Abstract
Objective
To determine the expression and clinical significance of plasma miR-335 in patients with acute ischemic stroke (AIS) and investigate its association with calmodulin (CaM) expression.
Methods
Plasma miR-335 and CaM expression levels in patients with AIS and healthy controls were examined. Correlations between miR-335, CaM, and National Institutes of Health Stroke Scale scores were also analysed. Furthermore, the potential regulatory function of miR-335 on CaM expression was investigated.
Results
Plasma miR-335 levels were significantly lower in AIS and negatively correlated with NIHSS scores. The converse was observed for plasma CaM levels. Plasma miR-335 and CaM levels were negatively correlated. Plasma miR-335 was confirmed as a novel biomarker for AIS diagnosis and an independent predictor of AIS. Up-regulation of miR-335 suppressed CaM protein expression, and CaM was confirmed as a direct target of miR-335.
Conclusions
Plasma miR-335 was down-regulated in AIS patients and represents a potential noninvasive circulating biomarker.
Keywords: MicroRNA-335, calmodulin, acute ischemic stroke, diagnosis
Introduction
Stroke is a leading cause of long-term disability and mortality worldwide.1 Acute ischemic stroke (AIS) is a major subtype of stroke and requires time-sensitive intervention.2 Although administration of thrombolytics within 3 hours from the onset of symptoms improves the clinical outcome,3 only a small proportion of AIS patients receive thrombolytic treatment.4,5 One of the important limitations for thrombolytic use is the narrow time window for treatment. There is evidence associating diagnostic uncertainty with underuse of fibrinolytic treatment.6 However, blood samples can be easily obtained in clinical practice and could be used for diagnostic purposes. Previous studies have revealed numerous molecular changes in the blood of AIS patients.7,8 Additional research is required to better understand the molecular biology of AIS and identify potential biomarkers for early diagnosis and accurate assessment.
MicroRNAs (miRNAs) are a class of endogenous, noncoding, single stranded small regulatory RNA molecules, which are approximately 22 nucleotides in length.9 Through base-pairing to the 3′ untranslated region (3′UTR) of target messenger RNA (mRNA), miRNAs can regulate gene expression at the posttranscriptional level by inducing mRNA degradation or translational repression.10 miRNAs are important regulators of brain development and function. Changes in miRNA expression are closely related to stroke pathogenesis.11,12 miRNA dysregulation has been detected following ischemic stroke.13,14 Since miRNAs are stably expressed in human serum or plasma,15 several miRNAs such as miR-26b, miR-146a, and miR-145 have been reported as blood biomarkers for ischemic stroke.7,16,17
Recent studies have demonstrated that miR-335 is involved in the regulation of neuronal growth and development.18 Dharap et al.19 observed decreased miR-335 expression in the adult rat brain after transient middle cerebral artery occlusion. However, the role of miR-335 in human AIS remains unknown. In the present study, plasma miR-335 levels were measured in AIS patients and healthy controls, and the correlation between plasma miR-335 levels and stroke severity was explored. Furthermore, calmodulin (CaM), which plays an important role in the mechanisms of ischemic brain injury, was identified as a direct target of miR-335 in human umbilical vein endothelial cells (HUVECs).
Patients and methods
Patients and samples
A total of 168 AIS patients who were admitted to the Department of Neurology, Tianjin Medical University General Hospital within 24 hours after the onset of symptoms between January 2012 and October 2015, were prospectively enrolled. The diagnosis of AIS was based on patient history, laboratory and neurological examination, magnetic resonance imaging, and magnetic resonance angiography. Neurological deficits were evaluated using the National Institutes of Health Stroke Scale (NIHSS). Exclusion criteria included recurrent stroke, blood disorders, acute infectious diseases, renal or liver failure, and tumours. Healthy volunteers (104) were included as the healthy control group. The current study was approved by the medical ethics committee of Tianjin Medical University General Hospital (No. 2015011). All subjects provided signed informed consent forms.
Peripheral blood samples (5 mL) were collected in EDTA-K2 anti-coagulant tubes immediately after patient admission, and centrifuged at 1500 g for 20 min and at 12 000 rpm for 10 min at 4℃. Then, the prepared supernatant was transferred to RNase/DNase-free tubes and stored at −80℃ until further processing.
RNA extraction and quantitative real-time PCR
Total RNA was extracted using TRIzol solution (Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized with the ReverTra Ace qPCR RT Kit (Toyobo, Japan). Real-time qPCR was performed using the SYBR Premix Ex Taq kit (Takara, Dalian, China) on a 7500HT analyser (Applied Biosystems, USA) with cycling conditions of 95℃ for 5 min, followed by 40 cycles of 95℃ for 15 s and 60℃ for 60 s. Because of the lack of universal endogenous controls for plasma samples, synthetic cel-miRNA-39 was spiked into each sample as an internal control as previously described.20,21 The relative amount of miR-335 to cel-miR-39 was calculated using the equation 2−ΔCt, where ΔCT = (CTmiR-335 - CTcel-miR-39).
Plasma CaM protein measurements
CaM was determined using an enzyme-linked immunosorbent assay kit (Biocalvin, Jiangsu, China) according to the manufacturer’s instructions. All samples were analysed in triplicate and the mean CaM protein level was used for statistical analysis.
Cell culture and transfection
HUVECs were purchased from American Type Culture Collection (Rockville, MD, USA) and maintained in human endothelial serum free medium (Gibco-BRL, Rockville, MD, USA) at 37℃ in 5% CO2. Transfection with miR-335 mimics or negative control (miR-NC; GenePharma, Shanghai, China) was performed using Lipofectamine 2000 (Invitrogen, California, USA) according to the manufacturer’s instructions.
Luciferase Reporter Assays
The pGL3-reporter luciferase vector was used for the construction of the pGL3-CaM or pGL3-CaM-mut vectors. For the luciferase assay, cells were seeded into 24-well plates and cultured for 24 h. The cells were then co-transfected with the plasmids and miR-335 mimics or miR-NC using Lipofectamine 2000. After 2 days, the cells were harvested, and luciferase activity was measured using a dual-luciferase reporter assay system (Promega, USA).
Western blot analysis
The HUVEC cells were lysed in an ice-cold Radio-Immunoprecipitation Assay (RIPA) buffer, and the protein samples were separated using 12% SDS-PAGE and transferred to a PVDF membrane. Proteins were then blocked using 5% non-fat milk in TBST for 1 h. Later, the membranes were incubated with rabbit anti-human polyclonal primary antibody (ABclonal, USA) at 4℃ overnight. The following day, the membranes were washed with TBST and incubated with HRP-conjugated goat anti-rabbit IgG (ABclonal, USA). The blots were processed using an ECL reagent (GE healthcare, USA), and signals were quantified using Quantity One software (Bio-Rad). β-actin was used as an internal reference.
Statistical Analyses
Statistical analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL, USA) and P < 0.05 was considered statistically significant. Differences between groups were analysed using t-test for parametric variables and Mann–Whitney U-test for nonparametric variables. Pearson’s correlation analysis was performed to analyse the relationship between two variables. A receiver-operating characteristic (ROC) curve was used to assess the diagnostic value of plasma miR-335 for AIS. A multiple logistic regression analysis was carried out to test whether plasma miR-335 was associated with the presence of AIS.
Results
Plasma miR-335 and CaM levels in AIS patients and their association with stroke severity
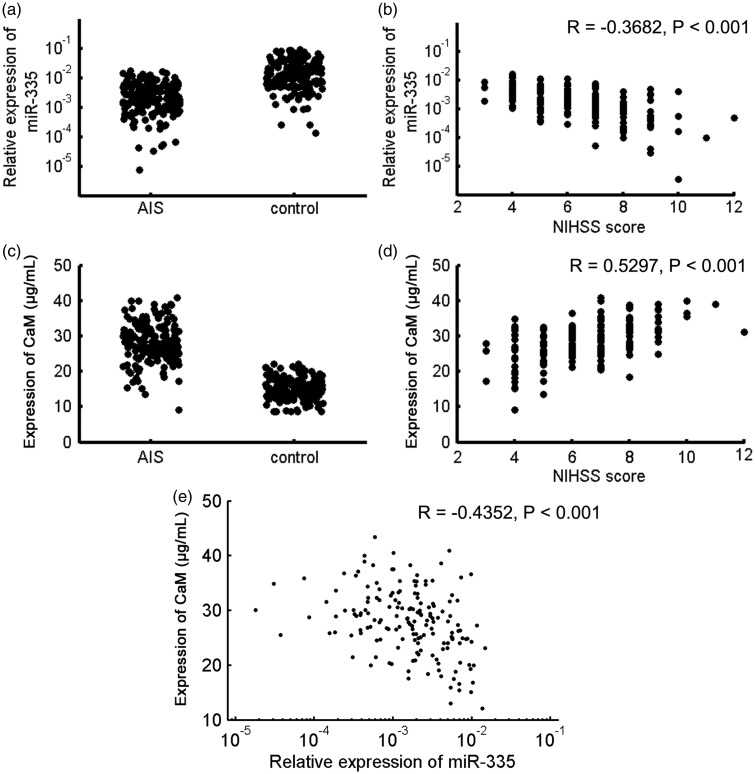
The characteristics of all participants are summarized in Table 1. Plasma miR-335 levels in AIS patients were significantly lower than in healthy controls (P < 0.001, Figure 1(a)), and were negatively correlated with NIHSS scores (R = −0.3682, P < 0.001; Figure 1(b)). Plasma CaM levels in AIS patients were significantly higher than in healthy controls (P < 0.001, Figure 1(c)), and were positively correlated with NIHSS scores (R = 0.5297, P < 0.001; Figure 1(d)). Moreover, plasma miR-335 and CaM levels were negatively correlated (R = −0.4352, P < 0.001; Figure 1(e)). Logistic multiple regression analysis revealed that decreased plasma miR-335 was independently correlated to the risk of AIS (Table 2).
Table 1.
Clinical characteristics of the whole study cohort at study entrance.
| Groups | Stroke patients | Controls | Statistical significance |
|---|---|---|---|
| Age (years) | 70 ± 8 | 69 ± 9 | P = 0.629 |
| Males/females (n) | 88/80 | 55/49 | P = 0.518 |
| Hypertension (n) | 115 (68.5%) | — | — |
| Diabetes (n) | 63 (37.5%) | — | — |
| Dyslipidaemia (n) | 91 (54.2%) | — | — |
| Coronary artery disease (n) | 59 (35.1%) | — | — |
| Smokers (n) | 82 (48.8%) | 47 (45.2%) | P = 0.358 |
Figure 1.
Plasma miR-335 and Calmodulin (CaM) levels in acute ischemic stroke (AIS) patients and healthy controls. (a) Plasma miR-335 levels in AIS patients were significantly lower than in healthy controls. (b) Plasma miR-335 levels were negatively correlated with NIHSS scores in AIS patients. (c) Plasma CaM levels in AIS patients were significantly higher than in healthy controls. (d). Plasma CaM levels were positively correlated with NIHSS scores in AIS patients. (e) Plasma miR-335 and CaM levels were negatively correlated.
Table 2.
Logistic regression analysis for risk factors of acute ischemic stroke.
| Risk factors | OR | 95% CI | Statistical significance |
|---|---|---|---|
| Age | 2.03 | 1.56 – 2.61 | P = 0.011 |
| Hypertension | 2.36 | 1.89 – 2.95 | P = 0.008 |
| Diabetes | 1.65 | 1.21 – 2.33 | P = 0.026 |
| Dyslipidaemia | 1.59 | 1.19 – 2.15 | P = 0.032 |
| Coronary artery disease | 1.72 | 1.28 – 2.47 | P = 0.015 |
| miR-335 | 0.79 | 0.68 – 0.87 | P = 0.028 |
Diagnostic value of plasma miR-335 for AIS
ROC curve analysis was performed to assess the diagnostic value of plasma miR-335 for AIS. The area under the curve was 0.898 (95% CI, 0.855 – 0.931; Figure 2). The optimal sensitivity and specificity were 97.6% and 69.2%, respectively.
Figure 2.
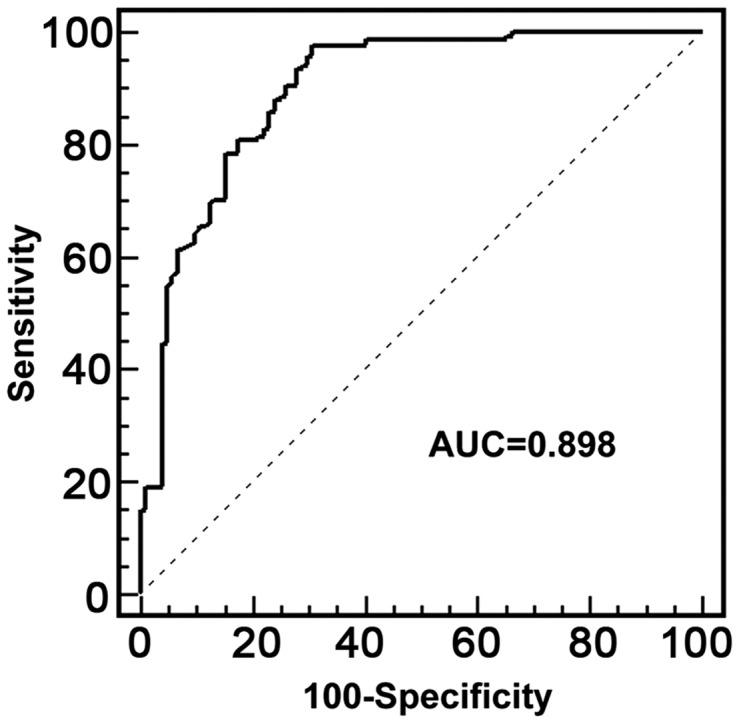
Receiver operating characteristics (ROC) curve of plasma miR-335 for the diagnosis of acute ischemic stroke.
CaM is the direct target of miR-335
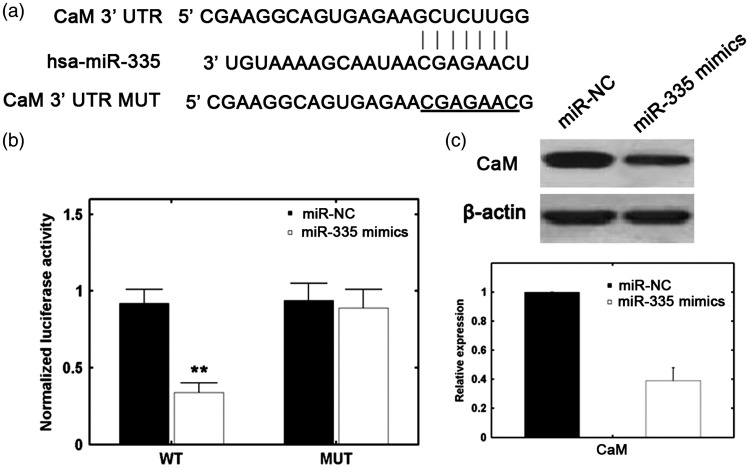
Using the TargetScan bioinformatics software, CaM was identified as one of the potential targets of miR-335. The predicted binding of miR-335 with the CaM 3′UTR is illustrated in Figure 3(a). Figure 3(b) shows that miR-335 mimics significantly reduced CaM protein levels. In addition, a reporter assay revealed that transfection with miR-335 mimics triggered a marked decrease in the luciferase activity of a pGL3-CaM plasmid, without a change in the luciferase activity of a pGL3-CaM-mut plasmid (Figure 3(c)). These data indicate that CaM is a direct target of miR-335.
Figure 3.
CaM is a direct target of miR-335. (a) Predicted miR-335 target sequence in the 3′UTR of CaM. (b) Analysis of relative luciferase activities of CaM-WT and CaM-mut (**P > 0.01 versus control [one-way analysis of variance]). (c) miR-335 upregulation in HUVECs was associated with decreased CaM protein levels.
Discussion
Early diagnosis and evaluation would improve the prognosis of AIS patients. miRNAs are stable in human serum/plasma and have been identified as circulating biomarkers in numerous diseases including ischemic stroke.22,23 miR-335 has been reported to be associated with the teratogenic effects of ethanol in a foetal mouse cerebral cortex-derived neurosphere culture model.24 Samaraweera et al.18 showed that miR-335 may be involved in neuronal differentiation through regulation of HAND1 and JAG1, two known modulators of neuronal differentiation. In addition, miR-335 levels in the adult rat brain were downregulated after transient focal ischemia.19 The current study presents several novel findings. Decreased plasma miR-335was detected in AIS patients within 24 h of the onset of stroke. This is in accordance with the results reported by Yuan et al.25 The current study also determined that a low level of miR-335 expression could distinguish AIS patients from healthy controls and was correlated with NIHSS scores. Using multiple logistic regression analysis, it was confirmed that decreased plasma miR-335 is an independent risk factor for AIS.
It is now clear that miRNAs exert their functions by the regulation of target gene expression.26 Calcium overload plays an important role during ischemic brain damage and is mediated by CaM.27 Tang et al.28 revealed increased CaM activity in the rat brain in a cerebral ischemia-reperfusion injury model. Yuan et al.16 found increased plasma CaM expression in patients with acute cerebral infarction and showed a correlation between plasma CaM levels and the severity of neurological deficits. Using PCR and western blotting, Yuan’s study showed that miR-335 overexpression suppressed CaM at both mRNA and protein levels in HUVEC cells.25 However, the association between plasma miR-335 and CaM levels in clinical AIS samples and the direct targeting of CaM by miR335 were not analysed. The current study confirmed high plasma levels of CaM in AIS patients and their association with high NIHSS scores. Furthermore, plasma miR-335 and CaM levels were negatively correlated. A luciferase reporter assay revealed that up-regulation of miR-335 significantly decreased the relative luciferase activity of the 3′UTR of wild-type CaM but had no effect on the 3′UTR of mutant CaM, indicating that miR-335 directly targets CaM expression by binding to its 3′UTR region. Therefore, miR-335 may play a role in stroke-related pathophysiological processes by targeting CaM. However, a ‘one-to-one’ connection between miRNAs and target mRNAs does not exist. An average miRNA can have more than 100 targets.29 In addition, several miRNAs can target a single transcript.30 Therefore, the potential regulatory effects afforded by miR-335 are numerous, and the link between miR-335 and AIS may not be limited to CaM and calcium overload. The involvement of miR-335 in the pathophysiological processes of AIS may be complex and multifaceted and needs further clarification.
There are some limitations in this work. First, this is a retrospective study, and the sample size is relatively small. Second, the source of circulating miRNAs and the mechanisms controlling the biogenesis of circulating miRNAs are not yet fully understood. It is widely believed that miRNAs released from damaged cells or circulating cells lead to increased levels of circulating miRNA.31 However, the precise reason for the down-regulation of circulating miRNAs after ischemic stroke is not clear. Further studies are needed to explore these mechanisms.
The present study has shown that plasma miR-335 is down-regulated in AIS patients and might serve as a useful noninvasive circulating biomarker. Large-scale prospective studies are needed to confirm this conclusion.
Declaration of conflicting interest
The Authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Alonso de Leciñana M, Gutiérrez-Fernández M, Romano M, et al. Strategies to improve recovery in acute ischemic stroke patients: Iberoamerican stroke group consensus. Int J Stroke 2014; 9: 503–513. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin KA, McCoy SL. Making a case for acute ischemic stroke. J Pharm Pract 2010; 23: 387–397. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Jr., del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American heart association/American stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: the American academy of neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007; 115: e478–e534. [DOI] [PubMed] [Google Scholar]
- 4.Wang DZ, Rose JA, Honings DS, et al. Treating acute stroke patients with intravenous tPA. The OSF stroke network experience. Stroke 2000; 31: 77–81. [DOI] [PubMed] [Google Scholar]
- 5.Katzan IL, Hammer MD, Hixson ED, et al. Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol 2004; 61: 346–350. [DOI] [PubMed] [Google Scholar]
- 6.Barber PA, Zhang J, Demchuk AM, et al. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 2001; 56: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 7.Jia L, Hao F, Wang W, et al. Circulating miR-145 is associated with plasma high-sensitivity C-reactive protein in acute ischemic stroke patients. Cell Biochem Funct 2015; 33: 314–319. [DOI] [PubMed] [Google Scholar]
- 8.Sørensen SS, Nygaard AB, Nielsen MY, et al. miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res 2014; 5: 711–718. [DOI] [PubMed] [Google Scholar]
- 9.Osman A. MicroRNAs in health and disease–basic science and clinical applications. Clin Lab 2012; 58: 393–402. [PubMed] [Google Scholar]
- 10.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 2012; 148: 1172–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics 2011; 43: 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan JR, Koo YX, Kaur P, et al. microRNAs in stroke pathogenesis. Curr Mol Med 2011; 11: 76–92. [DOI] [PubMed] [Google Scholar]
- 13.Zeng Y, Liu JX, Yan ZP, et al. Potential microRNA biomarkers for acute ischemic stroke. Int J Mol Med 2015; 36: 1639–1647. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Zhang J, Han R, et al. Downregulation of serum brain specific microRNA is associated with inflammation and infarct volume in acute ischemic stroke. J Clin Neurosci 2015; 22: 291–295. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008; 18: 997–1006. [DOI] [PubMed] [Google Scholar]
- 16.Yuan M, Tang Y, Zhou C, et al. Elevated plasma CaM expression in patients with acute cerebral infarction predicts poor outcomes and is inversely associated with miR-26b expression. Int J Neurosci 2016; 126: 408–414. [DOI] [PubMed] [Google Scholar]
- 17.Bao MH, Xiao Y, Zhang QS, et al. Meta-Analysis of miR-146a Polymorphisms association with coronary artery diseases and ischemic stroke. Int J Mol Sci 2015; 16: 14305–14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samaraweera L, Grandinetti KB, Huang R, et al. MicroRNAs define distinct human neuroblastoma cell phenotypes and regulate their differentiation and tumorigenicity. BMC Cancer 2014; 14: 309–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dharap A, Bowen K, Place R, et al. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab 2009; 29: 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105: 10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Kowdley KV. Method for microRNA isolation from clinical serum samples. Anal Biochem 2012; 431: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng H, Shen C, Hirokawa G, et al. Plasma miR-124 as a biomarker for cerebral infarction. Biomed Res 2011; 32: 135–141. [DOI] [PubMed] [Google Scholar]
- 23.Zeng L, Liu J, Wang Y, et al. MicroRNA-210 as a novel blood biomarker in acute cerebral ischemia. Front Biosci (Elite Ed) 2011; 3: 1265–1272. [DOI] [PubMed] [Google Scholar]
- 24.Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci 2007; 27: 8546–8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan M, Tang Y, Yuan H, et al. Negative Regulation of Calmodulin by miR-335 in Acute Ischemic Stroke. Medical Science Journal of Central South China 2015; 43: 276–280. [Google Scholar]
- 26.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 2010; 79: 351–379. [DOI] [PubMed] [Google Scholar]
- 27.Moha Ou Maati H, Widmann C, Sedjelmaci D, et al. Mapacalcine protects mouse neurons against hypoxia by blocking cell calcium overload. PLoS One 2013; 8: e66194–e66194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang LH, Xia ZY, Zhao B, et al. Phosphocreatine preconditioning attenuates apoptosis in ischemia-reperfusion injury of rat brain. J Biomed Biotechnol 2011; 2011: 107091–107091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brennecke J, Stark A, Russell RB, et al. Principles of microRNA-target recognition. PLoS Biol 2005; 3: e85–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krek A, Grün D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet 2005; 37: 495–500. [DOI] [PubMed] [Google Scholar]
- 31.Mayr M, Zampetaki A, Kiechl S. MicroRNA biomarkers for failing hearts? Eur Heart J 2013; 34: 2782–2783. [DOI] [PubMed] [Google Scholar]