Abstract
Objective
The results of segmental venous resection (VR) combined with pancreatoduodenectomy (PD) are controversial but may be promising. Few studies have described reconstruction of the portal/superior mesenteric vein (PV/SMV) with the iliac vein harvested from donation after cardiac death (DCD).
Methods
From January 2014 to April 2016, PD combined with segmental excision of the PV/SMV (VR group) was performed in 21 patients with adenocarcinoma of the head of the pancreas (ADHP). The authors established a new technique of venous reconstruction using the iliac vein from DCD and analysed patients’ long-term survival.
Results
The tumour dimensions and tumour staging were greater and the operation time was longer in the VR than PD group; however, no differences in the resection degree, blood loss, complications, reoperation rate, or mortality rate were found. The median survival was similar between the VR and PD groups. The long-term patency of the donor iliac vein was 90%. The degree of resection was a strong predictor of long-term survival.
Conclusion
Segmental PV/SMV resection combined with PD is applicable to selective patients with venous invasion by ADHP if R0 resection has probably been achieved. An iliac vein obtained by DCD provides an effective graft for venous reconstruction.
Keywords: Pancreatoduodenectomy, venous resection, venous reconstruction, iliac vein, donation after cardiac death
Abbreviations
- PD
pancreatoduodenectomy
- VR
venous resection
- PV/SMV
portal/superior mesenteric vein
- DCD
donation after cardiac death
- ADHP
adenocarcinoma of the head of the pancreas
- PV
portal vein
- PD group
pancreatoduodenectomy group
- VR group
pancreatoduodenectomy combined with venous resection group
- SMV
superior mesenteric vein
- SV
splenic vein
- PF
pancreatic fistula
Introduction
According to the NCCN guidelines, solid pancreatic tumour invasion to the superior mesenteric vein (SMV) or portal vein (PV) is considered borderline resectable or unresectable cancer.1 Increasingly more studies are showing that venous resection (VR) combined with pancreatoduodenectomy (PD) is safe and has a long-term survival comparable with that of standard PD.2–4 VR is classified into four types: type 1 involves partial venous excision with direct closure, type 2 involves partial venous excision using a patch, type 3 involves segmental resection with primary venovenous anastomosis, and type 4 involves segmental resection with an interposed venous conduit.5 In clinical practice, venous graft interposition following segmental resection of the PV/SMV remains only a small part of vascular reconstruction,6,7 and the operative procedure, venous status, and long-term outcomes still require detailed evaluation. A recent report from the authors’ department presented a new venous reconstruction technique using the iliac vein harvested from donation after cardiac death (DCD) and addressed the feasibility of this method.8 Further exploration of the long-term survival, safety and R0 resection is needed, especially in comparison with PD in patients with adenocarcinoma of the head of the pancreas (ADHP). On the basis of this previous work, the current study was performed to evaluate the safety and long-term results of excision of the PV/SMV combined with PD and identify the potential factors influencing survival.
Materials and methods
Ethics statement
The need for the iliac vein as a homograft was registered in the China Organ Transplant Response System and then harvested by DCD by the assigned coordinator of this system. This study was approved by the ethics committee of Chaoyang Hospital Affiliated to Capital Medical University. After obtaining a full understanding of the details of the vascular allografts, all patients provided written informed consent to undergo the operation.
Patients
From January 2014 to April 2016, patients who underwent PD for treatment of ADHP were reviewed in the Hepatobiliary and Pancreatic Department of Chaoyang Hospital. The enrolled patients were divided into two groups: the PD group and the VR combined with PD group (VR group). In the PD group, PD was performed without venous reconstruction. In the VR group, venous graft interposition following segmental resection of the PV/SMV was performed in combination with PD. The three exclusion criteria were as follows. First, arterial invasion is associated with a lower survival rate and higher perioperative mortality rate; therefore, arterial invasion was regarded as unrespectable, and such patients were excluded.9 Second, patients who had received neoadjuvant chemotherapy were excluded. Finally, patients who underwent other venous reconstruction techniques such as direct suturing, end-to-end anastomosis, or patch repair were excluded. The demographic data, perioperative details, pathologic findings, and survival data were collected from our database. Enhanced computed tomography (CT) or three-dimensional vascular reconstruction was performed to determine the local status of the PV/SMV and tumour before the operation. Survival was evaluated from the date of postoperation to death or the end of the follow-up period.
Surgical management
En bloc PD was performed in both groups. Dissection of the retropancreatic lymph nodes and skeletonization of the hepatoduodenal ligament were routine procedures. Child’s type digestive tract reconstruction was performed. The anastomotic pancreatojejunostomy techniques in the two groups included the placement of running sutures from the pancreatic parenchyma to jejunal serosa, the placement of sutures from the duct of Wirsung to the jejunal mucosa, and stenting of the main pancreatic duct. A >1-mm negative resection margin, <1-mm negative resection margin, and the macroscopic presence of a residual tumour were considered R0, R1, and R2 resection, respectively.
Venous reconstruction
The iliac vein was harvested by DCD and preserved at −80℃ in accordance with the existing standards in the authors’ hospital.10 The duration of cryopreservation did not exceed 6 months. On the day of surgery, the cryopreserved donor iliac vein was immerged in saline at 37℃. After thawing for 5 minutes, the iliac vein was rinsed and placed in lactated Ringer’s solution at 4℃ for subsequent use.
After segmental excision of the PV/SMV together with the specimen, frozen pathologic examination of the ends of the resected vein was performed to ensure that the venous extremities were cancer-free.
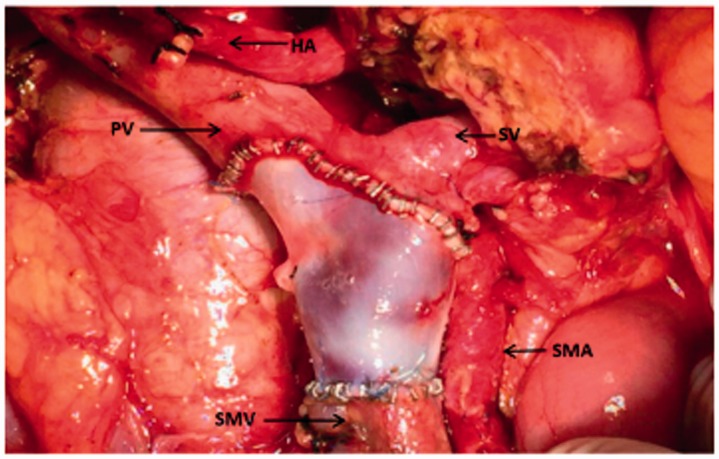
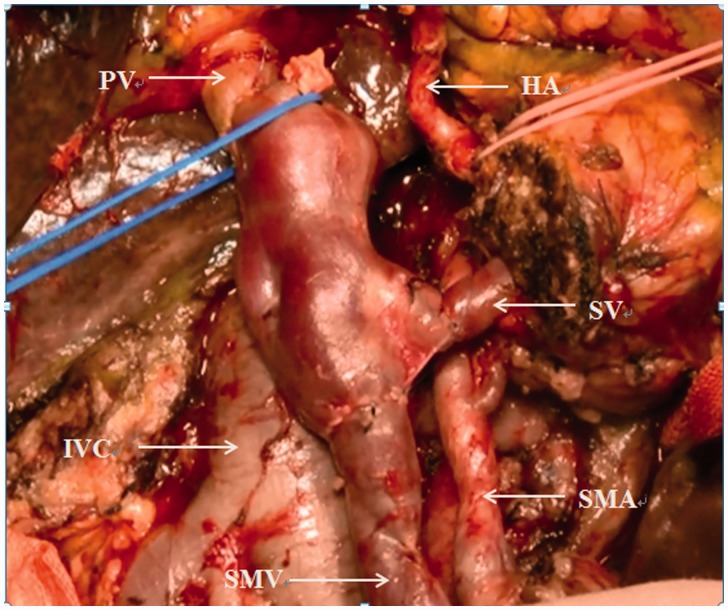
The patients in the VR group were classified into two types based on the sites of PV/SMV invasion. In type I, the preoperative enhanced CT images showed that the pancreatic tumour had invaded the lumen of the SMV and that the splenic vein (SV) was intact. Intraoperatively, after cutting off the pancreas, we confirmed invasion of the SMV and clamped this vein. The tumour combined with the SMV was removed, and end-to-end anastomosis of the internal or external iliac vein (harvested by DCD) to the SMV was performed (Figure 1). In type II, invasion of the pancreatic malignancy to the junction of the SMV and SV was suspected on the preoperative CT angiography images and then verified by surgical exploration. Thus, occlusion of the PV, SV, and SMV was performed simultaneously, and the tumour was resected together with the invaded segmental vessels. Finally, the allograft was pruned into three branches, and the common iliac vein, external iliac vein, and internal iliac vein were connected to the recipient PV, SMV, and SV, respectively (Figure 2). Both the anterior and posterior walls of the recipient’s PV/SMV were anastomosed to appropriately interposition the iliac vein with running 6-0 polypropylene sutures. Approximately 100 ml of static blood was exsanguinated to prevent aeroembolism and acidosis before the last suture was placed.
Figure 1.
The external iliac vein was pruned and anastomosed to the ends of the SMV. SMV, superior mesenteric vein; PV, portal vein; SMA, superior mesenteric artery; HA, hepatic artery; SV, splenic vein.
Figure 2.
Anastomosis of the donor common iliac vein, external iliac vein, and internal iliac vein to the recipient portal vein, superior mesenteric vein, and splenic vein, respectively. SMV, superior mesenteric vein; PV, portal vein; SV, splenic vein; SMA, superior mesenteric artery; HA, hepatic artery; IVC, inferior vena cava.
Anticoagulation strategy
Low-molecular-weight heparin (0.3 ml, 2850 IU anti-Xa activity) was subcutaneously injected twice a day during the first week after surgery for anticoagulation treatment. This drug was then changed to oral enteric-coated aspirin tablets (100 mg) once a day for up to 6 months postoperatively. Ultrasonography on day 3 postoperatively and enhanced CT on day 7 postoperatively were applied to evaluate the patency of the homograft.
Statistical analysis
An independent-samples t-test and a chi-square test were used to compare the continuous and categorical variables, respectively, of the two groups. The log-rank test based on the Kaplan–Meier curve was applied to evaluate survival in the two groups. Multivariate Cox regression analysis was performed to identify factors impacting survival. A P value of 0.05 was considered statistically significant. SPSS version 13.0 (SPSS Inc., Chicago, IL) was used to perform the data analysis.
Results
In total, 106 patients were enrolled in this retrospective study (21 in the VR group, 85 in the PD group). The overall survival duration was 20.8 months during the average follow-up period of 18.2 months. The clinical, laboratory, and pathologic results are summarized in Table 1. The tumour dimensions were significantly greater and the rate of regional lymph node metastasis was significantly lower in the VR than PD group (3.7 vs. 2.8 cm, P < 0.01 and 14% vs. 45%, P = 0.01, respectively). The tumour stage was significantly more severe in the VR than PD group (P = 0.01). The duration of the operation was significantly prolonged due to VR and venous reconstruction (510 vs. 407 min, P < 0.01); however, no significant differences were found between the VR and PD groups in terms of blood loss (938 vs. 731 ml), complications (29% vs. 16%), reoperation (14% vs. 7%), or mortality (5% vs. 2%). Pancreatic fistula (PF) was the most common and serious complication in the current study. PF was diagnosed in seven patients of the PD group and in three patients of the VR group following the International Study Group of Pancreatic Fistula criteria.11 In the PD group, four patients with a grade C PF developed a subsequent abdominal infection that resulted in intra-abdominal bleeding in three patients. Similarly, two patients in the VR group underwent an emergent reoperation because of infection caused by a grade C PF. Delayed gastric emptying, mild abdominal infection, and grade A and B PFs prolonged the hospitalization duration without inducing serious consequences in the two groups. The overall R0 resection rate was 70.9% (73 of 103 patients). The degree of resection, especially the R0 resection rate, was similar between the two groups. According to the Kaplan–Meier curve (Figure 3), there was no significant difference between the VR and PD groups in the median survival time (15 vs. 19 months, respectively). In the Cox regression model, the degree of resection was the only predictor of long-term survival (P = 0.004) (Table 2). There were fewer risks following R0 (OR 0.151, P = 0.001) and R1 (OR 0.317, P = 0.035) resection than following R2 resection.
Table 1.
Patients’ clinical and pathological details.
| PD group (n = 85) | VR group (n = 21) | P value | |
|---|---|---|---|
| Age (y) | 63.5 ± 10.7 | 63.0 ± 7.5 | 0.83 |
| Sex (male/female) | 44/41 | 13/8 | 0.404 |
| TBil (µmol/L) | 125.7 ± 110.4 | 103.4 ± 110.9 | 0.409 |
| PV/SMV invasion (−/+) | 85/0 | 5/16 | Null |
| Tumour dimension (cm) | 2.8 ± 0.9 | 3.7 ± 0.6 | <0.01 |
| RLM (−/+) | 38/47 | 3/18 | 0.01 |
| Stage (IA/IB/IIA/IIB) | 17/17/4/47 | 0/1/2/18 | 0.021 |
| Resection (R0/R1/R2) | 60/19/6 | 14/5/2 | 0.91 |
| Blood loss (ml) | 731.8 ± 568.9 | 938.1 ± 664.4 | 0.153 |
| Duration of operation (min) | 407.0 ± 104.8 | 510.2 ± 146.2 | <0.01 |
| Complications (−/+) | 71/14 | 15/6 | 0.204 |
| Pancreatic fistula (n) | 7 | 3 | Null |
| Intra-abdominal bleeding (n) | 3 | 2 | Null |
| Abdominal infection (n) | 4 | 2 | Null |
| Delayed gastric emptying (n) | 2 | 1 | Null |
| Reoperation (−/+) | 79/6 | 18/3 | 0.287 |
| Mortality (−/+) | 83/2 | 20/1 | 0.551 |
| Adjuvant therapy (−/+) | 27/58 | 7/14 | 0.890 |
Portal vein, PV; SMV, superior mesenteric vein; RLM, regional lymph node metastasis; TBil, total bilirubin; PD group, pancreatoduodenectomy group; VR group, venous resection combined with pancreatoduodenectomy group.
Figure 3.
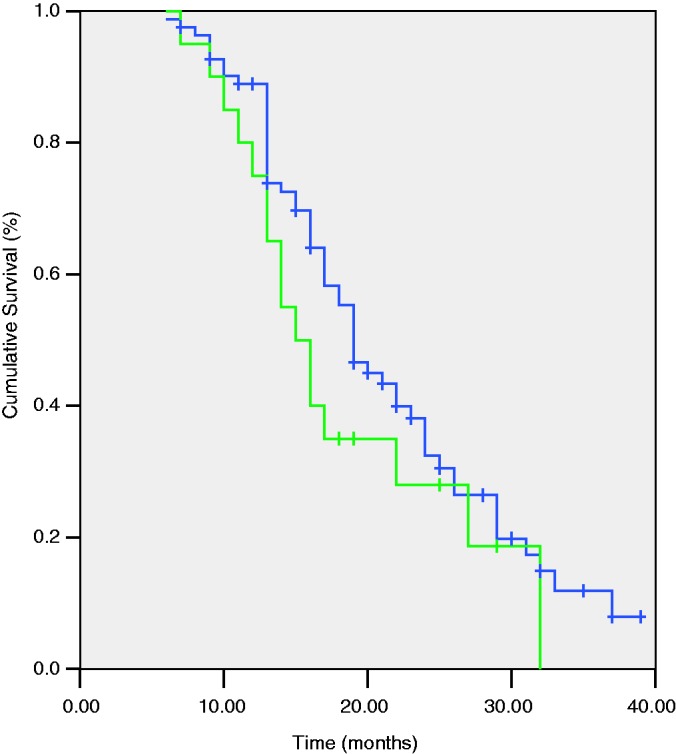
Kaplan–Meier survival curves (PD group vs. VR group) PD group, pancreatoduodenectomy group; VR group, pancreatoduodenectomy combined with venous resection group.
Table 2.
Cox regression model for long-term survival.
| n | Hazard ratio | Odds ratio (95% confidence interval) | P value | |
|---|---|---|---|---|
| Resection | 0.004 | |||
| R2 | 7 | 1.000 | ||
| R1 | 23 | 0.317 | (0.109, 0.922) | 0.035 |
| R0 | 73 | 0.151 | (0.047, 0.479) | 0.001 |
In the VR group, type I and II venous reconstruction was performed in 14 (66.7%) and 7 (33.3%) patients, respectively. No anastomotic bleeding or stenosis occurred in association with type I or II venous reconstruction as indicated by enhanced CT in the early postoperative period. By the end of follow-up period, the graft patency rate was 90% (19 of 21 patients). Two patients were hospitalized for transient ascites following type I venous reconstruction and subsequently diagnosed with SMV thrombosis by CT angiography at 6 and 8 months after surgery, respectively. Because of the development of collateral circulation, these patients survived without serious complications. In addition, no patients in the VR group showed symptoms of infection or rejection caused by the allograft vein during the follow-up period.
Discussion
Venous resection combined with PD provides a radical treatment opportunity for patients with borderline resectable ADHP; however, its outcomes remain controversial.12 Although this technique has been shown to be safe and feasible without increasing the postoperative mortality and morbidity rates,13,14 the R0 resection rate and long-term survival following VR are undesirable.15 Osamu et al.16 reported that bilateral and >1.2-cm invasion of the PV/SMV by a pancreatic tumour was associated with a very poor prognosis. In contrast, Kure et al.17 showed that even in patients with PV involvement, >5-year survival could be achieved if complete tumour extirpation (R0 resection) was achieved by PV resection. Perhaps PV/SMV involvement is not correlated with tumour invasion and a poor prognosis but can be regarded as functional adjacency.18 In the present study, tumour invasion of the PV/SMV was histopathologically confirmed in 16 of 21 (76%) patients in the VR group. There were some differences in venous involvement between the imaging and histological examination results. Consequently, Reddy and Hoffman19 questioned whether venous involvement should be included in the category “borderline resectable disease.” Similar to a previous study,20 the median survival time in patients of the VR group in the present study was not significantly lower than that in patients of the PD group. Several studies have found that the long-term survival was similar between patients who underwent PV/SMV resection combined with PD and patients who underwent PD alone.4,5,21–23 R0 resection has been widely accepted as a strong predictive factor for long-term survival.1 The R0 resection rate was comparable between the VR and PD groups in the present study (P = 0.726), which perhaps resulted in the comparable long-term survival. In a recent report, the International Study Group of Pancreatic Surgery strongly recommended the limitation of VR and venous reconstruction to high-volume centers.5
Generally, venous grafting is considered a necessary method if the length of the resected segment is >5 cm.24 In the current study, if the PV/SMV was involved by a malignancy of >3 cm, venous conduits were interposed to achieve a negative margin and tension-free anastomosis. Two sources of interpositional venous grafts can be used for revascularization: an autologous vein graft, such as that taken from the great saphenous vein; or a superficial femoral vein, external iliac vein, or internal jugular vein and synthetic graft.25–27 The iliac vein has been successfully applied as an allograft in living-donor liver transplantation to resolve congestion of the anterior sector of right-lobe grafts.28 Inspired by this surgical management, we used a portion of the iliac vein harvested by DCD as an autologous conduit to restore the PV/SMV continuity. Compared with a synthetic graft, the external iliac vein more closely matches the PV in terms of its diameter and thickness. Other advantages of external iliac vein grafts include better histocompatibility, a lower incidence of thrombosis, and no need for long-term anticoagulation. However, external iliac vein grafts also have some disadvantages. First, the unavailability of donor iliac vein grafts restricts the application of this technique, especially for centres without DCD. Additionally, in type II reconstruction, the branch of the external and internal iliac vein may not match the branch angle of the PV and SV. In contrast, a suitable synthetic graft can be used for venous reconstruction in most cases because of the broad choices available. Second, the long-term patency of iliac vein grafting remains unclear because of the shortage of long-term observation data and few reported cases. Third, the use of an allograft inevitably leads to additional infectious and epidemical risks compared with the use of a synthetic graft. Overall, however, use of the donor iliac veins in the present study achieved satisfactory long-term patency and did not lead to stenosis following antithrombotic treatment. Because of the lack of antigenicity in cryopreserved grafts, blood matching and immunosuppression are not needed. The technique performed in the present study decreased the operation time and avoided edema and deep venous thrombosis because an autologous vein graft was not used. In addition, the cryopreserved iliac vein was long-lasting; therefore, it offered a ready-to-use conduit for venous grafting. One limitation of the present study is that the results of using the iliac vein as a graft were not compared with the results of using an autologous or synthetic graft. Additionally, the difference in thrombosis between the two types of venous reconstruction could not be estimated because of the small number of patients.
The iliac vein can serve as a patch when a portion of the PV/SMV has been resected. An experienced specialist operator and meticulous perivascular anatomy are indispensable factors in ensuring high safety and smooth performance of this surgical approach. To the best of our knowledge, this is the first study to evaluate the long-term results of reconstruction of the PV/SMV using the iliac vein obtained by DCD in patients undergoing PD for treatment of ADHP.
Conclusion
Segmental PV/SMV resection combined with PD is comparable with PD in terms of R0 resection and long-term survival. Therefore, this technique is applicable to patients with ADHP if R0 resection has probably been achieved. The iliac vein obtained by DCD is appropriate as a conduit for PV/SMV reconstruction.
Declaration of conflicting interest
The Authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2012; 10: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravikumar R, Sabin C, Abu Hilal M, et al. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg 2014; 218: 401–411. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Zhang Z, Liu Y, et al. Pancreatectomy combined with superior mesenteric vein-portal vein resection for pancreatic cancer: a meta-analysis. World J Surg 2012; 36: 884–891. [DOI] [PubMed] [Google Scholar]
- 4.Tang D, Zhang JQ, Wang DR. Long term results of pancreatectomy with portal-superior mesenteric vein resection for pancreatic carcinoma: a systematic review. Hepatogastroenterology 2011; 58: 623–631. [PubMed] [Google Scholar]
- 5.Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the international study group of pancreatic surgery (ISGPS). Surgery 2014; 155: 977–988. [DOI] [PubMed] [Google Scholar]
- 6.Wang F, Arianayagam R, Gill A, et al. Grafts for mesenterico-portal vein resections can be avoided during pancreatoduodenectomy. J Am Coll Surg 2012; 215: 569–579. [DOI] [PubMed] [Google Scholar]
- 7.Ravikumar R, Holroyd D, Fusai G. Is there a role for arterial reconstruction in surgery for pancreatic cancer. World J Gastrointest Surg 2013; 5: 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing-Mao Z, Hua F, Jian-Tao K, et al. Resection of portal and/or superior mesenteric vein and reconstruction by using allogeneic vein for pT3 pancreatic cancer. J Gastroenterol Hepatol 2016. [DOI] [PubMed] [Google Scholar]
- 9.Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011; 254: 882–893. [DOI] [PubMed] [Google Scholar]
- 10.Heng WL, Madhavan K, Wee P, et al. Banking of cryopreserved iliac artery and vein homografts: clinical uses in transplantation. Cell Tissue Bank 2015; 16: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005; 138: 8–13. [DOI] [PubMed] [Google Scholar]
- 12.Delpero JR, Boher JM, Sauvanet A, et al. Pancreatic adenocarcinoma with venous involvement: is up-front synchronous portal-superior mesenteric vein resection still justified? A survey of the association francaise de chirurgie. Ann Surg Oncol 2015; 22: 1874–1883. [DOI] [PubMed] [Google Scholar]
- 13.Sgroi MD, Narayan RR, Lane JS, et al. Vascular reconstruction plays an important role in the treatment of pancreatic adenocarcinoma. J Vasc Surg 2015; 61: 475–480. [DOI] [PubMed] [Google Scholar]
- 14.Martin RC, 2nd, Scoggins CR, Egnatashvili V, et al. Arterial and venous resection for pancreatic adenocarcinoma: operative and long-term outcomes. Arch Surg 2009; 144: 154–159. [DOI] [PubMed] [Google Scholar]
- 15.Delpero JR, Bachellier P, Regenet N, et al. Pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a French multicentre prospective evaluation of resection margins in 150 evaluable specimens. HPB (Oxford) 2014; 16: 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa O, Ohigashi H, Imaoka S, et al. Preoperative indications for extended pancreatectomy for locally advanced pancreas cancer involving the portal vein. Ann Surg 1992; 215: 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kure S, Kaneko T, Takeda S, et al. Analysis of long-term survivors after surgical resection for invasive pancreatic cancer. HPB (Oxford) 2005; 7: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuhrman GM, Leach SD, Staley CA, et al. Rationale for en bloc vein resection in the treatment of pancreatic adenocarcinoma adherent to the superior mesenteric-portal vein confluence. Pancreatic tumor study group. Ann Surg 1996; 223: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy SS, Hoffman JP. “Vein involvement during pancreaticoduodenectomy: is there a need for redefinition of borderline resectable disease”: a commentary on the article published by Kelly et al. In the journal of gastrointestinal surgery 17:1209 (2013). J Gastrointest Surg 2014; 18: 1719–1719. [DOI] [PubMed] [Google Scholar]
- 20.Kulemann B, Hoeppner J, Wittel U, et al. Perioperative and long-term outcome after standard pancreaticoduodenectomy, additional portal vein and multivisceral resection for pancreatic head cancer. J Gastrointest Surg 2015; 19: 438–444. [DOI] [PubMed] [Google Scholar]
- 21.Yekebas EF, Bogoevski D, Cataldegirmen G, et al. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg 2008; 247: 300–309. [DOI] [PubMed] [Google Scholar]
- 22.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg 2004; 8: 935–949. discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 23.Gong Y, Zhang L, He T, et al. Pancreaticoduodenectomy combined with vascular resection and reconstruction for patients with locally advanced pancreatic cancer: a multicenter, retrospective analysis. PLoS One 2013; 8: e70340–e70340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirono S, Kawai M, Tani M, et al. Indication for the use of an interposed graft during portal vein and/or superior mesenteric vein reconstruction in pancreatic resection based on perioperative outcomes. Langenbecks Arch Surg 2014; 399: 461–71. [DOI] [PubMed] [Google Scholar]
- 25.Norton JA, Harris EJ, Chen Y, et al. Pancreatic endocrine tumors with major vascular abutment, involvement, or encasement and indication for resection. Arch Surg 2011; 146: 724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adham M, Mirza DF, Chapuis F, et al. Results of vascular resections during pancreatectomy from two European centres: an analysis of survival and disease-free survival explicative factors. HPB (Oxford) 2006; 8: 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao K, Wang H, Chen Q, et al. Prosthetic graft for superior mesenteric-portal vein reconstruction in pancreaticoduodenectomy: a retrospective, multicenter study. J Gastrointest Surg 2014; 18: 1452–1461. [DOI] [PubMed] [Google Scholar]
- 28.Kilic M, Aydin U, Sozbilen M, et al. Comparison between allogenic and autologous vascular conduits in the drainage of anterior sector in right living donor liver transplantation. Transpl Int 2007; 20: 697–701. [DOI] [PubMed] [Google Scholar]