Abstract
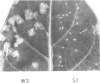
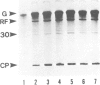
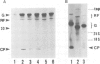
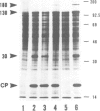
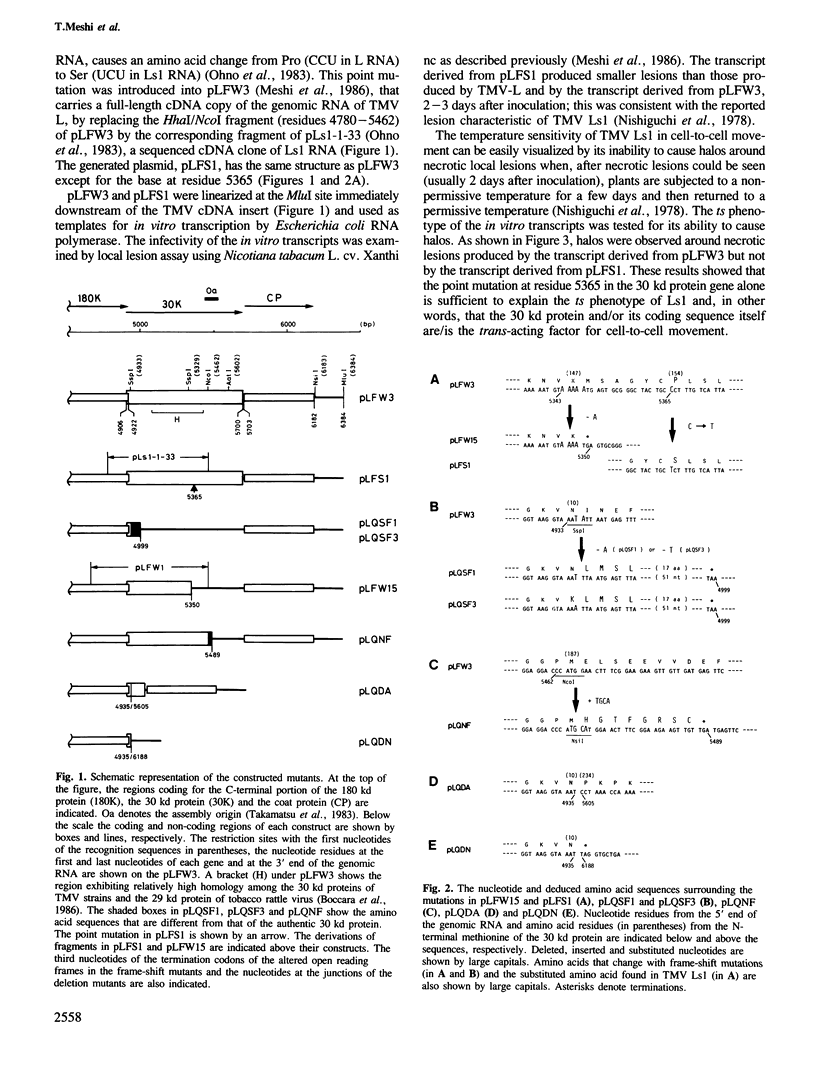
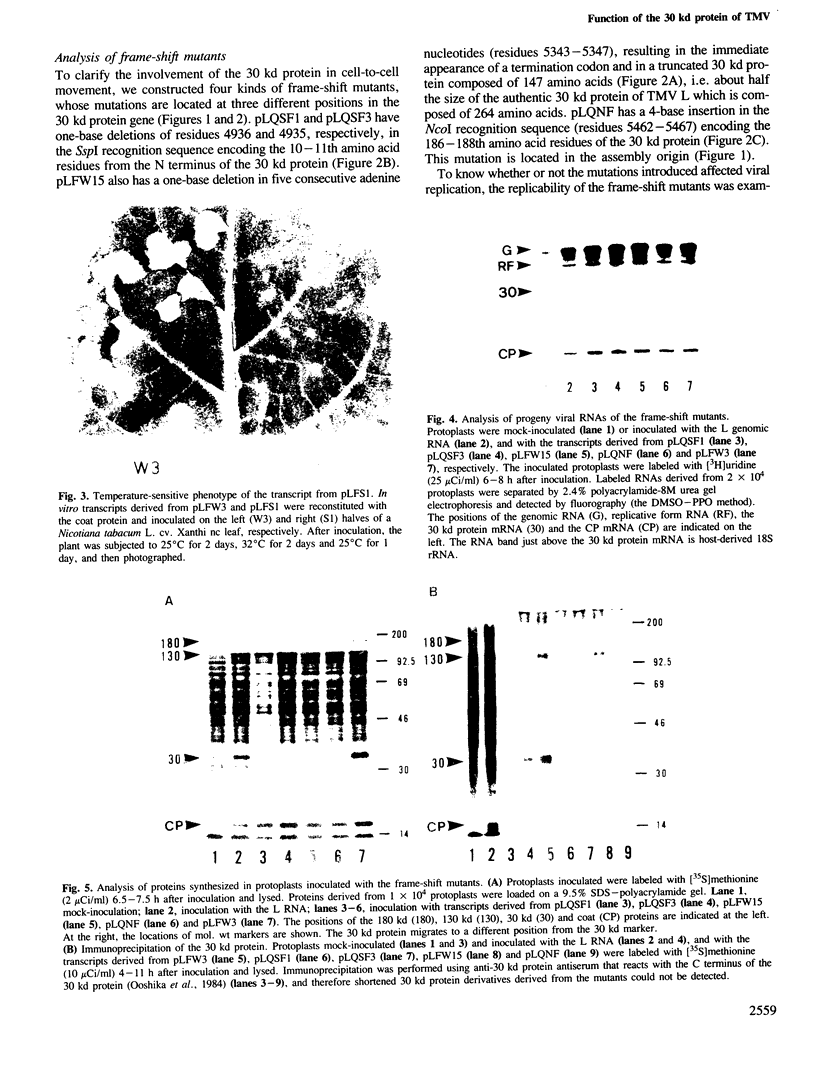
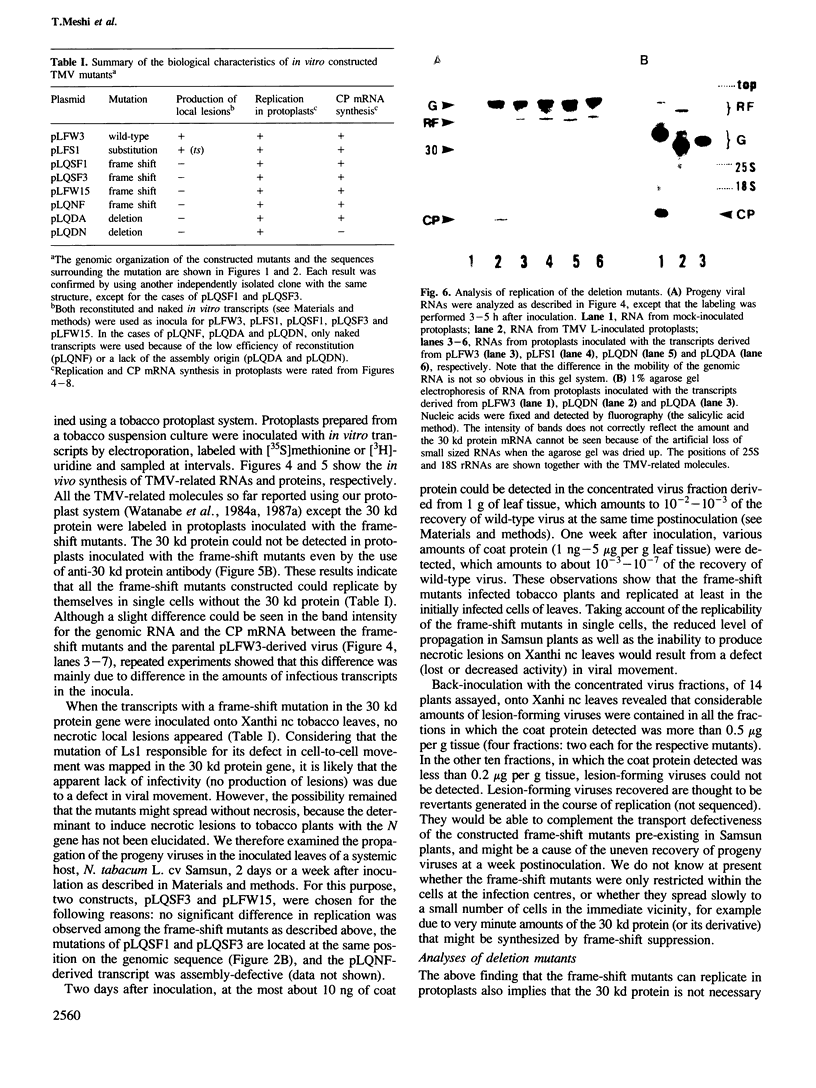
We have investigated the function of the 30 kd protein of tobacco mosaic virus (TMV) by a reverse genetics approach. First, a point mutation of TMV Ls1 (a temperature-sensitive mutant defective in cell-to-cell movement), that causes an amino acid substitution in the 30 kd protein, was introduced into the parent strain, TMV L. The generated mutant showed the same phenotype as TMV Ls1, and therefore the one-base substitution in the 30 kd protein gene adequately explains the defectiveness of TMV Ls1. Next, four kinds of frame-shift mutants were constructed, whose mutations are located at three different positions of the 30 kd protein gene. All the frame-shift mutants were replication-competent in protoplasts but none showed infectivity on tobacco plants. From these observations the 30 kd protein was confirmed to be involved in cell-to-cell movement. To clarify that the 30 kd protein is not necessary for replication, two kinds of deletion mutants were constructed; one lacking most of the 30 kd protein gene and the other lacking both the 30 kd and coat protein genes. Both mutants replicated in protoplasts and the former still produced the subgenomic mRNA for the coat protein. These results clearly showed that the 30 kd protein, as well as the coat protein, is dispensable for replication and that no cis-acting element for replication is located in their coding sequences. It is also suggested that the signal for coat protein mRNA synthesis may be located within about 100 nucleotides upstream of the initiation codon of the coat protein gene.
Keywords: cell-to-cell movement, replication, reverse genetics, 30 kd protein, tobacco mosaic virus
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atabekov J. G., Dorokhov YuL Plant virus-specific transport function and resistance of plants to viruses. Adv Virus Res. 1984;29:313–364. doi: 10.1016/s0065-3527(08)60412-1. [DOI] [PubMed] [Google Scholar]
- Boccara M., Hamilton W. D., Baulcombe D. C. The organisation and interviral homologies of genes at the 3' end of tobacco rattle virus RNA1. EMBO J. 1986 Feb;5(2):223–229. doi: 10.1002/j.1460-2075.1986.tb04202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening G., Beachy R. N., Scalla R., Zaitlin M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology. 1976 Jun;71(2):498–517. doi: 10.1016/0042-6822(76)90377-9. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Cornelissen B. J., Linthorst H. J., Brederode F. T., Bol J. F. Analysis of the genome structure of tobacco rattle virus strain PSG. Nucleic Acids Res. 1986 Mar 11;14(5):2157–2169. doi: 10.1093/nar/14.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W. O., Beck D. L., Knorr D. A., Grantham G. L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1832–1836. doi: 10.1073/pnas.83.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Meshi T., Okada Y., Otsuki Y., Takebe I. Correlation between particle multiplicity and location on virion RNA of the assembly initiation site for viruses of the tobacco mosaic virus group. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4231–4235. doi: 10.1073/pnas.78.7.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Jonard G., Kukla B., Richards K. E. Sequence of 1000 nucleotides at the 3' end of tobacco mosaic virus RNA. Nucleic Acids Res. 1979 Apr;6(4):1287–1308. doi: 10.1093/nar/6.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. I., Dodds J. A. Infection of barley by tobacco mosaic virus in single and mixed infection. Virology. 1970 Sep;42(1):266–268. doi: 10.1016/0042-6822(70)90267-9. [DOI] [PubMed] [Google Scholar]
- Hirth L., Richards K. E. Tobacco mosaic virus: model for structure and function of a simple virus. Adv Virus Res. 1981;26:145–199. doi: 10.1016/s0065-3527(08)60423-6. [DOI] [PubMed] [Google Scholar]
- Hull R., Sadler J., Longstaff M. The sequence of carnation etched ring virus DNA: comparison with cauliflower mosaic virus and retroviruses. EMBO J. 1986 Dec 1;5(12):3083–3090. doi: 10.1002/j.1460-2075.1986.tb04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. R., Hunt T., Knowland J., Zimmern D. Messenger RNA for the coat protein of tobacco mosaic virus. Nature. 1976 Apr 29;260(5554):759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Jackson R., Zimmern D. Multiple proteins and subgenomic mRNAs may be derived from a single open reading frame on tobacco mosaic virus RNA. Nucleic Acids Res. 1983 Feb 11;11(3):801–821. doi: 10.1093/nar/11.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Motoyoshi F., Takamatsu N., Okada Y. In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 1986 Nov 11;14(21):8291–8305. doi: 10.1093/nar/14.21.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Motoyoshi F., Semba K., Okada Y. In vitro transcription of infectious RNAs from full-length cDNAs of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5043–5047. doi: 10.1073/pnas.83.14.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ohno T., Okada Y. Nucleotide sequence and its character of cistron coding for the 30 K protein of tobacco mosaic virus (OM strain). J Biochem. 1982 Apr;91(4):1441–1444. doi: 10.1093/oxfordjournals.jbchem.a133833. [DOI] [PubMed] [Google Scholar]
- Meshi T., Ohno T., Okada Y. Nucleotide sequence of the 30K protein cistron of cowpea strain of tobacco mosaic virus. Nucleic Acids Res. 1982 Oct 11;10(19):6111–6117. doi: 10.1093/nar/10.19.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Ohno T., Aoyagi M., Yamanashi Y., Saito H., Ikawa S., Meshi T., Okada Y. Nucleotide sequence of the tobacco mosaic virus (tomato strain) genome and comparison with the common strain genome. J Biochem. 1984 Dec;96(6):1915–1923. doi: 10.1093/oxfordjournals.jbchem.a135026. [DOI] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y., Nishiguchi M., Kiho Y. Single amino acid substitution in 30K protein of TMV defective in virus transport function. Virology. 1983 Nov;131(1):255–258. doi: 10.1016/0042-6822(83)90551-2. [DOI] [PubMed] [Google Scholar]
- Takamatsu N., Ishikawa M., Meshi T., Okada Y. Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J. 1987 Feb;6(2):307–311. doi: 10.1002/j.1460-2075.1987.tb04755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamatsu N., Ohno T., Meshi T., Okada Y. Molecular cloning and nucleotide sequence of the 30K and the coat protein cistron of TMV (tomato strain) genome. Nucleic Acids Res. 1983 Jun 11;11(11):3767–3778. doi: 10.1093/nar/11.11.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Meshi T., Okada Y. The initiation site for transcription of the TMV 30-kDa protein messenger RNA. FEBS Lett. 1984 Jul 23;173(1):247–250. doi: 10.1016/0014-5793(84)81056-x. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Morita N., Nishiguchi M., Okada Y. Attenuated strains of tobacco mosaic virus. Reduced synthesis of a viral protein with a cell-to-cell movement function. J Mol Biol. 1987 Apr 20;194(4):699–704. doi: 10.1016/0022-2836(87)90247-6. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Ooshika I., Meshi T., Okada Y. Subcellular localization of the 30K protein in TMV-inoculated tobacco protoplasts. Virology. 1986 Jul 30;152(2):414–420. doi: 10.1016/0042-6822(86)90143-1. [DOI] [PubMed] [Google Scholar]
- Zimmern D., Hunter T. Point mutation in the 30-K open reading frame of TMV implicated in temperature-sensitive assembly and local lesion spreading of mutant Ni 2519. EMBO J. 1983;2(11):1893–1900. doi: 10.1002/j.1460-2075.1983.tb01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]