Abstract
Objective
To investigate the association between arginine vasopressin (AVP) levels and loop diuretic (LD) therapy in patients with heart failure and to determine if AVP levels are a prognostic indicator of treatment failure.
Methods
Patients with stable heart failure and reduced (< 40%) left ventricular ejection fraction (LVEF) were divided into those treated with (LD) or without LD (NLD). The LD group was separated into subgroups of high (> 6.5 pg/dl) and low (≤ 6.5 pg/dl) AVP levels. The clinical and biochemical characteristics of the two groups were compared and the prognostic value of AVP levels in heart failure evaluated.
Results
Of the 63 patients enrolled into the study, 41 (65.1%) were in the LD group and 22 (34.9%) were in the NLD group. Despite no differences between groups in LVEF, creatinine clearance, or brain natriuretic peptide, the LD group had significantly higher AVP levels compared with the NLD group. A Cox proportional-hazards model showed that AVP was an independent predictor of adverse events. In addition, the elevation in AVP in the LD group was inversely correlated with an increase in free water clearance but not serum osmolality and was related to poor outcome.
Conclusions
Elevated AVP levels in patients with heart failure who received LD therapy were associated with a poor prognosis. Loop diuretics may induce non-osmolar AVP release, which can worsen heart failure.
Keywords: Heart failure, arginine vasopressin, loop diuretic therapy
Introduction
Guidelines from the European Society of Cardiology (ESC) and American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) recommend loop diuretic therapy for the management of fluid overload in heart failure.1,2 Most patients with decompensated heart failure are treated primarily with intravenous loop diuretics at a dose that achieves a favourable response, but a proportion of these patients show definite diuretic resistance.3 Diuretic resistance can lead to refractory fluid overload, which requires a larger dose of loop diuretic administration and can result in worsening of renal function and a poor prognosis for patients with heart failure.4–8
Several studies have examined diminished renal response to diuretic therapy as loop diuretics can have post-diuretic effects on renal tubular function (e.g., rebound sodium retention).9–12 Indeed, renal adaptation to forced diuresis has been considered as a potential mechanism underlying loop diuretic resistance.9–12 However, the mechanisms of diuretic resistance have not been fully elucidated and remain an important issue in the management of patients with heart failure.13,14
Chronic heart failure is associated with increased neurohormonal activation and alterations in autonomic control and these compensatory mechanisms have a key role in the development and progression of congestion in heart failure.15,16 For example, the secretion of arginine vasopressin (AVP) causes increased reabsorption of free water that can contribute to fluid overload.17,18 Loop diuretic therapy is reported to elevate AVP levels, but the clinical importance of this effect is not clear.19–21 We hypothesized that an elevation in AVP associated with loop diuretic administration in patients with heart failure may be indicative of diuretic resistance and so lead to a poor prognosis. Therefore, the objective of this study was to investigate the association of AVP levels with loop diuretic therapy in patients with heart failure and determine if they were a prognostic indicator of treatment failure.
Patients and methods
Study population
This prospective, observational study took place at the Department of Cardio-Renal Medicine and Hypertension, Nagoya City University Hospital, Nagoya, Japan between 1 July 2009 and 31 March 2014. Ambulatory patients with heart failure were eligible for the study providing they had reduced (< 40%) left ventricular ejection fraction (LVEF) and were in a stable condition (i.e., no dose changes in heart failure medication for more than 1 month prior to enrolment). Exclusion criteria included the following: (i) uncontrolled heart failure; (ii) haemodynamically-significant valvular heart disease; (iii) renal dysfunction; (iv) endocrine disorders; (v) uncontrolled diabetes mellitus; (vi) thiazide-type diuretic therapy; (vii) a non-cardiac condition that was likely to cause death within the following 6 months.
The study was conducted in full accordance with the Declaration of Helsinki and received approval from the Institutional Review Boards and Ethics Committees of the Nagoya City University Graduate School of Medical Sciences. Written informed consent was provided by all study participants.
Study design and methodology
Baseline demographic details and clinical characteristics including medication for heart failure were recorded at enrolment by one of three physicians (S.K., S.K., and H.S.). Blood samples were collected after more than 1 h of complete rest with the patient in the sitting position. Ethylenediaminetetra-acetic acid tubes were used and samples were incubated on ice until centrifugation at 4 ℃. Plasma was stored at −80℃ for subsequent analyses of blood biochemistry and hormone levels. Cardiac function parameters (i.e., LVEF, left ventricular (LV) end systolic volume, LV end diastolic volume and stroke volume) were measured on the day of enrolment by one of the three physicians using cardiac magnetic resonance imaging. Urine analysis was performed on specimens collected over the first 24 h after enrolment.
With the exception of AVP levels, blood and urinary biochemistry and blood hormones were analysed at a local laboratory and included assessments of serum osmolality, brain natriuretic peptide (BNP) levels, AVP levels and renal function (i.e., creatinine clearance [CrCl], fractional excretion of sodium [FENa], and free water clearance [FWC]). Free water clearance was calculated using the formula: FWC = Output of urine for 24 h × (1 - (urine osmolality/serum osmolality). The AVP levels were measured at an outside laboratory using a radioimmunoassay method (Bühlman Laboratories AG, Schonenbuch, Switzerland).
Patients were separated into those with (LD) and without (NLD) loop diuretic therapy. In addition, the LD group was further subdivided into two groups depending on the AVP level at the time of enrolment. Patients were separated into those who had high AVP values (i.e., above the upper limit of the normal range [> 6.5 pg/dl] and those who had low AVP values [≤ 6.5 pg/dl]).
Statistical analyses
Statistical analyses were performed using the SPSS® statistical package, version 23.0 (SPSS Inc., Chicago, IL, USA) for Windows®. A P-value < 0.05 was considered to indicate statistical significance. The Student’s unpaired t-test was used to compare continuous variables between groups. Categorical variables were summarized as frequencies and percentages and compared with Pearson’s χ2-test or Fisher’s exact test.
To investigate the impact of AVP elevations on the prognosis for patients with heart failure, the study endpoints (i.e. adverse events) were defined as cardiovascular death, cardiopulmonary resuscitation due to ventricular tachycardia or fibrillation and hospitalization for heart failure. Patients that received placement of an implantable cardiac defibrillator for primary prevention of death from ventricular arrhythmias during the observational period were excluded from the analysis. However, patients with successful resuscitation with a defibrillator following near fatal ventricular arrhythmias were included in the analysis. The time from enrolment to the occurrence of an adverse event or to study cut-off point (i.e., 31 March 2014) was defined as the duration of observation. The cumulative event-free survival was calculated using Kaplan–Meier product limit estimators. Survival rates were compared between groups using a log-rank test.
Associations between AVP and the other clinical variables for the whole study population and the two loop diuretic groups were determined using linear regression analyses. A Cox proportional hazards model was used to evaluate the relationship between clinical variables measured at enrolment and an adverse event. The model was adjusted for age, sex, beta blocker therapy, renin-angiotensin system inhibitor therapy and selected variables that showed a significant association with adverse events in the univariate analysis.
Results
Of the 105 patients who were eligible for enrolment, 42 were excluded for the following reasons: uncontrolled heart failure (n = 9); haemodynamically significant valvular heart disease (n = 7); renal dysfunction (n = 10); endocrine disorders (n = 6); thiazide-type diuretic therapy (n = 7); non-cardiac condition that was likely to cause death within six months (n = 3). Among the 63 patients enrolled into the study, 41 (65.1%) received loop diuretic therapy (LD group) and 22 (34.9%) did not receive loop diuretic therapy (NLD group). The mean ± SD daily loop diuretic dose in the LD group was 29.5 ± 12.3 mg/day, expressed as the furosemide equivalent dose.
Patient characteristics at the time of enrolment are summarized in Table 1. There were no differences between groups in terms of most baseline characteristics, laboratory parameters or cardiac function including LVEF, BNP, CrCl, FENa, or 24-h urine output. However, AVP levels were significantly higher in the LD group compared with the NLD group (P = 0.005). In addition, the LD group had significantly higher serum creatinine levels (P = 0.016) and urinary acid (P = 0.003) compared with the NLD group. In contrast, the NLD group had significantly higher chlorine levels compared with the LD group (P = 0.032). Linear regression analyses involving the whole population showed that serum osmolality was significantly associated with the AVP level (data not shown; r = 0.279, P = 0.031).
Table 1.
Baseline demographic and clinical characteristics of patients with stable heart failure and reduced (<40%) left ventricular ejection fraction that received loop diuretic therapy (LD) or did not receive loop diuretic therapy (NLD).
| Characteristic | All patients | NLD group | LD group | Statistical significancea |
|---|---|---|---|---|
| (n = 63) | (n = 22) | (n = 41) | ||
| Age, years | 66.8 ± 11.6 | 66.3 ± 12.2 | 67.4 ± 11.2 | NS |
| Men | 45 (71.4) | 13 (59.1) | 32 (78.0) | NS |
| BMI, kg/m2 | 23.0 ± 3.2 | 22.7 ± 3.0 | 23.0 ± 3.3 | NS |
| Systolic BP, mmHg | 118 ± 15 | 121 ± 13 | 116 ± 16 | NS |
| Ischaemic heart disease | 14 (22.2) | 8 (36.4) | 6 (14.6) | NS |
| Blood biochemistry | ||||
| Sodium, mmol/l | 140.8 ± 3.6 | 141.9 ± 2.1 | 140.3 ± 4.0 | NS |
| Potassium, mmol/l | 4.4 ± 0.4 | 4.4 ± 0.3 | 4.3 ± 0.5 | NS |
| Chlorine, mmol/l | 103.0 ± 3.5 | 104.5 ± 2.8 | 102.4 ± 3.8 | P = 0.032 |
| BUN, mg/dl | 19.6 ± 9.0 | 17.1±5.2 | 20.9±10.1 | NS |
| Creatinine, mg/dl | 0.98 ± 0.35 | 0.84 ± 0.22 | 1.05 ± 0.38 | P = 0.016 |
| Serum osmolality, mOsm/kg | 287.0 ± 7.3 | 287.5 ± 4.5 | 286.9 ± 8.4 | NS |
| Urinary acid, mg/dl | 7.2 ± 1.8 | 6.3 ± 1.1 | 7.7 ± 1.9 | P = 0.003 |
| Total protein, g/dl | 7.1 ± 0.6 | 7.1 ± 0.5 | 7.0 ± 0.6 | NS |
| Albumin, g/dl | 4.1 ± 0.5 | 4.1 ± 0.4 | 4.1 ± 0.5 | NS |
| Total bilirubin, mg/dl | 0.7 ± 0.3 | 0.8 ± 0.4 | 0.7 ± 0.3 | NS |
| Haemoglobin, g/dl | 13.7 ± 1.9 | 13.7 ± 1.9 | 13.6 ± 2.0 | NS |
| Glucose, mg/dl | 109 ± 25 | 107 ± 19 | 109 ± 28 | NS |
| CRP, mg/dl | 0.33 ± 0.65 | 0.30 ± 0.61 | 0.35 ± 0.68 | NS |
| Blood hormone level | ||||
| TSH, µI | 2.56 ± 2.54 | 2.12 ± 1.14 | 2.85 ± 3.05 | NS |
| FT3, pg/ml | 3.22 ± 0.44 | 3.30 ± 0.31 | 3.16 ± 0.49 | NS |
| FT4, ng/dl | 1.01 ± 0.20 | 0.99 ± 0.17 | 1.01 ± 0.21 | NS |
| Cortisol, µg/dl | 14.5 ± 4.8 | 13.9 ± 4.9 | 14.7 ± 4.7 | NS |
| Renin, ng/ml per h | 4.53 ± 15.25 | 1.40 ± 1.68 | 6.25 ± 18.81 | NS |
| Aldosterone, pg/ml | 199.2 ± 722.1 | 95.8 ± 63.8 | 248.8 ± 882.7 | NS |
| Noradrenaline, pg/ml | 581.7 ± 317.1 | 541.0 ± 305.5 | 590.0 ± 326.1 | NS |
| AVP, pg/ml | 2.05 (1.38, 4.15) | 1.65 (1.13, 1.90) | 2.45 (1.88, 5.05) | P = 0.005 |
| BNP, pg/ml | 133 (56, 287) | 121 (86, 214) | 158 (31, 368) | NS |
| Urinary biochemistry NS | ||||
| Output of urine, ml/day | 1439 ± 493 | 1308 ± 400 | 1471 ± 552 | NS |
| CrCl, ml/min | 67.2 ± 25.8 | 73.7 ± 29.1 | 62.5 ± 23.1 | NS |
| FENa, % | 0.93 ± 0.50 | 0.81 ± 0.24 | 0.98 ± 0.58 | NS |
| Albumin, mg/day | 40.7 ± 72.7 | 28.8 ± 45.7 | 45.4 ± 82.4 | NS |
| Osmolality, mOsm/kg | 404.2 ± 138.6 | 444.0 ± 156.0 | 386.9 ± 126.3 | NS |
| FWC, ml/dayb | –466.7 ± 586.5 | –640.2 ± 633.9 | –371.0 ± 535.2 | NS |
| Cardiac function | ||||
| LVEF, % | 30.8 ± 14.1 | 33.0 ± 15.4 | 29.7 ± 13.1 | NS |
| SV, ml | 47.9 ± 15.0 | 45.9 ± 11.6 | 49.8 ± 18.4 | NS |
| LVEDV, ml | 177.7 ± 70.5 | 158.7 ± 58.4 | 188.1 ± 74.9 | NS |
| LVESV, ml | 129.8 ± 68.9 | 112.7 ± 59.3 | 138.3 ± 71.3 | NS |
| Medication for heart failure | ||||
| Beta blocker | 51 (81.0) | 18 (81.8) | 33 (80.5) | NS |
| ACEI/ARB | 42 (66.7) | 11 (50.0) | 31 (75.6) | NS |
| Aldosterone blocker | 25 (39.7) | 6 (27.3) | 19 (46.3) | NS |
Data are presented as mean ± SD, n of patients (%) or median (25th, 75th percentile).
Student’s unpaired t-test for continuous variables and Pearson’s χ2-test or Fisher’s exact test for categorical variables.
Negative values for free water clearance (FWC) indicate reabsorption of excess free water. FWC was calculated using the formula: FWC = Output of urine for 24 h × (1 - (urine osmolality/serum osmolality).
BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CRP, C-reactive protein; TSH, thyroid-stimulating hormone; FT3, free thyroid 3 hormone; FT4, free thyroid 4 hormone; AVP, arginine vasopressin; BNP, brain natriuretic peptide; CrCl, creatinine clearance; FENa, fractional excretion of sodium; FWC, free water clearance; LVEF, left ventricular ejection fraction; SV, stroke volume; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; NS, no statistically significant between-group difference (P ≥ 0.05).
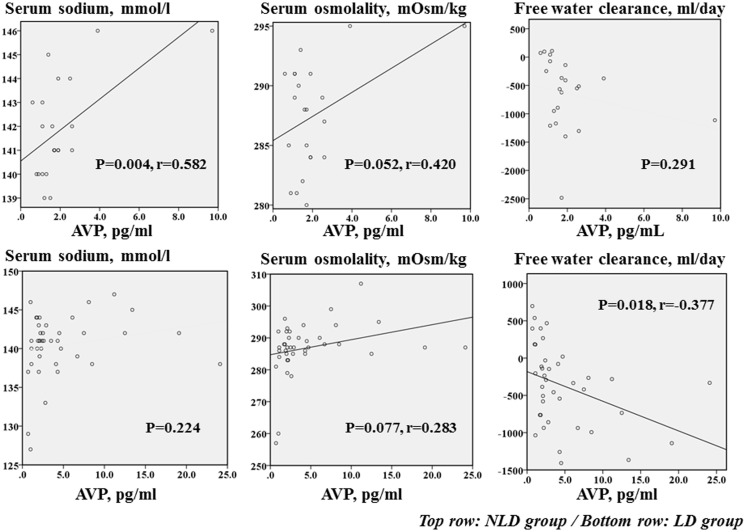
In the LD group, serum sodium and serum osmolality were not correlated with the AVP level but free water clearance was significantly inversely correlated with the AVP level (r = –0.377, P = 0.018). In the NLD group, serum sodium was significantly, positively correlated with the AVP level (r = 0.582, P = 0.004) (Figure 1). However, in the NLD group, although serum osmolality tended to be positively correlated with the AVP level it showed no correlation with free water clearance. No significant correlations were observed between AVP levels and LVEF, BNP, or CrCl in both groups (data not shown).
Figure 1.
Linear regression analyses showing the correlations between arginine vasopressin (AVP) levels and serum sodium, serum osmolality and free water clearance in patients with heart failure on loop diuretic therapy (LD; bottom row) or without loop diuretic therapy (NLD; top row).
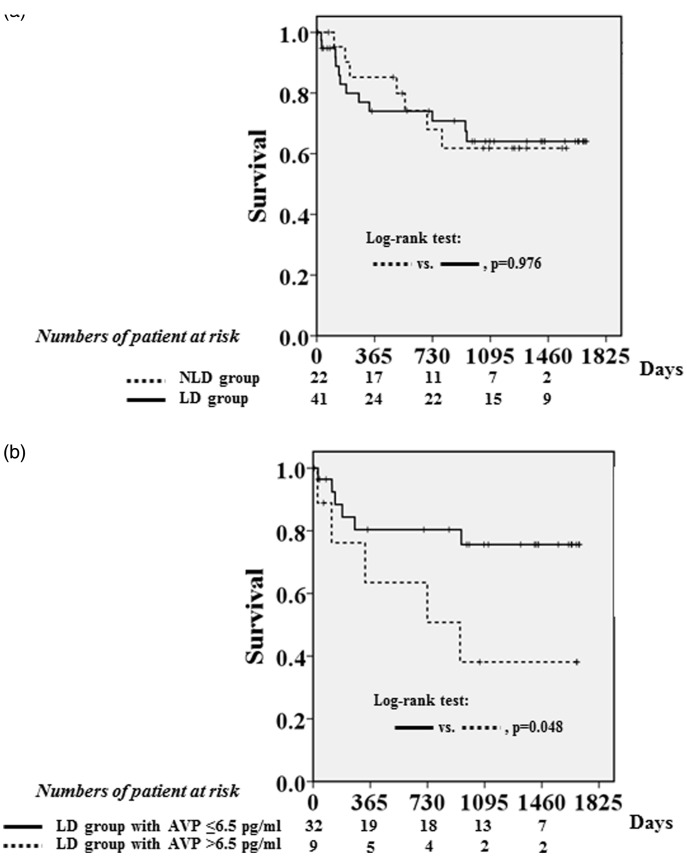
Four cardiovascular deaths, one cardioversion for the occurrence of sustained ventricular tachycardia and 14 hospitalizations for heart failure were documented during the follow-up period (median: 938 days, 25th, 75th percentile: 186 to 1325 days). There was no significant difference in the event-free survival between the LD and NLD groups (Figure 2a). The cumulative event-free survival was compared between the two LD subgroups. Patients with a high AVP level had a significantly lower event-free survival rate than patients with a low AVP level (P = 0.048) (Figure 2b).
Figure 2.
(a) Adverse event-free survival in patients treated with loop diuretic therapy (LD; solid line) or without loop diuretic therapy (NLD; dashed line). There was no significant difference in event-free survival between the LD and NLD groups. (b) Adverse event-free survival in patients treated with loop diuretic therapy (LD) and elevated arginine vasopressin (AVP) levels (>6.5 pg/ml; dashed line) or low AVP levels (≤6.5 pg/ml; solid line). Patients with a high AVP level had a significantly lower event-free survival rate than patients with a low AVP level (P = 0.048). Adverse events included cardiovascular death, cardiopulmonary resuscitation due to ventricular tachycardia or fibrillation and hospitalization for heart failure.
A univariate Cox proportional hazards model analysis involving all patients showed that the LVEF, BNP, renin-angiotensin system inhibitor therapy and AVP levels were significantly associated with adverse events (P < 0.05 for all) (Table 2). In addition, after adjusting for age, sex, beta blocker therapy, renin-angiotensin system inhibitor therapy, LVEF, and BNP levels, a multivariate analysis showed that AVP levels were significant independent predictors of adverse events (P = 0.002).
Table 2.
Univariate and multivariate regression analyses of the association between baseline clinical characteristics and adverse events in patients with heart failure.a
| Characteristic | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | Statistical significance | HR (95% CI) | Statistical significance | |
| Age, years | 1.00 (0.96, 1.04) | NS | NA | |
| Male sex | 0.62 (0.24, 1.57) | NS | NA | |
| LVEF, % | 0.93 (0.88, 0.98) | P = 0.004 | 0.91 (0.85, 0.98) | P = 0.018 |
| BNP, pg/ml | 1.00 (1.00, 1.00) | P = 0.002 | 1.001 (1.00, 1.00) | NS |
| AVP, pg/ml | 1.09 (1.01, 1.19) | P = 0.032 | 1.218 (1.08, 1.38) | P = 0.002 |
| CrCl, ml/min | 0.99 (0.97, 1.01) | NS | ||
| Beta blocker | 0.94 (0.31, 2.83) | NS | NA | |
| ACEI/ARB | 0.28 (0.11, 0.70) | P = 0.006 | 0.22 (0.08, 0.62) | P = 0.004 |
Adverse events included cardiovascular death, cardiopulmonary resuscitation due to ventricular tachycardia or fibrillation and hospitalization for heart failure.
HR, hazard ratio; CI, confidence interval, LVEF, left ventricular ejection fraction; BNP, brain natriuretic peptide; AVP, arginine vasopressin; CrCl, creatinine clearance; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; NA, not applicable; NS, not statistically significant.
Although there were no significant differences between LD subgroups in LVEF, BNP, CrCl, FENa or daily dose of LD, compared with the low AVP group, patients in the high AVP group demonstrated significantly lower daily urinary output (P = 0.029) and lower free water clearance (P = 0.025) (Table 3). The high AVP group also had a significantly higher serum osmolality compared with the low AVP group (P = 0.031).
Table 3.
Characteristics of patients with heart failure on loop diuretic therapy with high (> 6.5 pg/ml) or low (≤ 6.5 pg/ml) arginine vasopressin (AVP) levels.
| Variable | High AVP levels | Low AVP levels | Statistical significancea |
|---|---|---|---|
| (n = 9) | (n = 32) | ||
| Age, years | 63.1 ± 13.9 | 68.3 ± 10.3 | NS |
| Men | 6 (66.7) | 26 (81.3) | NS |
| BMI, kg/m2 | 24.4 ± 3.3 | 22.8 ± 3.2 | NS |
| Systolic BP, mmHg | 116 ± 18 | 116 ± 16 | NS |
| Heart rate, beats/min | 76 ± 18 | 72 ± 11 | NS |
| Ischaemic heart disease | 1 (11.1) | 5 (15.6) | NS |
| Blood biochemistry | |||
| Sodium, mmol/l | 142.1 ± 3.4 | 139.9 ± 4.1 | NS |
| Potassium, mmol/l | 4.3 ± 0.3 | 4.4 ± 0.5 | NS |
| Chlorine, mmol/l | 104.1 ± 2.9 | 102.0 ± 4.0 | NS |
| BUN, mg/dl | 24.9 ± 14.9 | 19.7 ± 8.4 | NS |
| Creatinine, mg/dl | 1.13 ± 0.52 | 1.03 ± 0.34 | NS |
| Serum osmolality, mOsm/kg | 292.2 ± 7.2 | 285.4 ± 8.3 | P = 0.031 |
| Urinary acid, mg/dl | 8.8 ± 2.2 | 7.4 ± 1.8 | NS |
| Total protein, g/dl | 7.3 ± 0.4 | 6.9 ± 0.7 | NS |
| Albumin, g/dl | 4.2 ± 0.6 | 4.1 ± 0.5 | NS |
| Total bilirubin, mg/dl | 0.8 ± 0.4 | 0.7 ± 0.3 | NS |
| Haemoglobin, g/dl | 13.1 ± 2.4 | 13.7 ± 1.9 | NS |
| Glucose, mg/dl | 103 ± 15 | 110 ± 30 | NS |
| CRP, mg/dl | 0.27 ± 0.48 | 0.36 ± 0.74 | NS |
| Blood hormone level | |||
| TSH, µI | 4.01 ± 4.81 | 2.51 ± 2.33 | NS |
| FT3, pg/ml | 3.34 ± 0.27 | 3.11 ± 0.53 | NS |
| FT4, ng/dl | 0.92 ± 0.16 | 1.03 ± 0.22 | NS |
| Cortisol, µg/dl | 15.7 ± 6.3 | 14.5 ± 4.2 | NS |
| Renin, ng/ml per h | 3.31 ± 3.80 | 7.10 ± 21.28 | NS |
| Aldosterone, pg/ml | 105.1 ± 73.9 | 290.5 ± 1001.8 | NS |
| Noradrenaline, pg/ml | 746.8 ± 235.7 | 544.0 ± 337.5 | NS |
| AVP, pg/ml | 11.20 (8.10, 13.40) | 2.10 (1.75, 2.85) | P < 0.001 |
| BNP, pg/ml | 158 (13, 397) | 152 (40, 362) | NS |
| Urinary biochemistry | |||
| Output of urine, ml/day | 1244 ± 214 | 1541 ± 611 | P = 0.029 |
| CrCl, ml/min | 66.5 ± 27.8 | 61.7 ± 22.3 | NS |
| FENa, % | 1.04 ± 0.39 | 0.97 ± 0.64 | NS |
| Albumin, mg/day | 49.9 ± 87.5 | 40.4 ± 80.8 | NS |
| Osmolality, mOsm/kg | 457.4 ± 79.2 | 366.7 ± 132.9 | NS |
| FWC, ml/dayb | –719.2 ± 411.7 | –264.3 ± 537.5 | P = 0.025 |
| Cardiac function | |||
| LVEF, % | 32.3 ± 14.2 | 29.4 ± 12.9 | NS |
| SV, ml | 51.6 ± 13.6 | 49.5 ± 19.9 | NS |
| LVEDV, ml | 185.0 ± 78.9 | 186.6 ± 75.1 | NS |
| LVESV, ml | 133.4 ± 80.8 | 137.1 ± 69.3 | NS |
| Medication for heart failure | |||
| Beta blocker | 9 (100.0) | 23 (71.9) | NS |
| ACEI/ARB | 7 (77.8) | 24 (75.0) | NS |
| Aldosterone blocker | 3 (33.3) | 15 (46.9) | NS |
| Dose of loop diuretic, mg/dayc | 33.3 ± 14.1 | 28.6 ± 11.9 | NS |
Data are presented as mean ± SD, n of patients (%) or median (25th, 75th percentile).
Student’s unpaired t-test for continuous variables and Pearson’s χ2-test or Fisher’s exact test for categorical variables.
Negative values for free water clearance (FWC) indicate reabsorption of excess free water. FWC was calculated using the formula: FWC = Output of urine for 24 h × (1 - (urine osmolality/serum osmolality).
The daily loop diuretic dose is expressed as the furosemide equivalent dose.
BMI, body mass index; BP, blood pressure; BUN, blood urea nitrogen; CRP, C-reactive protein; TSH, thyroid-stimulating hormone; FT3, free thyroid 3 hormone; FT4, free thyroid 4 hormone; AVP, arginine vasopressin; BNP, brain natriuretic peptide; CrCl, creatinine clearance; FENa, fractional excretion of sodium; FWC, free water clearance; LVEF, left ventricular ejection fraction; SV, stroke volume; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; NS, no statistically significant between-group difference (P ≥ 0.05).
Discussion
The current study demonstrated four major findings. First, patients receiving loop diuretic therapy had significantly higher AVP levels than those who did not receive this therapy. Secondly, the AVP level was an independent predictor of adverse events in patients with stable heart failure and reduced LVEF. Thirdly, among patients on loop diuretic therapy, those with elevated AVP levels (> 6.5 pg/dl) had a significantly worse prognosis than those with low AVP levels. Finally, patients on loop diuretic therapy with high AVP levels demonstrated reduced daily urine output and decreased free water clearance (i.e., increased free water reabsorption) compared with those with low AVP levels.
Arginine vasopressin is synthesized in the hypothalamus and stored in the posterior pituitary gland.22 An increase in serum osmolality, as well as a variety of non-osmolar stimuli can cause AVP release.23,24 Indeed, several vasopressin receptor subtypes exist and include the V1a receptor, which can be found on blood vessels and the myocardium; and the V2 receptor, which is present on renal collecting tubules.25,26 Both V1a-mediated vasoconstriction and V2-mediated free water retention are pathophysiologically associated with worsening congestion in heart failure.27,28 Several reports have shown that oral AVP V2 receptor antagonists can increase the urine volume in patients with heart failure and reduce volume overload, which in turn relieves the symptoms of heart failure.29–32 These observations suggest that worsening congestion and heart failure symptoms may be profoundly affected by the activation of the AVP system. In addition, previous studies have demonstrated that plasma AVP levels were increased in patients with heart failure and elevated AVP levels were correlated with symptoms, disease severity and increased morbidity and mortality.17,18,19,33–35 In a subgroup analysis of the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) study, investigators demonstrated that an elevated baseline AVP level (> 8 pg/dl) was independently associated with poor long-term outcomes, including death, in patients with heart failure and reduced LVEF.36 In addition, another study showed that elevated AVP levels (≥ 5.3 pg/dl) due to the non-osmotic AVP secretion pathway were significantly associated with a poor prognosis in patients with hyponatraemia and severe heart failure.37 These observations are consistent with these current findings that an elevation in AVP was a predictor of adverse events in heart failure.
Although AVP levels are elevated in patients with heart failure on loop diuretics,20,38 the clinical implications of an increase in AVP associated with loop diuretic therapy are not known. In the current study, despite the lack of differences between groups in baseline LVEF, BNP, or CrCl, patients in the LD group with relatively high baseline AVP levels (> 6.5 pg/dl) had a significantly worse prognosis than those with low AVP levels. These current observations suggest that patients with stable heart failure and high AVP levels are likely to have a poor prognosis following loop diuretic therapy.
Arginine vasopressin secretion is regulated by osmolality receptors and other receptors unrelated to osmosis. For example, in healthy subjects, AVP secretion is closely regulated by receptors that are sensitive to small variations in serum osmolality.39 However, in patients with heart failure, significantly high AVP levels are thought to be associated with a non-osmotic AVP release pathway that involves baroreceptors that detect arterial underfilling.20,36,40 Accordingly, this present study found serum sodium and serum osmolality were positively correlated with AVP levels in the NLD group but not in the LD group. In contrast, serum AVP levels were inversely correlated with free water clearance in the LD group, but not in the NLD group. Overall, in the LD group, patients with high AVP levels had significantly less 24-h urine output and significantly lower free water clearance than patients with low AVP levels. These current observations suggest that LD administration may increase AVP release via a non-osmotic pathway that worsens fluid retention and overload and results in a poor prognosis in patients with heart failure.
The present study had several limitations. First, the cohort size was small, the study was conducted at a single institution and it did not assess inter-assessor reliability; these factors may well have affected the study outcome. More multicentre, prospective, randomized studies are required to confirm these current findings. Secondly, several exclusion criteria may have inadvertently influenced the results and created a patient selection bias. Thirdly, circulating AVP has a short half-life and it is primarily bound to platelets; these features have rendered AVP measurements troublesome for clinical use.41,42 Therefore, repeated measurements of AVP levels and analysis of continuous changes in AVP may be required to clarify the importance of AVP as a predictor of adverse outcomes in heart failure. Finally, the present study did not address patient clinical history (e.g., duration of heart disease, number of hospitalizations). Therefore, it failed to determine the optimal timing of AVP measurements following onset of heart failure, which may have been crucial in determining a reliable prognostic indicator.
In conclusion, this present study showed that the administration of a loop diuretic was associated with an elevation in AVP levels, which was independent of serum sodium and osmolality, and reduced urine output and decreased free water clearance (i.e., increased free water reabsorption). Therefore, loop diuretics may induce non-osmolar AVP release, which can worsen heart failure. Although further studies are needed to confirm the current findings, these results indicated that an elevation in AVP may be a useful predictor of adverse events in patients with stable heart failure, reduced LVEF and receiving loop diuretic therapy.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
The authors have no financial relationship with any commercial entity that has an interest in the subject of this manuscript. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology: developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation 2013; 128: e240–e327. [DOI] [PubMed] [Google Scholar]
- 3.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006; 97: 1759–1764. [DOI] [PubMed] [Google Scholar]
- 4.Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 2004; 147: 331–338. [DOI] [PubMed] [Google Scholar]
- 5.Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol 2006; 47: 1987–1996. [DOI] [PubMed] [Google Scholar]
- 6.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail 2007; 13: 599–608. [DOI] [PubMed] [Google Scholar]
- 7.Peacock WF, Costanzo MR, De Marco T, et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology 2009; 113: 12–19. [DOI] [PubMed] [Google Scholar]
- 8.Metra M, Davison B, Bettari L, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 2012; 5: 54–62. [DOI] [PubMed] [Google Scholar]
- 9.Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology 2001; 96: 132–143. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki T, Fujiki H, Yamamura Y, et al. Tolvaptan, an orally active vasopressin V(2)-receptor antagonist - pharmacology and clinical trials. Cardiovasc Drug Rev 2007; 25: 1–13. [DOI] [PubMed] [Google Scholar]
- 11.Ernst ME, Moser M. Use of diuretics in patients with hypertension. N Engl J Med 2009; 361: 2153–2164. [DOI] [PubMed] [Google Scholar]
- 12.Jentzer JC, DeWald TA, Hernandez AF. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol 2010; 56: 1527–1534. [DOI] [PubMed] [Google Scholar]
- 13.Goldsmith SR, Bart BA, Burnett J. Decongestive therapy and renal function in acute heart failure: time for a new approach? Circ Heart Fail 2014; 7: 531–535. [DOI] [PubMed] [Google Scholar]
- 14.Testani JM, Brisco MA, Turner JM, et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014; 7: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. JACC 1992; 20: 248–254. [DOI] [PubMed] [Google Scholar]
- 16.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med 1999; 8: 577–585. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Funayama H, Yoshimura A, et al. Possible vascular role of increased plasma arginine vasopressin in congestive heart failure. Int J Cardiol 2006; 106: 191–195. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol 2005; 95: 8B–13B. [DOI] [PubMed] [Google Scholar]
- 19.Francis GS, Benedict C, Johnstone DE, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the studies of left ventricular dysfunction (SOLVD). Circulation 1990; 82: 1724–1729. [DOI] [PubMed] [Google Scholar]
- 20.Szatalowicz VL, Arnold PE, Chaimovitz C, et al. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med 1981; 305: 263–266. [DOI] [PubMed] [Google Scholar]
- 21.Goldsmith SR, Francis GS, Cowley AW, Jr, et al. Increased plasma arginine vasopressin levels in patients with congestive heart failure. JACC 1983; 1: 1385–1390. [DOI] [PubMed] [Google Scholar]
- 22.Koshimizu TA, Nakamura K, Egashira N, et al. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev 2012; 92: 1813–64. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith SR, Cowley AW, Jr, Francis GS, et al. Reflex control of osmotically stimulated vasopressin in normal humans. Am J Physiol 1985; 248(6 Pt 2): R660–R663. [DOI] [PubMed] [Google Scholar]
- 24.Ambrosy A, Goldsmith SR, Gheorghiade M. Tolvaptan for the treatment of heart failure: a review of the literature. Expert Opin Pharmacother 2011; 12: 961–976. [DOI] [PubMed] [Google Scholar]
- 25.Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol 2005; 46: 1785–1791. [DOI] [PubMed] [Google Scholar]
- 26.Finley JJ, 4th, Konstam MA, Udelson JE. Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia. Circulation 2008; 118: 410–421. [DOI] [PubMed] [Google Scholar]
- 27.Penit J, Faure M, Jard S. Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol 1983; 244: E72–E82. [DOI] [PubMed] [Google Scholar]
- 28.Goldsmith SR. Vasopressin receptor antagonists: mechanisms of action and potential effects in heart failure. Cleve Clin J Med 2006; 73(Suppl 3): S20–S23. [DOI] [PubMed] [Google Scholar]
- 29.Gassanov N, Semmo N, Semmo M, et al. Arginine vasopressin (AVP) and treatment with arginine vasopressin receptor antagonists (vaptans) in congestive heart failure, liver cirrhosis and syndrome of inappropriate antidiuretic hormone secretion (SIADH). Eur J Clin Pharmacol 2011; 67: 333–346. [DOI] [PubMed] [Google Scholar]
- 30.Kinugawa K, Sato N, Inomata T, et al. Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ J 2014; 78: 844–852. [PubMed] [Google Scholar]
- 31.Gheorghiade M, Konstam MA, Burnett JC, Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007; 297: 1332–1343. [DOI] [PubMed] [Google Scholar]
- 32.Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 2008; 371: 1624–1632. [DOI] [PubMed] [Google Scholar]
- 33.Benedict CR, Johnstone DE, Weiner DH, et al. Relation of neurohumoral activation to clinical variables and degree of ventricular dysfunction: a report from the registry of studies of left ventricular dysfunction. SOLVD Investigators. J Am Coll Cardiol 1994; 23: 1410–1420. [DOI] [PubMed] [Google Scholar]
- 34.Rouleau JL, Packer M, Moyé L, et al. Prognostic value of neurohumoral activation in patients with an acute myocardial infarction: effect of captopril. J Am Coll Cardiol 1994; 24: 583–591. [DOI] [PubMed] [Google Scholar]
- 35.Funayama H, Nakamura T, Saito T, et al. Urinary excretion of aquaporin-2 water channel exaggerated dependent upon vasopressin in congestive heart failure. Kidney Int 2004; 66: 1387–1392. [DOI] [PubMed] [Google Scholar]
- 36.Lanfear DE, Sabbah HN, Goldsmith SR, et al. Association of arginine vasopressin levels with outcomes and the effect of V2 blockade in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail 2013; 6: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imamura T, Kinugawa K, Hatano M, et al. Low cardiac output stimulates vasopressin release in patients with stage d heart failure. Circ J 2014; 78: 2259–2267. [DOI] [PubMed] [Google Scholar]
- 38.Balling L, Kistorp C, Schou M, et al. Plasma copeptin levels and prediction of outcome in heart failure outpatients: relation to hyponatremia and loop diuretic doses. J Card Fail 2012; 18: 351–358. [DOI] [PubMed] [Google Scholar]
- 39.Robertson GL, Mahr EA, Athar S, et al. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest 1973; 52: 2340–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa SE, Schrier RW. Pathophysiological roles of arginine vasopressin and aquaporin-2 in impaired water excretion. Clin Endocrinol (Oxf) 2003; 58: 1–17. [DOI] [PubMed] [Google Scholar]
- 41.Robertson GL, Athar S. The interaction of blood osmolality and blood volume in regulating plasma vasopressin in man. J Clin Endocrinol Metab 1976; 42: 613–620. [DOI] [PubMed] [Google Scholar]
- 42.Preibisz JJ, Sealey JE, Laragh JH, et al. Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension 1983; 5: I129–138. [DOI] [PubMed] [Google Scholar]