Abstract
Objective
To investigate the effect of sperm DNA fragmentation on the fertilization rate, embryo development and pregnancy outcome of in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in a cohort of Chinese couples.
Methods
Infertile couples that had undergone assisted reproductive technology at our centre between January 2011 and December 2013 were included in this retrospective study. Fractions of prepared sperm samples were evaluated for sperm DNA fragmentation on the day of oocyte recovery.
Results
Of the 550 couples selected, 415 had undergone IVF and 135 ICSI. Sperm DNA fragmentation rate was significantly negatively correlated with the fertilization rate in the ICSI cycles but not the IVF cycles. No association was found between sperm DNA fragmentation and cleavage rate or good quality embryo formation rates in IVF or ICSI cycles. Receiver operating characteristic (ROC) curve analysis showed that the sperm DNA fragmentation rate was a statistically significant prognostic indicator of the clinical fertilization rate in ICSI cycles; a rate > 22.3% was associated with a lower fertilization rate following ICSI compared with a rate ≤ 22.3%.
Conclusions
High values of sperm DNA fragmentation were associated with a low fertilization rate following ICSI but were not associated with alterations in pregnancy or live birth rates in either ICSI or IVF in this cohort of Chinese couples.
Keywords: Sperm DNA fragmentation, in vitro fertilization, intracytoplasmic sperm injection, fertilization rate
Introduction
Assisted reproductive technology has revolutionized reproductive medicine and the treatment of infertility.1 However, several factors may affect the fertilization rate, embryo development and process of embryo transplantation.2,3 For example, women undergoing in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) may benefit from recombinant human luteinizing hormone supplementation during the early follicular phase,2 and intrauterine human chorionic gonadotropin (hCG) injections before embryo transfer.3 For men, semen parameters, semen quality and sperm function may affect the success of IVF/ICSI.4–6 Sperm DNA fragmentation is an important parameter that has been suggested as a potential prognostic indicator of fertilization rate, embryo development, pregnancy rate and outcome.7–9
Sperm DNA integrity plays an important role in mammalian fertilization and subsequent embryo development.10 Studies have suggested that sperm DNA fragmentation may be a useful predictor of outcome in IVF or ICSI.10–12 Indeed, several systematic reviews and meta-analyses concluded that sperm DNA damage in assisted reproductive technology was associated with a decrease in pregnancy and live birth rates and an increase in miscarriage rates.13–17 However, other studies found that sperm DNA fragmentation was not associated with pregnancy rates or clinical outcomes.18–20 In addition, a recent meta-analysis of 20 studies found no association between sperm DNA fragmentation and IVF or ICSI outcomes.21 Indeed, guidelines published by the American Society for Reproductive Medicine do not recommend the assessment of sperm DNA integrity as a predictor of treatment outcomes in assisted reproductive technology.22
Differences in study methodologies such as age and ovarian function of the women and/or use of frozen semen samples may explain inconsistencies in findings.23 Therefore, we believe that the role of sperm DNA fragmentation in IVF/ICSI warrants further study. To this end, we investigated the effect of sperm DNA fragmentation on fertilization rate, embryo development and pregnancy outcomes for IVF/ICSI cycles in in a cohort of Chinese couples.
Patients and methods
Study population
Infertile couples that had undergone assisted reproductive technology at the Reproductive and Genetic Centre, People’s Hospital of Guangxi Zhuang Autonomous Region between January 2011 and December 2013 were included in this retrospective study. The choice of the fertilization method was based on the infertility diagnosis and semen quality. For IVF this was the first procedure. Indications for ICSI were as follows: severe oligoasthenozoospermia diagnosed as a sperm concentration <5 × 106/mL and/or progressive motility less than 10%; one previous conventional IVF attempt with total fertilization failure or low fertilization rate (<30%); a total progressive motile sperm count of <1 × 106 after purification by density gradient centrifugation. A physician (B.H.) chose suitable couples for the study and inclusion criteria were: (1) female age <37 years; (2) no indication of female infertility except for simple tubal factors; (3) freshly ejaculated semen had been used for the sperm suspensions; (4) the number of metaphase II (MII) oocytes retrieved was ≥4. Men with azoospermia were excluded.
The study protocol was approved by the Ethics Committee of People’s Hospital of Guangxi Zhuang Autonomous Region and all participants provided written informed consent.
Sperm DNA fragmentation
Sperm DNA fragmentation was performed by a physician (W-Y.M.) using the Halosperm® kit (INDAS Laboratories, Madrid, Spain) according to the manufacturer’s instructions. A sample aliquot was taken from each washed semen sample. Briefly, the prepared spermatozoa were mixed with melted agarose, pipetted onto pre-coated slides and covered with a 22 × 22 mm coverslip. The slide was placed on a cold plate (4℃) for 5 min to allow the agarose to set into a microgel with sperm cells embedded. The coverslip was removed and the slide was incubated in an acid solution for 7 min, and then in lysing solution for 25 min. After washing the slide for 5 minutes with an excess of distilled water, the sample was dehydrated in increasing concentrations of ethanol (70%, 90%, 100%) each for 2 min and air-dried. After Giemsa staining, a minimum of 500 spermatozoa per sample were scored under a 400 × objective of the microscope.24 Spermatozoa with small halos (i.e., width similar to/smaller than a third of the minor diameter of the nucleus) and without halos as well as degraded sperm cells were scored as containing fragmented DNA.25,26
IVF and ICSI procedures
Ovarian stimulation was performed using gonadotropin-releasing hormone (GnRH) agonist, a GnRH antagonist and recombinant follicle-stimulating hormone/human menopausal gonadotropin.2 Monitoring was based on plasma oestradiol levels and vaginal ultrasound and was performed by two physicians (X-B.M. and J-P.C.). Ovulation was triggered with an injection of human chorionic gonadotrophin (hCG) when the ovarian follicles had reached a diameter of ≥18 mm and oocyte retrieval was performed using transvaginal ultrasound guidance 36 h later. Retrieved oocytes were incubated in G-IVFTM (Vitrolife, Göteborg, Sweden) medium supplemented with 10% human serum albumin (Vitrolife). IVF or ICSI was performed at 40 h and 42 h post-hCG, respectively. The cumulus–corona–oocyte complex was dispersed 6 h after insemination in the IVF procedure. Two physicians (L.H. and S-K W.) checked the maturity of oocytes and evaluated the presence of an extruded second polar body; those lacking a second polar body were subjected to early ‘rescue’ ICSI (data excluded from this study).
Oocytes were assessed to determine whether fertilization had occurred at 16–18 h after insemination or ICSI.27 Fertilization was considered to be normal if two pronuclei (PN) and two polar bodies were identified. The fertilization rate was calculated as the percentage of metaphase II oocytes forming two PN. At 72 hours after oocyte retrieval, embryos were classified according to cleavage and morphology score.28 Embryo quality was assessed based on the blastomeric number and symmetry and cytoplasm fragmentation. Day 3 embryos were given grades from I to IV. Grade I: embryo with 7–9 equal blastomeres and with ≤5% fragmentation. Grade II: embryo with ≥6 slightly unequal blastomeres and with 5–20% fragmentation. Grade III: embryo with ≥6 obviously unequal blastomeres and with 21–50% fragmentation or embryo with 4–5balstomeres. Grade IV: embryo with ≥6 significantly unequal blastomeres and with >50% fragmentation or embryo with <4 blastomeres. The embryo cleavage rate was calculated as the percentage of cleaved embryos based on the number of fertilized oocytes. Embryo morphology was evaluated by assessing the number of blastomeres, the degree of any fragmentation and the presence of multinuclei. Day-3 embryos were defined as ‘good’ quality if they consisted of at least six cells without multinucleation and had less than 20% fragmentation.28
Two embryos were transferred into the woman’s uterine cavity on day 3 after oocyte retrieval. Serum hCG concentrations were measured 14 days after embryo transfer. Clinical pregnancy was defined as the presence of a gestational sac confirmed by transvaginal ultrasound examination at the 4th week after embryo transfer. Live birth was defined as the birth of at least one living child after a gestation of ≥25 weeks.
Statistical analyses
Statistical analyses were performed using SPSS software (version 17.0 for Windows®; Chicago SPSS Inc. USA) and MedCalc software (version 15.6; MedCalc Software bvba Ostend, Belgium).
Continuous variables were presented as mean and standard deviation (SD). Intergroup comparisons were evaluated using the Mann–Whitney nonparametric U test. Correlations of sperm functional parameters with fertilization and embryo development rates were tested using Spearman’s coefficient of correlation. In addition, using the predicted probability as the analysed variable, a receiver operating characteristic (ROC) curve was generated to evaluate the predictive values of sperm DNA fragmentation on the fertilization rate in ICSI cycles. All statistical tests were two-sided and a P-value < 0.05 was considered to indicate statistical significance.
Results
Of the 550 consecutive infertile couples that were eligible for this retrospective study, 415 had undergone IVF and 135 had undergone ICSI during the study period. The clinical characteristics of subjects in the IVF and ICSI groups are shown in Table 1. There were no significant differences between IVF and ICSI cycles in terms of female age, male age, number of retrieved oocytes, metaphase II oocytes, fertilization rate, cleavage rate, or good quality embryo rates.
Table 1.
Baseline characteristics and clinical outcomes of the 550 Chinese couples included in the study that had undergone assisted reproductive technology.
| Characteristic | IVF | ICSI |
|---|---|---|
| No couples | 415 | 135 |
| Cycles | 415 | 135 |
| Female age, years | 30.5 ± 3.2 | 30.7 ± 3.6 |
| Male age, years | 33.0 ± 4.6 | 33.7 ± 5.3 |
| Retrieved oocytes, n | 12.7 ± 5.7 | 12.0 ± 5.3 |
| Metaphase II oocytes, n | 11.0 ± 5.2 | 9.2 ± 4.3 |
| Fertilization rate, % | 70.6 ± 18.6 | 69.0 ± 20.7 |
| Cleavage rate, % | 97.5 ± 7.4 | 96.6 ± 9.2 |
| Good quality embryo rate, % | 52.8 ± 27.2 | 46.5 ± 26.1 |
Data are presented as mean ± SD or n.
Abbreviations: IVF, vitro fertilization, ICSI, intracytoplasmic sperm injection
Spearman’s correlation analysis showed that sperm DNA fragmentation rate was negatively correlated with the fertilization rate in the ICSI cycles (r = −0.433, P < 0.001) but there was no association in the IVF cycles. No significant correlations were observed between sperm DNA fragmentation rate and cleavage rate or good quality embryo rate in either the ICSI or IVF cycles (Table 2).
Table 2.
Correlation of sperm DNA fragmentation rate with fertilization rate and embryo development status in the IVF and ICSI cycles.
| sperm DNA fragmentation |
||
|---|---|---|
| Spearman’s correlation coefficient (R) | Statistical significance | |
| IVF (n = 415) | ||
| Fertilization rate | −0.012 | ns |
| Cleavage rate | 0.081 | ns |
| Good quality embryo rate | −0.053 | ns |
| ICSI (n = 135) | ||
| Fertilization rate | −0.433 | P < 0.001 |
| Cleavage rate | −0.029 | ns |
| Good quality embryo rate | 0.086 | ns |
Abbreviations: IVF, vitro fertilization, ICSI, intracytoplasmic sperm injection; ns, not statistically significant
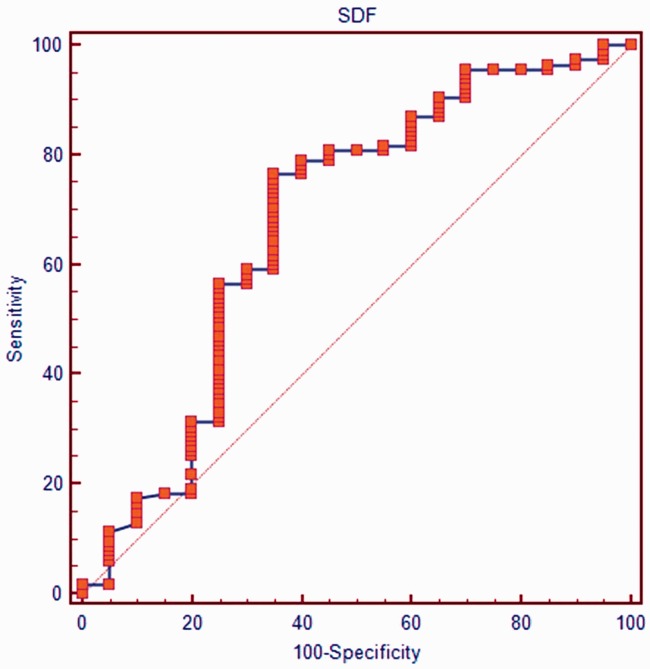
According to the ROC curve analysis of data from the 135 ICSI cycles, the sperm DNA fragmentation rate was statistically significant as a prognostic indicator of the clinical fertilization rate, with the area under the curve (AUC) 0.68 (P = 0.05; 95% Confidence Intervals, 0.59,0.77). Using this P value, the value with the best ratio of sensitivity and specificity was evaluated (Figure 1) and was found to be 22.3%, which was used as the cutoff value for predicting fertilization rates. Using this cut-off value, fertilization rates following ICSI were significantly lower in men with sperm DNA fragmentation > 22.3% than in men with sperm DNA fragmentation ≤ 22.3% (P < 0.001; Table 3). However, there were no differences between high and low sperm DNA fragmentation groups in cleavage rates, good quality embryo rates and clinical pregnancy or live birth rates. There were also no differences between groups in sperm concentration, progressive motility or normal morphology (Table 4).
Figure 1.
Receiver operating characteristic (ROC) curve for sperm DNA fragmentation (SDF) rates in men undergoing ICSI cycles. Area under the curve (AUC) = 0.68 (P = 0.05; 95% Confidence Intervals, 0.59, 0.77). The value with the best ratio of sensitivity and specificity was 22.3% which was used as the cutoff value for predicting fertilization rates in subsequent analyses.
Table 3.
Comparison of clinical outcomes for couples who underwent ICSI in relation to different sperm DNA fragmentation values.
| Outcome | Sperm DNA fragmentation* ≤ 22.3% | Sperm DNA fragmentation* > 22.3% | Statistical significance |
|---|---|---|---|
| Cycles | 95 | 40 | |
| Fertilization rate, % | 74.9 ± 18.6 | 55.1 ± 19.0 | P < 0.001 |
| Cleavage rate, % | 97.4 ± 6.9 | 94.7 ± 13.0 | ns |
| Good quality embryo rate, % | 47.0 ± 24.6 | 45.3 ± 29.6 | ns |
| Clinical pregnancy | 38 (40%) | 16 (40%) | ns |
| Live birth | 33 (35%) | 14 (35%) | ns |
Data are presented as mean ± SD, n or n (%).
Abbreviations: ICSI, intracytoplasmic sperm injection; ns, not statistically significant
According to the Receiver operating characteristic (ROC) curve analysis, the value for sperm DNA fragmentation with the best ratio of sensitivity and specificity was 22.3%, which was used as the cutoff value for predicting fertilization rates.
Table 4.
Comparison of sperm parameters in couples undergoing ICSI with different sperm DNA fragmentation rates.
| Outcome | Sperm DNA fragmentation ≤22.3% | Sperm DNA fragmentation >22.3% | Statistical significance |
|---|---|---|---|
| Cycles | 95 | 40 | |
| Sperm concentration, ×106/ml | 21.8 ± 18.8 | 26.56 ± 20.3 | ns |
| Progressive motility, % | 22.0 ± 16.4 | 22.12 ± 16.3 | ns |
| Normal morphology, % | 3.9 ± 2.9 | 3.70 ± 3.3 | ns |
Data are presented as mean ± SD or n
Discussion
According to the process of natural selection, successful conception can only occur following the fertilization of an oocyte by sperm with intact DNA. However, assisted reproductive technology has increased the possibility that abnormal spermatozoons can be used to fertilize oocytes.29 Sperm DNA fragmentation is an important parameter of sperm quality and can be used to assess sperm nuclear integrity which plays an important role in fertilization and embryo development.10 Although sperm DNA fragmentation using the Halosperm® kit has been developed as a diagnostic test of male infertility, its correlation with the outcomes of IVF/ICSI has not been established.21
Although our sample sizes were different (415 IVF vs 135 ICSI) we found no differences between the IVF and ICSI groups in terms of baseline characteristics or clinical outcomes. However, we did observe a significant negative correlation between the sperm DNA fragmentation rate and the fertilization rate in the ICSI cycles but not in the IVF cycles. These results are consistent with previous findings.7,30 For example, one study found that the concentration of sperm DNA adducts significantly diminished the fertilization rate following ICSI but not after IVF.30 Another study observed a statistically significant negative relationship between the percentage of sperm DNA fragmentation and fertilization rate during ICSI.7 By contrast, a meta-analysis of six studies concluded that men with high sperm DNA fragmentation values were more likely to benefit from ICSI treatment than IVF treatment.16 In addition, although sperm DNA fragmentation has been reported to be a stronger predictor of outcome compared with free sperm plasma DNA in both IVF and ICSI,31 some studies have reported no correlation between sperm DNA fragmentation rates and fertilization rates following ICSI.32,33 These disparities in findings may have arisen because of differences in the indications for IVF and ICSI.16
Several investigators have attempted to determine a sperm DNA fragmentation threshold that would predict clinical outcomes of IVF/ICSI. A sperm DNA fragmentation rate >25.5% was found to be associated with high probability of failure in IVF in one study,11 and a high sperm DNA fragmentation rate has been reported to affect post-implantation embryo development adversely in ICSI procedures.34 However, no difference was found between the outcomes of ICSI and IVF in a group with a DNA fragmentation index ≤30% but in a group with a value >30%, outcomes of ICSI were significantly better than those of IVF.10 In the present study, we used a threshold of 22.3% as the cutoff value and found that fertilization rates following ICSI were significantly lower in men with sperm DNA fragmentation rates >22.3% than in those with rates ≤22.3%. Nevertheless, no differences were detected between high and low threshold sperm DNA fragmentation groups in terms of sperm concentration, progressive motility or normal morphology.
We found no association between sperm DNA fragmentation and the cleavage rate or good quality embryo formation rates in the IVF and ICSI cycles. These findings are consistent with other studies that have also shown sperm DNA fragmentation is not associated with pregnancy rates or clinical outcomes of assisted reproductive technology.18–20 Another study reported that sperm DNA fragmentation did not affect embryo quality.35 One study reported that sperm DNA fragmentation had a significant negative impact on the chance of pregnancy in an infertile population using the women’s own oocytes but this disappeared when donated oocytes were used.23
A possible limitation of our study was the small sample size. Additionally, we only investigated the effect of a single variable of sperm DNA fragmentation on outcomes of IVF and ICSI, therefore, further research is required to substantiate our findings.
In conclusion, a sperm DNA fragmentation rate >22.3% was associated with a lower fertilization rate following ICSI but was not associated with alterations in pregnancy or live birth rates in this cohort of Chinese couples.
Acknowledgements
We are sincerely grateful to all of the staff of the Andrology Laboratory, Reproductive Medical and Genetic Centre, People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, China, for their excellent work.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This research was supported by a National Natural Science Fund (No. 81471515).
References
- 1.Gomel V. Reconstructive tubal microsurgery and assisted reproductive technology. Fertil Steril 2016; 105: 887–890. [DOI] [PubMed] [Google Scholar]
- 2.Hu L, Bu Z, Wang K, et al. Recombinant luteinizing hormone priming in early follicular phase for women undergoing in vitro fertilization: systematic review and meta-analysis. J Int Med Res 2014; 42: 261–269. [DOI] [PubMed] [Google Scholar]
- 3.Ye H, Hu J, He W, et al. The efficacy of intrauterine injection of human chorionic gonadotropin before embryo transfer in assisted reproductive cycles: Meta-analysis. J Int Med Res 2015; 43: 738–746. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Zhang W, Luo Y, et al. Predictive value of semen parameters in in vitro fertilisation pregnancy outcome. Andrologia 2009; 41: 111–117. [DOI] [PubMed] [Google Scholar]
- 5.Kini S, Morrell D, Thong KJ, et al. Lack of impact of semen quality on fertilization in assisted conception. Scott Med J 2010; 55: 20–32. [DOI] [PubMed] [Google Scholar]
- 6.Oehninger S, Franken DR, Sayed E, et al. Sperm function assays and their predictive value for fertilization outcome in IVF therapy: a meta-analysis. Hum Reprod Update 2000; 6: 160–168. [DOI] [PubMed] [Google Scholar]
- 7.Benchaib M, Lornage J, Mazoyer C, et al. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril 2007; 87: 93–100. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, Pan C, Fei Q, et al. Effect of sperm DNA fragmentation on the clinical outcomes for in vitro fertilization and intracytoplasmic sperm injection in women with different ovarian reserves. Fertil Steril 2015; 103: 910–916. [DOI] [PubMed] [Google Scholar]
- 9.Simon L, Liu L, Murphy K, et al. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum Reprod 2014; 29: 904–917. [DOI] [PubMed] [Google Scholar]
- 10.Bungum M, Humaidan P, Axmon A, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod 2007; 22: 174–179. [DOI] [PubMed] [Google Scholar]
- 11.López G, Lafuente R, Checa MA, et al. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J Androl 2013; 15: 790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon L, Lutton D, McManus J, et al. Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and in vitro fertilization success. Fertil Steril 2011; 95: 652–657. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Wang L, Cai J, et al. Correlation of sperm DNA damage with IVF and ICSI outcomes: a systematic review and meta-analysis. J Assist Reprod Genet 2006; 23: 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zini A, Jamal W, Cowan L, et al. Is sperm DNA damage associated with IVF embryo quality? A systematic review. J Assist Reprod Genet 2011; 28: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zini A, Boman JM, Belzile E, et al. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod 2008; 23: 2663–2668. [DOI] [PubMed] [Google Scholar]
- 16.Osman A, Alsomait H, Seshadri S, et al. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: a systematic review and meta-analysis. Reprod Biomed Online 2015; 30: 120–127. [DOI] [PubMed] [Google Scholar]
- 17.Simon L, Zini A, Dyachenko A, et al. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J Androl 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ni W, Xiao S, Qiu X, et al. Effect of sperm DNA fragmentation on clinical outcome of frozen-thawed embryo transfer and on blastocyst formation. PLoS One 2014; 9: e94956–e94956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smit M, Wissenburg OG, Romijn JC, et al. Increased sperm DNA fragmentation in patients with vasectomy reversal has no prognostic value for pregnancy rate. J Urol 2010; 183: 662–665. [DOI] [PubMed] [Google Scholar]
- 20.Anifandis G, Bounartzi T, Messini CI, et al. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia 2015; 47: 295–302. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Zhu L, Jiang H, et al. Sperm DNA fragmentation index and pregnancy outcome after IVF or ICSI: a meta-analysis. J Assist Reprod Genet 2015; 32: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril 2013; 99: 673–677. [DOI] [PubMed] [Google Scholar]
- 23.Meseguer M, Santiso R, Garrido N, et al. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril 2011; 95: 124–128. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization: World Health Organization laboratory manual for the examination and processing of human semen. Geneva, Swtizerland, 2010.
- 25.Meseguer M, Santiso R, Garrido N, et al. The effect of cancer on sperm DNA fragmentation as measured by the sperm chromatin dispersion test. Fertil Steril 2008; 90: 225–227. [DOI] [PubMed] [Google Scholar]
- 26.Fernández JL, Muriel L, Rivero MT, et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl 2003; 24: 59–66. [PubMed] [Google Scholar]
- 27.García J, Noriega-Hoces L, Gonzales GF. Sperm chromatin stability and its relationship with fertilization rate after intracytoplasmic sperm injection (ICSI) in an assisted reproduction program. J Assist Reprod Genet 2007; 24: 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu HB, Zhang ZH, Fadlalla E, et al. Culturing surplus poor-quality embryos to blastocyst stage have positive predictive value of clinical pregnancy rate. Iran J Reprod Med 2014; 12: 609–616. [PMC free article] [PubMed] [Google Scholar]
- 29.Tavukçuoğlu IŞ, Al-Azawi T, Khaki AA, et al. Clinical value of DNA fragmentation evaluation tests under ART treatments. J Turk Ger Gynecol Assoc 2012; 13: 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horák S, Olejek A, Widłak P. Sperm DNA adducts impair fertilization during ICSI but not during IVF. Folia Histochem Cytobiol 2007; 45(Suppl 1): S99–S104. [PubMed] [Google Scholar]
- 31.Bounartzi T, Dafopoulos K, Anifandis G, et al. Pregnancy prediction by free sperm DNA and sperm DNA fragmentation in semen specimens of IVF/ICSI-ET patients. Hum Fertil (Camb) 2016; 19: 56–62. [DOI] [PubMed] [Google Scholar]
- 32.Simon L, Brunborg G, Stevenson M, et al. Clinical significance of sperm DNA damage in assisted reproduction outcome. Hum Reprod 2010; 25: 1594–1608. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Wang H, Wang L, et al. The clinical significance of sperm DNA damage detection combined with routine semen testing in assisted reproduction. Mol Med Rep 2008; 1: 617–624. [DOI] [PubMed] [Google Scholar]
- 34.Borini A, Tarozzi N, Bizzaro D, et al. Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod 2006; 21: 2876–2881. [DOI] [PubMed] [Google Scholar]
- 35.Benchaib M, Braun V, Lornage J, et al. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod 2003; 18: 1023–1028. [DOI] [PubMed] [Google Scholar]