Abstract
Objective
To investigate the diagnostic value of lung ultrasonography for neonatal meconium aspiration syndrome (MAS).
Methods
This prospective observational study enrolled patients diagnosed with MAS based on medical history, clinical manifestations and chest X-ray and control newborns without MAS. During ultrasonography, each lung was divided into three regions (front, lateral, and back), using anterior and posterior axillary lines as the boundary. While scanning each region of the lungs, the hand piece was perpendicular or parallel to the ribs.
Results
This study enrolled 117 newborns with MAS and 100 controls. The main lung ultrasonographic findings in patients with MAS were: (i) pulmonary consolidation with air bronchogram was found in all patients; (ii) pleural line anomalies and the disappearance of the A-line was found in all patients; (iii) atelectasis was found in 19 (16.2%) severe cases, who demonstrated severe massive atelectasis and visible lung pulse; (iv) pleural effusion was found in 16 patients (13.7%); and (v) alveolar-interstitial syndrome or B-line in the non-consolidation area was found in all patients with MAS.
Conclusion
Ultrasonography can be used routinely to diagnose MAS in an accurate, reliable, convenient, and non-invasive manner.
Keywords: Meconium aspiration syndrome, neonate, lung ultrasound
Introduction
Meconium aspiration syndrome (MAS) is a common cause of severe respiratory distress in neonates especially in full-term or post-term infants, with an associated highly variable morbidity and mortality.1 MAS accounts for about 10% of all cases of respiratory failure with a 39% mortality rate in developing and newly industrialized countries.1 Early diagnosis and early treatment are important for improving the prognosis.2–4 Historically, MAS has been mainly diagnosed based on medical history, clinical manifestations, and chest X-ray, and ultrasonography has not been used as a diagnostic tool. In recent years, however, ultrasonography has been used successfully to diagnose many types of lung diseases, such as neonatal respiratory distress syndrome (RDS), transient tachypnoea of the newborn, pneumonia, and atelectasis.5–9 This previous experience showed that lung ultrasonography is an accurate and reliable method for the early diagnosis and identification of neonatal lung diseases. This present study investigated the diagnostic value of lung ultrasonography for MAS, as well as presenting the ultrasonographic characteristics of MAS.
Patients and methods
Patients
This prospective observational study enrolled consecutive patients who were admitted to the Neonatal Intensive Care Unit, Bayi Children’s Hospital, the Army General Hospital of the Chinese People's Liberation Army, Beijing, China and diagnosed with MAS based on medical history, clinical manifestations, arterial blood gas analysis and chest X-ray between January 2014 and August 2015. Those patients with MAS and the complications of pneumothorax and/or pneumomediastinum were excluded from the study. The diagnostic criteria for MAS were as follows:1,10 (i) respiratory distress with elevated oxygen dependence; (ii) presence of meconium in the amniotic fluid; (iii) the classic radiographic findings in MAS, which are overexpansion of the lungs with widespread coarse, massive bilateral patchy infiltrates with or without pleural fluid; and (iv) abnormal blood gas analysis results. A group of newborns without lung and heart disease who were hospitalized at Bayi Children’s Hospital during the same time period and who underwent lung ultrasonography were used as the control subjects.
This study obtained approval from the Ethics Committee of the Army General Hospital of the Chinese People's Liberation Army (no. 2011-LC-Ped-01). The parents of the patients and control subjects participating in the study provided verbal informed consent.
Ultrasonography methods
A Voluson™ E8 or a LOGIC™ C9 ultrasound machine (GE Healthcare, Piscataway, NJ, USA) was used with a hand piece frequency of 10–14 MHz. Newborns were placed in a supine, lateral or prone position while at rest. During ultrasonography, each lung was divided into three regions (front, lateral and back), using anterior and posterior axillary lines as the boundary. While scanning each region of the lungs, the hand piece was perpendicular or parallel to the ribs. The lung ultrasonography examinations were performed by one physician (J.L.), while the clinical data were collected by different physicians, and the ultrasound operator was blinded to the clinical condition of the neonates. The lung ultrasound for MAS patients was performed on admission to the Neonatal Intensive Care Unit or within 2 h after receiving mechanical ventilation. The lung ultrasound for control newborns was performed by the same physician (J.L.) on admission to hospital.
Observational indices
The observational indices included pleural lines, lung sliding, A-lines, B-lines, interstitial syndrome, white lung, lung consolidation with air bronchogram or fluid bronchogram, double lung point, and pleural effusion.11–15
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 16.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Fisher’s exact test was used to compare the rate of positive neonatal ultrasound findings between the two groups. The specificity and sensitivity of the primary examination results for diagnosing MAS were calculated based on this test. A P-value < 0.05 was considered statistically significant.
Results
A total of 117 paediatric patients diagnosed with MAS based on typical medical history, clinical manifestations, abnormal blood gas analysis results and chest X-ray findings were enrolled in this study and underwent lung ultrasonography. Their gestational age ranged from 35+5 to 42+3 weeks (including seven late preterm newborns); 69 were male, and 48 were female. Sixty-six newborns were delivered vaginally, and 51 were delivered via caesarean section. Their birth weight ranged from 2280 to 5010 g. A total of 100 newborns with no lung diseases were included in the control group; 59 were male, and 41 were female. Among the controls, 57 were delivered vaginally, and 43 were delivered via caesarean section. Their gestational age ranged from 35+2 to 41+5 weeks and their birth weight ranged from 2550 to 4780 g, which were similar to those of the MAS patients.
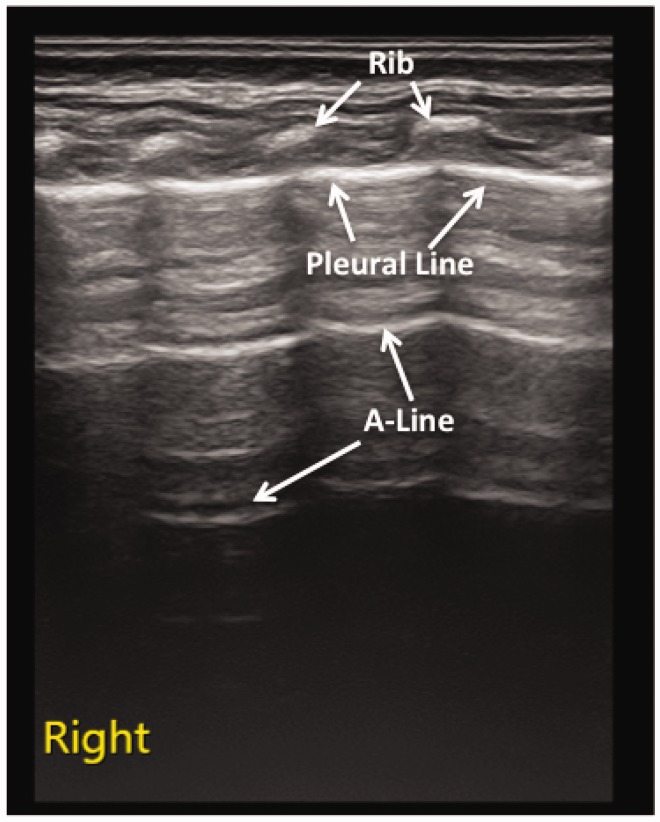
In normal lung tissue, the pleural line and A-line were clear, parallel and equally spaced, with smooth, clear and regular high echoes. The lung sliding was clearly present, with no or a few B-lines. B-lines were found in 25 of the 100 newborns; and at follow-up at 2–3 weeks after birth, five of them still had significant B-lines. There was no pleural effusion or lung consolidation (Figure 1).
Figure 1.
Normal neonatal lung ultrasonographic appearance of the right lung of a newborn in the control group. In normal newborns without lung disease, the lungs were hypoechoic; the pleural line and A-line were hyperechoic, clear, smooth, regular, and parallel and equally spaced, and the echoes gradually became weaker and eventually disappeared as the pleural line and the A-line extended deep into the lungs.
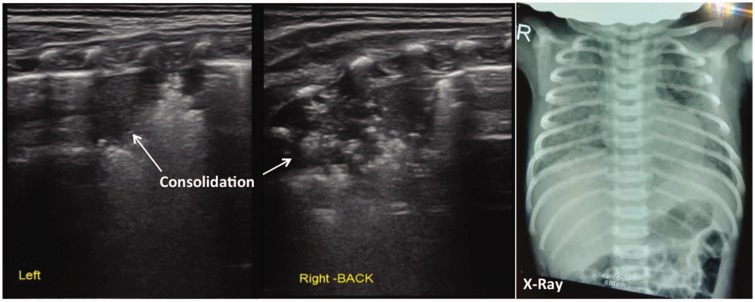
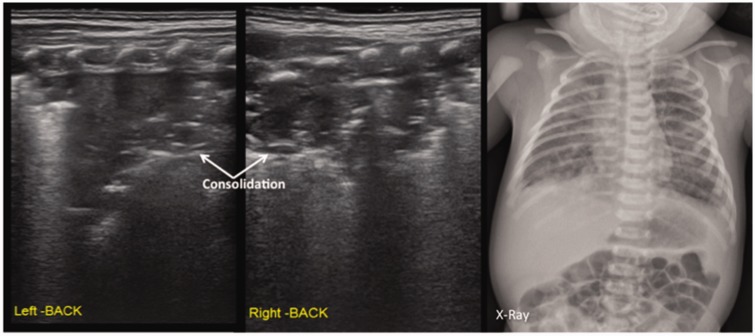
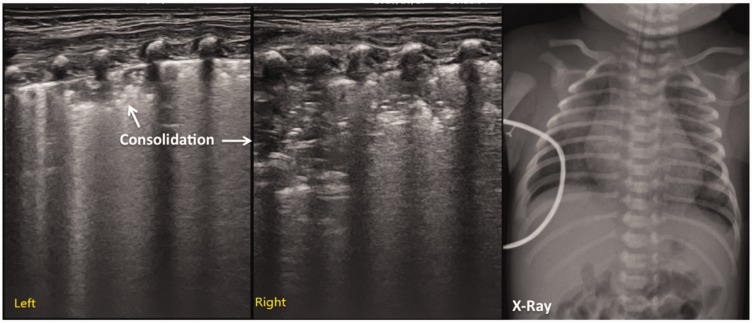
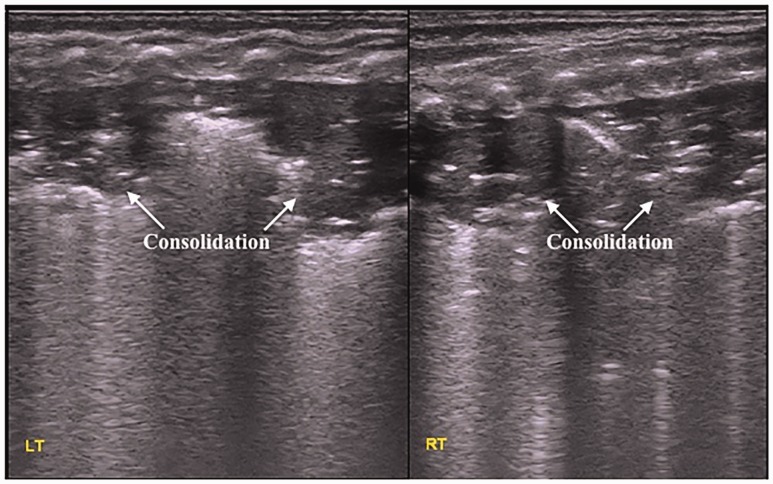
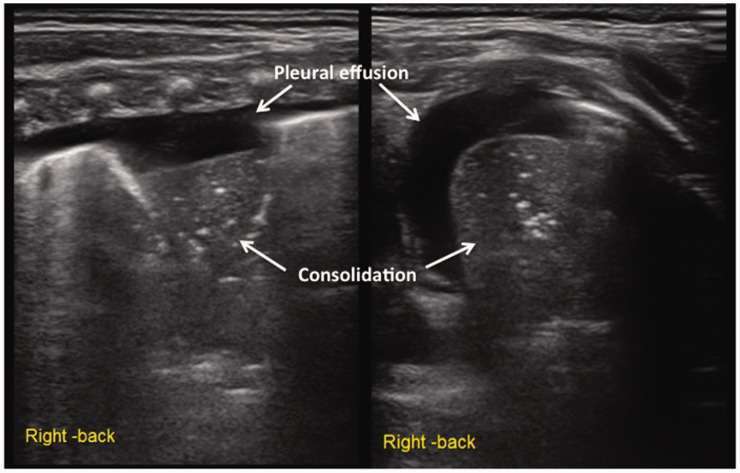
In this study, the main lung ultrasonographic findings in MAS patients were as follows: (i) lung consolidation with air bronchogram was found in all patients, usually with a large consolidation area with irregular edges (Figures 2 and 3). The lung consolidation area varied between the lungs, and different sizes of consolidation area were present in the same lung (Figures 4 and 5); (ii) pleural line anomalies and the disappearance of the A-line was found in all patients. Pleural line anomalies presented as the disappearance of the pleural line or as thickened and blurred pleural lines in the lesions (Figures 1–4); (iii) atelectasis is a manifestation of severe lung consolidation that was found in 19 severe cases (16.2%) in this study (Figure 6). These patients showed severe massive atelectasis and visible lung pulse. All of the patients with atelectasis had an absence of lung sliding; (iv) pleural effusion was found in 16 patients (13.7%) in this study (Figure 6); (v) alveolar-interstitial syndrome (AIS) or B-line in the non-consolidation area was found in all patients with MAS (Table 1).
Figure 2.
Lung ultrasonographic findings in a newborn with neonatal meconium aspiration syndrome (MAS). The newborn was delivered at 39+5 weeks of gestation and presented with meconium staining of the amniotic fluid, asphyxia at birth, and difficulty breathing after resuscitation. Lung ultrasonography showed large areas of pulmonary consolidation with air bronchogram and irregular edges in the lungs, especially in the right lung. The pleural line was abnormal, and the A-line disappeared in the consolidation area. Chest X-ray showed patchy and cloudy shadows that were consistent with MAS.
Figure 3.
Lung ultrasonographic findings in a newborn with neonatal meconium aspiration syndrome (MAS). The newborn was delivered at 41 weeks of gestation, weighed 3330 g and presented with meconium staining of the amniotic fluid, asphyxia at birth, and difficulty breathing after resuscitation. Lung ultrasonography showed large areas of pulmonary consolidation with air bronchogram and irregular edges in the lungs. The pleural line was abnormal, and the A-line disappeared in the consolidation area. Chest X-ray showed patchy and cloudy shadows that were consistent with MAS.
Figure 4.
Lung ultrasonographic findings in a newborn with neonatal meconium aspiration syndrome. The newborn was delivered at 39+6 weeks of gestation via a vaginal birth, weighted 3350 g and presented with fetal distress, meconium staining of the amniotic fluid, and decreased amniotic fluid. At 37 weeks, the umbilical cord was wrapped twice around the feet and was twisted. Apgar scores at birth were 7, 10, and 10 points at 1, 5, and 10 min, respectively, and heart rate was < 80 beats per min. There was no premature rupture of the fetal membranes according to the patient’s family. The newborn had difficulty breathing after birth. The patient was transferred to our hospital with endotracheal intubation and positive pressure ventilation. Lung ultrasonography showed large areas of pulmonary consolidation with irregular edges in the lungs, especially in the right lung.
Figure 5.
Lung ultrasonographic findings in a newborn with neonatal meconium aspiration syndrome. This patient had large areas of pulmonary consolidation with air bronchogram shown on lung ultrasonography; two consolidation areas of different sizes are shown in the right lung, the pleural line blurred or disappeared, and the A-line disappeared.
Figure 6.
Lung ultrasonographic findings in a newborn with neonatal meconium aspiration syndrome. This patient’s lung ultrasonography showed large areas of pulmonary consolidation with air bronchogram and clear edges in the right lung that were consistent with atelectasis. The pleural line blurred or disappeared, and the A-line disappeared. Apparent hyperechoic fluid-filled areas were present in the chest, suggesting pleural effusion (left: the hand piece was perpendicular to the ribs; right: the hand piece was parallel to the ribs).
Table 1.
The frequency of common ultrasonographic signs in newborns with neonatal meconium aspiration syndrome (MAS) and healthy control subjects.
| Ultrasonographic signs | MAS group n = 117 | Control group n = 100 | Statistical significancea |
|---|---|---|---|
| Lung consolidation | 117 (100.0) | 0 (0.0) | P < 0.001 |
| Pleural line anomalies | 117 (100.0) | 0 (0.0) | P < 0.001 |
| Disappearance of A-line | 117 (100.0) | 0 (0.0) | P < 0.001 |
| Pleural effusion | 16 (13.7) | 0 (0.0) | P < 0.001 |
| Atelectasis | 19 (16.2) | 0 (0.0) | P <0.001 |
| Lung pulse | 10 (8.5) | 0 (0.0) | P < 0.001 |
| Absence of lung sliding | 19 (16.2) | 0 (0.0) | P < 0.001 |
| B-line | 117 (100.0) | 25 (25.0) | NS |
Data presented as n of patients (%).
Between-group comparison; Fisher’s exact test.
NS, no significant between-group difference (P ≥ 0.05).
Based on the results of this study, lung consolidation with irregular margins were the common and specific ultrasound findings of MAS. Therefore, this study used the presence of lung consolidation with irregular margins as a parameter for calculating the sensitivity (a/a+c) and specificity (d/b + d) of ultrasonography for diagnosing MAS. The results indicate this sign exhibited both a sensitivity and specificity of 100% in diagnosing MAS (Table 2).
Table 2.
The sensitivity and specificity of lung consolidation in diagnosing neonatal meconium aspiration syndrome (MAS).
| Lung consolidationa | MAS group | Control group | Total |
|---|---|---|---|
| Existence | 117 (a) | 0 (b) | 117 (a + b) |
| Non-existence | 0 (c) | 100 (d) | 100 (c + d) |
| Total | 117 (a + c) | 100 (b + d) | 217 (a + b + c + d) |
Lung consolidation means large areas of lung consolidation with irregular, serrated margins.
Discussion
The incidence of meconium staining of the amniotic fluid (MSAF) increases with gestational age. For example, the overall incidence of MSAF is approximately 12%, while the incidence is ≥30% at gestational ages of >42 weeks; < 2% at gestational ages of <37 weeks; and rare at gestational ages of <34 weeks.16,17 Meconium excretion is a naturally occurring sign of the development of the gastrointestinal tract. For fetuses with mature nervous systems, umbilical cord compression may cause transient parasympathetic excitement and ensuing meconium excretion. Fetal distress may also trigger meconium excretion. After the baby is born and starts to breathe on their own, they may inhale meconium particles into the distal airways. Should a large amount of thick meconium be inhaled, the newborn may develop severe difficulty breathing within hours of birth, which may manifest as cyanosis, nasal flaring, signs of three depressions and shortness of breath and expiratory groaning; and in severe cases, the baby may die before birth or shortly after birth. Therefore, early diagnosis is important for guiding treatment and improving the patient’s prognosis. In the past, MAS was mainly diagnosed based on typical medical history, clinical manifestations, arterial blood gas analysis, and X-ray. However, many cases of MAS were misdiagnosed or had delayed diagnoses.18,19
A previous study had described the lung ultrasound signs in six patients with MAS, which included: (i) B-pattern coalescent or sparse; (ii) consolidations; (iii) atelectasis; and (iv) bronchograms.20 The authors reported that the lung ultrasound images corresponded well with the X-ray findings.20 This present study demonstrated that lung ultrasonography was accurate and reliable for the diagnosis of MAS. Based on the results of this study, the main ultrasonographic characteristics of MAS were as follows: (i) lung consolidation with air bronchogram was found in all patients, usually with a large consolidation area with irregular edges. According to this present study, the signs of lung consolidation with irregular margins on ultrasonography had a 100% sensitivity and 100% specificity for diagnosing MAS; (ii) there was variation in the nature and extent of pulmonary lesions between lungs or in the same lung. The area of lung consolidation varied between the lungs, and different sizes of consolidated areas were present in the same lung. A large consolidation was often seen in severely affected patients, while small consolidations and confluent B-lines were often seen in patients with less severe MAS; (iii) atelectasis was found in a few (16.2%) severe cases in this present study. Lung pulse was observed in severe cases (8.5%). There was an absence of lung sliding in all patients with atelectasis; (iv) pleural line anomalies were seen, including the disappearance of the pleural line or thickened and blurred pleural lines in the lesions; (v) the disappearance of the A-line was observed; (vi) the B-line or AIS was observed in the non-consolidation area; (vii) pleural effusion was found in 13.7% of patients in this present study. However, these ultrasonographic findings are also observed in lung diseases such as RDS, pneumonia and atelectasis, so they are not specific to MAS.5–7 In particular, large areas of pulmonary consolidation with irregular edges are also observed on lung ultrasonography in cases of pneumonia,6 which is useful for differentiating between MAS and other diseases. MAS and pneumonia may be differentiated because varying degrees of pulmonary consolidation are usually observed in both lungs in MAS but only in one lung in pneumonia.
Previous research suggests that in normal newborns a few B-lines may be present within 24–36 hours of birth but should disappear 48–72 hours after birth.14 However, this present study found that B-lines may still be present in healthy newborns after 3 days, and even 2–3 weeks after birth. This suggests that our knowledge and understanding of lung ultrasonography, a new technology, will deepen with more in-depth research and clinical experience.
In conclusion, lung ultrasonography appears to be an accurate, reliable, simple and non-invasive technology for the diagnosis of MAS, which can be performed at the bedside. Moreover, lung ultrasonography can be readily performed and is suitable for dynamic monitoring, and it can provide timely and valuable medical information when clinicians need to determine the causes of sudden changes in the patient’s condition. In particular, lung ultrasonography does not use radiation and thus prevents radiation-related injury in the patient, other patients in the same ward and the medical staff.21 In addition, it is very easy to distinguish MAS from other lung diseases, such as transient tachypnoea of the newborn, RDS, pneumonia, atelactesis, by using lung ultrasound.5–8 Lung ultrasonography could replace X-rays for the diagnosis and differential diagnosis of common lung diseases.22,23 In order for this new technology to be used more effectively, medical staff will require proper training in lung ultrasonography.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the Clinical Research Special Fund of Wu Jieping Medical Foundation (no. 320.6750.15072).
References
- 1.Swarnam K, Soraisham AS, Sivanandan S. Advances in the management of meconium aspiration syndrome. Int J Pediatr 2012; 2012: 359571–359571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Yerland Y, de Beaufort AJ. Why does meconium cause meconium aspiration syndrome? Current concepts of MAS pathophysiology. Early Hum Dev 2009; 85: 617–620. [DOI] [PubMed] [Google Scholar]
- 3.Dargaville PA, Copnell B. Australian and New Zealand Neonatal Network. The epidemiology of meconium aspiration syndrome: incidence, risk factors, therapies, and outcome. Pediatrics 2006; 117: 1712–1721. [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Hofmeyr J, Roy C, et al. Intrapartum amnioinfusion for meconium-stained amniotic fluid: a systematic review of randomised controlled trials. BJOG 2007; 114: 383–390. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Cao HY, Wang HW, et al. The role of lung ultrasound in diagnosis of respiratory distress syndrome in newborn infants. Iran J Pediatr 2014; 24: 147–154. [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Liu F, Liu Y, et al. Lung ultrasonography for the diagnosis of severe neonatal pneumonia. Chest 2014; 146: 383–388. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Chen SW, Liu F, et al. The diagnosis of neonatal pulmonary atelectasis using lung ultrasonography. Chest 2015; 147: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Wang Y, Fu W, et al. The diagnosis of neonatal transient tachypnea and its differentiation from respiratory distress syndrome using lung ultrasound. Medicine (Baltimore) 2014; 93: e197–e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Chen SW, Liu F, et al. BPD, not BPD, or iatrogenic BPD: findings of lung ultrasound examinations. Medicine (Baltimore) 2014; 93: e133–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubaltelli FF, Dani C, Reali MF, et al. Acute neonatal respiratory distress in Italy: a one-year prospective study. Italian Group of Neonatal Pneumology. Acta Paediatr 1998; 87: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995; 108: 1345–1348. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein DA, Lascols N, Prin S, et al. The “lung pulse”: an early ultrasound sign of complete atelectasis. Intensive Care Med 2003; 29: 2187–2192. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein DA, Lascols N, Mezière G, et al. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med 2004; 30: 276–281. [DOI] [PubMed] [Google Scholar]
- 14.Copetti R, Cattarossi L. The ‘double lung point’: an ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology 2007; 91: 203–209. [DOI] [PubMed] [Google Scholar]
- 15.Copetti R, Soldati G, Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound 2008; 6: 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker TA, Kinsella JP. Meconium Aspiration Syndrome. In: Gleason CA, Devaskar SU. (eds). Avery’s Diseases of the Newborn [M], 9th ed Philadelphia, USA: Elsevier Saunders, 2012, pp. 652–653. [Google Scholar]
- 17.Abu-Shaweesh JM. Meconium Aspiration Syndrome. In: Martin RJ, Fanaroff AA, Walsh MC. (eds). Fanaroff and Martin’s Neonatal-Perinatal Medicine [M], 9th ed Louis, USA: Elsevier Mosby, 2011, pp. 1157–1160. [Google Scholar]
- 18.Fischer C, Rybakowski C, Ferdynus C, et al. Population-based study of meconium aspiration syndrome in neonates born between 37 and 43 weeks of gestation. Int J Pediatr 2012; 2012: 321545–321545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meerkov M, Weiner G. Management of the meconium-stained newborn. NeoReviews 2016; 17: e471–e477. [Google Scholar]
- 20.Piastra M, Yousef N, Brat R, et al. Lung ultrasound findings in meconium aspiration syndrome. Early Hum Dev 2014; 90(Suppl 2): S41–S43. [DOI] [PubMed] [Google Scholar]
- 21.Smith-Bindman R. Is computed tomography safe? N Engl J Med 2010; 363: 1–4. [DOI] [PubMed] [Google Scholar]
- 22.Cattarossi L, Copetti R, Poskurica B. Radiation exposure early in life can be reduced by lung ultrasound. Chest 2011; 139: 730–731. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Cao HY, Wang XL, et al. The significance and the necessity of routinely performing lung ultrasound in the neonatal intensive care units. J Matern Fetal Neonatal Med 2016; 29: 1–6. . [DOI] [PubMed] [Google Scholar]