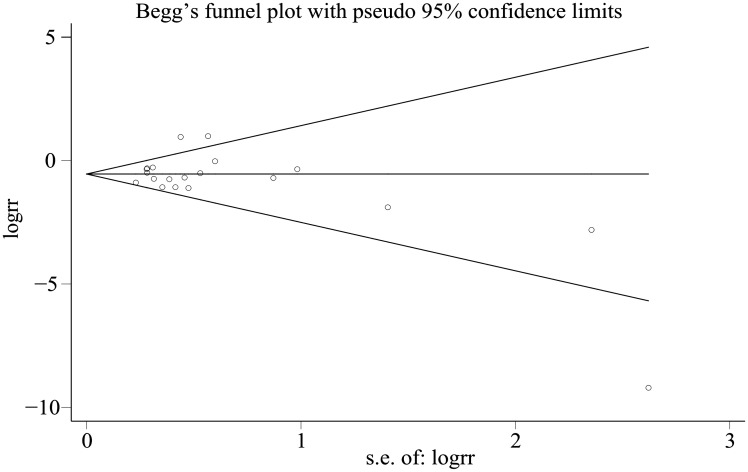
Outcomes were stable except for one study (Figure 3). No evidence of meaningful publication bias was indicated by the Begg funnel plot (Figure 4) and Egger’s test (PEgger = 0.396).
Figure 3.
Sensitivity analyses for the risk of diabetes mellitus and aneurysmal subarachnoid hemorrhage.
Figure 4.
Begg funnel plot for the risk of diabetes mellitus and aneurysmal subarachnoid hemorrhage.
Discussion
The incidence of cerebrovascular disease is 1.7 times higher in diabetic patients than non-diabetic persons.57 The cerebrovascular complications of DM are restricted to atherosclerotic disease and cerebral infarction, and the frequency of cerebral infarction is higher than expected in diabetic patients.58,59 However, the Harvard Cooperative Stroke Registry reported a low occurrence of DM in patients with aSAH.60 To date, the contribution of DM to the development of aSAH remains unclear. In our systematic review, all included studies except for two24,35 reported a decreased association between DM and aSAH risk. This meta-analysis using RRs from 15 case-control studies and three cohort studies included more than 2,000,000 individuals, therefore our findings indicate that DM reduces the risk of aSAH.
Strengths and limitations of this systematic review
To the best of our knowledge, this is the first systematic review and meta-analysis to focus on the association between DM and risk of aSAH. All available published studies with a sufficient number of participants for a qualitative and quantitative summary of the outcomes were included in this meta-analysis. Therefore, this study has more power than any individual study to investigate the relationship between DM and risk of aSAH.
Substantive heterogeneity between studies was monitored using the I2 statistic. The findings of observational studies should always be interpreted with caution. Subgroup analysis by study design showed that the majority of included studies were case-control studies, therefore our conclusions are chiefly based on case-control studies. Diabetic patients probably have a higher risk of dying from other diseases than non-diabetic individuals. Consequently, the likelihood of a SAH forming is reduced in DM patients compared with controls.57 At the same time, recall and selective bias may be increased.
The most important limitation highlighted by the current systematic review was the paucity of high quality studies. Only half of the included studies were of high quality. Insufficient study quality may be a source of heterogeneity according to the subgroup analysis of study quality. The CASP clearly demonstrated that recruited controls were unrepresentative, identified a large number of unadjusted potential confounders, and showed that unprecise results are the main cause of poor study quality. Because the study quality was limited and the heterogeneity was moderate, it was not possible to show a stronger association for population characteristics, such as geographical areas and sex.
Study quality may have been a contributing factor to heterogeneity, but there were other potential sources. For example, only a few studies10,27,39 specifically defined DM. The lack of a consistent standard definition for DM may have affected the number of diabetic patients recruited in each study. Similarly, some studies30,39,40 did not describe the methods of aSAH diagnosis, which may also have contributed to heterogeneity.
Prospective population-based cohort studies have shown that BMI and cholesterol levels may be related to DM and may protect against aSAH.10,61,62 Information about important risk factors such as BMI39,40 and cholesterol levels26,39,40 were only collected in a few studies. Other important risk factors such as cigarette smoking, hypotension, and alcohol consumption were generally inconsistent among the included studies. These factors have been associated with an increased risk of aSAH,10–14 but very few studies adjusted for them. This may explain the unprecise findings regarding risk of aSAH among the included studies.
Because the collected information was limited, we could not distinguish between type 1 and type 2 DM. This might have prevented identification of a genuine association between DM and risk of aSAH. For example, in type 1 diabetic patients, the prevalence of nonaneurysmal SAH was high in a prospective cohort study.55 Because the quality of the included studies was generally poor, we did not calculate the RR and 95% CI to detect more sources of heterogeneity, such as the methods for measuring diabetes, antidiabetic drugs, and duration of diabetes in each study. Notably, antidiabetic drugs were used by 9% of type 2 diabetic patients in a comparative study, but was not related to the risk of aSAH according to multivariate analysis.
We did not perform an exhaustive search of the published literature in all electronic databases; therefore we may have missed several pertinent published or unpublished studies. Finally, we found little proof of publication bias in our meta-analysis. However, potential publication bias might have had an impact on our findings, as statistical tests have shown.47,48
Comparison with previous studies
Feigin et al. performed a meta-analysis63 to evaluate various SAH risk factors published before 2005. Surprisingly, they found that DM was associated with a reduced risk of SAH [RR, 0.3 (0 to 2.2) including one10 longitudinal study [OR, 0.7 (0.5 to 0.8)] and six25,30,31,33,38,56 case-control studies]. However, the sample size and study quality were questionable in this study. The sample size and study quality 25,27,29,39,40 were better in the present meta-analysis [RR, 0.38 (0.27, 0.54)]. Our study yielded a narrower 95% CI of pooled aSAH RR associated with DM compared with the earlier meta-analysis, indicating that our results are more precise.
Only two of the included studies reported an increased association between DM and the risk of aSAH, largely because of small sample sizes and univariate analysis. If an increased association between DM and aSAH is confirmed by future studies, then special determination assessment will be affected, including cerebral angiography of patients with DM and recent symptoms of third nerve palsy.64
Hypothetical causes for the reduction of DM and aSAH risk
Although there are no confirmed biological theories to support our finding, several putative explanations exist. Patients with DM may modify their lifestyles (e.g., more exercise, better diet, less smoking and drinking) and receive medication for hypertension, which is an independent risk factor of aSAH.64 Inagawa suggested that an inverse association exists between DM and aneurysm rupture, which might be linked to the atherosclerotic wall because atherosclerotic aneurysms are less likely to rupture.29 This might also explain why the decreased association is stronger in older diabetic patients than younger diabetic patients.29 Similar to saccharification of the extracellular matrix and cross-linking between elastin and avitene in the aortic wall,65 diabetes-mediated alterations might exist in the hemal extracellular matrix.40 Furthermore, the 9p21.3 locus has been related to DM and saccular IAs,66,67 although it is not part of the linkage disequilibrium block. A genetic connection that enhances type 2 DM and reduces the risk of aSAH is likely to exist.23
Implication for future research
Future studies will need to be well-designed and carefully powered to address the following points: (1) consideration of more potential confounding factors, such as BMI, cholesterol level, smoking, hypertension, and drinking; (2) adjustment for or exclusion of chronic diseases (heart disease was associated with a decreased risk of aSAH, possibly due to reduced strenuous physical activity); (3) investigation of possible therapeutic mechanisms; (4) classification of DM as type 1 and type 2 to determine a potential causal relationship.
Conclusions and unanswered questions
In conclusion, most of the studies included in our systematic review are lacking in quality. Analysis of the high quality studies identified a potential decreased risk of aSAH in subjects with DM. However, the causal relationship between DM and aSAH remains to be elucidated. Further high quality epidemiological cohort and animal studies are necessary to investigate whether DM and aSAH are correlated and to elucidate the underlying biological mechanisms.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by Suzhou Key Medical Center (Szzx201501), grants from the National Natural Science Foundation of China (No. 81571115, 81422013, and 81471196), Scientific Department of Jiangsu Province (No. BL2014045), Suzhou Government (No. LCZX201301, SZS201413, and SYS201332), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- 1.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007; 369: 306–318. [DOI] [PubMed] [Google Scholar]
- 2.Teunissen LL, Rinkel GJ, Algra A, et al. Risk factors for subarachnoid hemorrhage: a systematic review. Stroke 1996; 27: 544–549. [DOI] [PubMed] [Google Scholar]
- 3.Menghini VV, Brown RD, Jr., Sicks JD, et al. Incidence and prevalence of intracranial aneurysms and hemorrhage in Olmsted County, Minnesota, 1965 to 1995. Neurology 1998; 51: 405–411. [DOI] [PubMed] [Google Scholar]
- 4.Cetas JS, McFarlane R, Kronfeld K, et al. Brainstem opioidergic system is involved in early response to experimental SAH. Transl Stroke Res 2015; 6: 140–147. [DOI] [PubMed] [Google Scholar]
- 5.Turan N, Heider RA, Zaharieva D, et al. Sex differences in the formation of intracranial aneurysms and incidence and outcome of subarachnoid hemorrhage: review of experimental and human studies. Transl Stroke Res 2016; 7: 12–19. [DOI] [PubMed] [Google Scholar]
- 6.Güresir E, Schuss P, Borger V, et al. Experimental subarachnoid hemorrhage: double cisterna magna injection rat model–assessment of delayed pathological effects of cerebral vasospasm. Transl Stroke Res 2015; 6: 242–251. [DOI] [PubMed] [Google Scholar]
- 7.Cheng C, Jiang L, Yang Y, et al. Effect of APOE gene polymorphism on early cerebral perfusion after aneurysmal subarachnoid hemorrhage. Transl Stroke Res 2015; 6: 446–450. [DOI] [PubMed] [Google Scholar]
- 8.Etminan N. Aneurysmal subarachnoid hemorrhage–status quo and perspective. Transl Stroke Res 2015; 6: 167–170. [DOI] [PubMed] [Google Scholar]
- 9.Inagawa T. Trends in surgical and management outcomes in patients with aneurysmal subarachnoid hemorrhage in Izumo city, Japan, between 1980–1989 and 1990–1998. Cerebrovasc Dis 2005; 19: 39–48. [DOI] [PubMed] [Google Scholar]
- 10.Knekt P, Reunanen A, Aho K, et al. Risk factors for subarachnoid hemorrhage in a longitudinal population study. J Clin Epidemiol 1991; 44: 933–939. [DOI] [PubMed] [Google Scholar]
- 11.Huttunen T, von und Zu Fraunberg M, Koivisto T, et al. Long-term excess mortality of 244 familial and 1502 sporadic one-year survivors of aneurysmal subarachnoid hemorrhage compared with a matched Eastern Finnish catchment population. Neurosurgery 2011; 68: 20–27. [DOI] [PubMed] [Google Scholar]
- 12.Feigin V, Parag V, Lawes CM, et al. Smoking and elevated blood pressure are the most important risk factors for subarachnoid hemorrhage in the Asia-Pacific region: an overview of 26 cohorts involving 306,620 participants. Stroke 2005; 36: 1360–1365. [DOI] [PubMed] [Google Scholar]
- 13.Inagawa T. Trends in incidence and case fatality rates of aneurysmal subarachnoid hemorrhage in Izumo City, Japan, between 1980–1989 and 1990–1998. Stroke 2001; 32: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 14.Inagawa T. Seasonal variation in the incidence of aneurysmal subarachnoid hemorrhage in hospital- and community-based studies. J Neurosurg 2002; 96: 497–509. [DOI] [PubMed] [Google Scholar]
- 15.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012; 380: 2197–2223. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Zheng Z, Huang P. Diabetes mellitus and the risk of glioma: a meta-analysis. Oncotarget 2016; 7: 4483–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010; 87: 4–14. [DOI] [PubMed] [Google Scholar]
- 18.Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med 2008; 359: 2220–2232. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011; 34(Suppl 1): S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003; 26: 725–731. [DOI] [PubMed] [Google Scholar]
- 21.Almdal T, Scharling H, Jensen JS, et al. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med 2004; 164: 1422–1426. [DOI] [PubMed] [Google Scholar]
- 22.O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010; 376: 112–123. [DOI] [PubMed] [Google Scholar]
- 23.Lindgren AE, Kurki MI, Rithinen A, et al. Type 2 diabetes and risk of rupture of saccular intracranial aneurysm in eastern Finland. Diabetes Care 2013; 36: 2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canhão P, Pinto AN, Ferro H, et al. Smoking and aneurysmal subarachnoid haemorrhage: a case-control study. J Cardiovasc Risk 1994; 1: 155–158. [DOI] [PubMed] [Google Scholar]
- 25.Ohkuma H, Tabata H, Suzuki S, et al. Risk factors for aneurysmal subarachnoid hemorrhage in Aomori, Japan. Stroke 2003; 34: 96–100. [DOI] [PubMed] [Google Scholar]
- 26.Inagawa T. Risk factors for aneurysmal subarachnoid hemorrhage in patients in Izumo City, Japan. J Neurosurg 2005; 102: 60–67. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz-Sandoval JL, Cantú C, Chiquete E, et al. Aneurysmal subarachnoid hemorrhage in a Mexican multicenter registry of cerebrovascular disease: the RENAMEVASC study. J Stroke Cerebrovasc Dis 2009; 18: 48–55. [DOI] [PubMed] [Google Scholar]
- 28.Koshy L, Easwer HV, Premkumar S, et al. Risk factors for aneurysmal subarachnoid hemorrhage in an Indian population. Cerebrovasc Dis 2010; 29: 268–274. [DOI] [PubMed] [Google Scholar]
- 29.Inagawa T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg 2010; 73: 155–164. [DOI] [PubMed] [Google Scholar]
- 30.Kubota M, Yamaura A, Ono J. Prevalence of risk factors for aneurysmal subarachnoid haemorrhage: results of a Japanese multicentre case control study for stroke. Br J Neurosurg 2001; 15: 474–478. [DOI] [PubMed] [Google Scholar]
- 31.Qureshi AI, Suri MF, Yahia AM, et al. Risk factors for subarachnoid hemorrhage. Neurosurgery 2001; 49: 607–613. [DOI] [PubMed] [Google Scholar]
- 32.Mhurchu CN, Anderson C, Jamrozik K, et al. Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: an international population-based, case-control study. Stroke 2001; 32: 606–612. [DOI] [PubMed] [Google Scholar]
- 33.Kissela BM, Sauerbeck L, Woo D, et al. Subarachnoid hemorrhage: a preventable disease with a heritable component. Stroke 2002; 33: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 34.Broderick JP, Viscoli CM, Brott T, et al. Major risk factors for aneurysmal subarachnoid hemorrhage in the young are modifiable. Stroke 2003; 34: 1375–1381. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto K, Horisawa R, Ohno Y. The relationships of gender, cigarette smoking, and hypertension with the risk of aneurysmal subarachnoid hemorrhage: a case-control study in Nagoya, Japan. Ann Epidemiol 2005; 15: 744–748. [DOI] [PubMed] [Google Scholar]
- 36.Shiue I, Arima H, Hankey GJ, et al. Modifiable lifestyle behaviours account for most cases of subarachnoid haemorrhage: a population-based case-control study in Australasia. J Neurol Sci 2012; 313: 92–94. [DOI] [PubMed] [Google Scholar]
- 37.Vlak MH, Rinkel GJ, Greebe P, et al. Lifetime risks for aneurysmal subarachnoid haemorrhage: multivariable risk stratification. J Neurol Neurosurg Psychiatry 2013; 84: 619–623. [DOI] [PubMed] [Google Scholar]
- 38.Kunze AK, Annecke A, Wigger F, et al. Recent infection as a risk factor for intracerebral and subarachnoid hemorrhages. Cerebrovasc Dis 2000; 10: 352–358. [DOI] [PubMed] [Google Scholar]
- 39.Cui R, Iso H, Yamagishi K, et al. Diabetes mellitus and risk of stroke and its subtypes among Japanese the Japan public health center study. Stroke 2011; 42: 2611–2614. [DOI] [PubMed] [Google Scholar]
- 40.Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol 2015; 3: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 42.Smith TO, Easton V, Bacon H, et al. The relationship between benign joint hypermobility syndrome and psychological distress: a systematic review and meta-analysis. Rheumatology (Oxford) 2014; 53: 114–122. [DOI] [PubMed] [Google Scholar]
- 43.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987; 9: 1–30. [DOI] [PubMed] [Google Scholar]
- 44.Rong Y, Chen L, Zhu T, et al. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ 2013; 346: e8539–e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 47.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 48.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macedo SK, de Siqueira CMP, Primo de Siqueira SB, et al. Frequency/prevalence analysis of risk factors on aneurysmal subarachnoid hemorrhage. Critical Care 2010; 14(Suppl 1): 337–337. [Google Scholar]
- 50.Kim YW, Neal D, Hoh BL. Risk factors, incidence, and effect of cardiac failure and myocardial infarction in aneurysmal subarachnoid hemorrhage patients. Neurosurgery 2013; 73: 450–457. [DOI] [PubMed] [Google Scholar]
- 51.Taylor CL, Yuan Z, Selman WR, et al. Cerebral arterial aneurysm formation and rupture in 20,767 elderly patients: hypertension and other risk factors. J Neurosurg 1995; 83: 812–819. [DOI] [PubMed] [Google Scholar]
- 52.Qureshi AI, Malik AA, Saeed O, et al. Hormone replacement therapy and the risk of subarachnoid hemorrhage in postmenopausal women. J Neurosurg 2016; 124: 45–50. [DOI] [PubMed] [Google Scholar]
- 53.Sankai T, Iso H, Shimamoto T, et al. Cohort study on risk factors for subarachnoid hemorrhage among Japanese men and women. Nihon Eiseigaku Zasshi 1999; 53: 587–595. [in Japanese, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 54.You SH, Kong DS, Kim JS, et al. Characteristics features of unruptured intracranial aneurysms: predictive risk factors for aneurysm rupture. J Neurol Neurosurg Psychiatry 2010; 81: 479–484. [DOI] [PubMed] [Google Scholar]
- 55.Korja M, Thorn LM, Hägg S, et al. Subarachnoid hemorrhage in type 1 diabetes: a prospective cohort study of 4,083 patients with diabetes. Diabetes Care 2013; 36: 3754–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson C, Ni Mhurchu C, Scott D, et al. Triggers of subarachnoid hemorrhage: role of physical exertion, smoking, and alcohol in the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS). Stroke 2003; 34: 1771–1776. [DOI] [PubMed] [Google Scholar]
- 57.Palumbo PJ, Elveback LR, Whisnant JP. Neurologic complications of diabetes mellitus: transient ischemic attack, stroke, and peripheral neuropathy. Adv Neurol 1978; 19: 593–601. [PubMed] [Google Scholar]
- 58.Alex M, Baron EK, Goldenberg S, et al. An autopsy study of cerebrovascular accident in diabetes mellitus. Circulation 1962; 25: 663–673. [DOI] [PubMed] [Google Scholar]
- 59.Roehmholdt ME, Palumbo PJ, Whisnant JP, et al. Transient ischemic attack and stroke in a community-based diabetic cohort. Mayo Clin Proc 1983; 58: 56–58. [PubMed] [Google Scholar]
- 60.Mohr JP, Caplan LR, Melski JW, et al. The Harvard cooperative stroke registry: a prospective registry. Neurology 1978; 28: 754–762. [DOI] [PubMed] [Google Scholar]
- 61.Sandvei MS, Lindekleiv H, Romundstad PR, et al. Risk factors for aneurysmal subarachnoid hemorrhage - BMI and serum lipids: 11-year follow-up of the HUNT and the Tromsø Study in Norway. Acta Neurol Scand 2012; 125: 382–388. [DOI] [PubMed] [Google Scholar]
- 62.Korja M, Silventoinen K, Laatikainen T, et al. Risk factors and their combined effects on the incidence rate of subarachnoid hemorrhage–a population-based cohort study. PloS One 2013; 8: e73760–e73760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005; 36: 2773–2780. [DOI] [PubMed] [Google Scholar]
- 64.Adams HP, Jr., Putman SF, Kassell NF, et al. Prevalence of diabetes mellitus among patients with subarachnoid hemorrhage. Arch Neurol 1984; 41: 1033–1035. [DOI] [PubMed] [Google Scholar]
- 65.Shantikumar S, Ajjan R, Porter KE, et al. Diabetes and the abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2010; 39: 200–207. [DOI] [PubMed] [Google Scholar]
- 66.Voight BF, Scott LJ, Steinthorsdottir V, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010; 42: 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007; 316: 1341–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]