Abstract
Objective
To identify the unique clinical features and biological markers of lung cancer-associated stroke.
Methods
We recruited 102 patients with lung cancer plus stroke, 102 with lung cancer, and 102 with stroke. Detailed information was analysed and compared among groups.
Results
The groups were age-matched. Patients with lung cancer plus stroke showed multiple lesions involving multiple cerebral artery territories on magnetic resonance imaging, compared with stroke-alone patients. These patients also had a poorer modified Rankin Scale score at 30 days, and high mortality (18.6%). Patients with lung cancer plus stroke had a higher incidence of metastasis, and higher blood levels of D-dimer, CA125 and CA199 compared with patients with lung cancer alone. Multivariate logistic regression analysis showed that levels of D-dimer, CA125 and CA199 were independently related to lung cancer-associated stroke.
Conclusion
Elevated plasma D-dimer, CA125 and CA199 may be independent risk factors for and biomarkers of lung cancer-associated stroke.
Keywords: Biological marker, clinical presentation, risk factors, pathogenesis, lung cancer, stroke
Introduction
Stroke is the second most common cause of death and the leading cause of disability worldwide.1–3 Ischemic stroke is the most common type of stroke, and its management and treatment differ from those of haemorrhagic stroke.4,5 Risk factors associated with ischaemic stroke include high blood pressure, obesity and diabetes mellitus, which require different management strategies.6,7
Cancer is a leading cause of death in both more and less economically developed countries.8,9 Although ischemic stroke is not a major cause of cancer-related death, it is frequently observed in cancer patients,10–12 and optimal stroke prevention and management measures in cancer patients may have significant bearing on their long-term survival.13 The incidence of ischemic stroke in cancer patients is significantly higher than in the general population,14–16 suggesting that cancer may contribute to stroke development. This specific stroke subset is referred to as “cancer-associated stroke”.13 Several studies have reported correlations between cancer-associated stroke and high blood levels of D-dimer, the occurrence of multiple lesions, and the absence of traditional stroke risk factors.13,14,17 Although cancers comprise a heterogeneous group of disorders with diverse pathologies and varied clinical characteristics, research into a specific type of cancer and its associated stroke might help us to understand the clinical features and pathogenesis of cancer-associated strokes in general.
Lung cancer is the most common type of cancer among men and is also associated with the highest mortality, while it has the third highest incidence and second highest mortality among women.18 Although the incidence of stroke is significantly higher in patients with lung cancer,19 the correlation is not universally accepted because of the small sample sizes of the relevant studies. Furthermore, studies investigating lung cancer-associated stroke (LCAS) have been limited. Nevertheless, it is reasonable to speculate that strokes occurring in some patients with lung cancer may be associated with the lung cancer itself, though it is unclear if such LCAS has unique clinical features and unique biomarkers.
To overcome this knowledge gap, the present study retrospectively investigated a relatively large dataset of patients with LCAS and analysed the clinical characteristics of this disease entity with respect to its clinical features and biological markers.
Patients and methods
Patient selection
All patients were recruited from the First Affiliated Hospital of Guangxi Medical University between January 2003 and December 2014. The diagnostic criteria for active lung cancer were adopted according to Navi et al.20. Active lung cancer was diagnosed on the basis of histopathology of lung biopsy specimens. The criteria for active disease included failure to achieve clinical cure standards, confirmation of cancer recurrence, or occurrence of metastatic lesions. The diagnosis of acute cerebral infarction was based on the American Heart Association diagnostic criteria for cerebral infarction,21 i.e., patients presenting with sudden-onset of slurring speech, paralysis and numbness of limbs, or other focal neurological deficits. Computed tomography (CT) images also showed no cerebral haemorrhage and magnetic resonance (MRI) images showed hyperintense lesions on T2 and diffusion-weighted images. The aetiology of cerebral infarction was determined according to TOAST criteria.22
The diagnosis of LCAS was slightly revised, based on several reports.14,17,23 The coexistence of both lung cancer and stroke in the same patient may be coincidental, and the stroke may be not associated with the lung cancer. To rule out this possibility as far as possible, lung cancer patients with stroke who also had high risk factors for stroke, such as elevated blood pressure, atrial fibrillation, diabetes or hypercholesterolemia were not considered to have LCAS, given that the observed stroke in such patients was more likely to have been caused by these factors than by their lung cancer. LCAS in the present study was therefore defined as the occurrence of stroke in patients with active lung cancer and an apparent absence of common risk factors. All patients were assessed by three independent experts from oncology, neurology and neuro-imaging, who were not otherwise associated with the planning and conduct of the study. The study protocol was approved by the Ethics Committee at the First Affiliated Hospital of Guangxi Medical University (No 2013 KY 108). Written informed consent was obtained from all the participants.
The inclusion criteria for patients in the LCAS group were as follows: (I) active lung cancer; (II) age > 18 years; (III) hospitalized between January 2003 and December 2014; and (IV) stroke occurred during treatment of lung cancer, or diagnosed with lung cancer during the treatment of stroke. The exclusion criteria were: (I) presence of conventional risk factors for stroke (such as hypertension, atrial fibrillation, diabetes or hypercholesterolaemia; or (II) lung cancer diagnosed > 5 years ago, with no evidence of recurrence or metastasis; (III) metastatic lesions in brain; (IV) presence of other neurological diseases; and (V) presence of any other malignant condition, in addition to lung cancer.
The inclusion criteria for patients with ischaemic stroke alone were: (I) presence of risk factors for stroke; (II) hospitalized between January 2003 and December 2014; and (III) age-and sex-matched to patients with LCAS. The exclusion criteria were: (I) cerebral haemorrhage, (II) other neurological diseases; (III) diagnosed with a malignant disorder, and (IV) presence of organ failure.
The inclusion criteria for patients with lung cancer alone were: (I) patients with active lung cancer; (II) hospitalized between January 2013 and December 2014; and (III) age- and sex-matched to patients with LCAS. The exclusion criteria were: (I) presence of brain metastasis; (II) absence of active lung cancer; (III) presence of other cancers; and (IV) organ failure.
Data collection and analysis
The medical records of the patients were scanned for relevant information, including demographic data, routine laboratory investigations, D-dimer levels, presence of cancer markers such as CA125, CA153 and CA199, presence of conventional risk factors for stroke, relevant clinical data, imaging reports (CT scan, MRI, transcranial Doppler, magnetic resonance angiography, computed tomography angiography, and digital subtraction angiography). A modified Rankin Scale (mRS) was used to reduce the potential impact of lung cancer on the prognosis of stroke, while the Barthel Index (BI) was used to assess the prognosis at 30 days post-stroke. Details of histological type, metastasis and treatment were also obtained in patients with lung cancer.
Statistical analyses
All statistical analyses were performed using SPSS 16.0. Quantitative data were analysed by analysis of variance or two independent t-tests. Frequencies of normally distributed variables were compared using χ2 or Fisher’s exact tests. For variables exhibiting a non-normal distribution (e.g., D-dimer, CA125, CA155 and CA199 levels), inter-group differences were evaluated using Mann – Whitney U tests. Multivariate logistic regression analysis was performed to identify independent risk factors. P < 0.05 was considered as statistically significant.
Results
Subject characteristics
A total of 102 patients with LCAS (mean age 52.74 ± 10.4 years), 102 patients with lung cancer alone (mean age 52.0 ± 11.0 years), and 102 patients with stroke alone (mean age 51.2 ± 9.7 years) were included in the study. Each group included 84 men (82.35%). The sex and age distributions were comparable among the three groups.
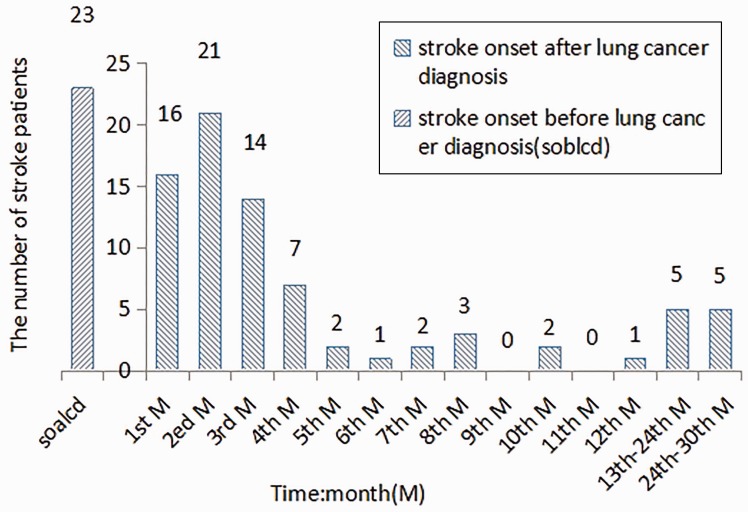
Twenty-three patients (22.55%) in the LCAS group were hospitalized as a result of acute cerebral infarction. These patients showed no other symptoms and the diagnosis of lung cancer was made incidentally during the management of stroke in the hospital. Fifty-eight patients (56.86%) developed cerebral infarction within 4 months of the diagnosis of lung cancer, 11 (10.78%) between 5 and 12 months of diagnosis, five (4.90%) from 1 – 2 years after diagnosis, and five patients (4.90%) 24–30 months after their diagnosis of lung cancer (Figure 1).
Figure 1.
Time interval between lung cancer diagnosis and stroke onset.
Clinical characterization of stroke in patients with lung cancer
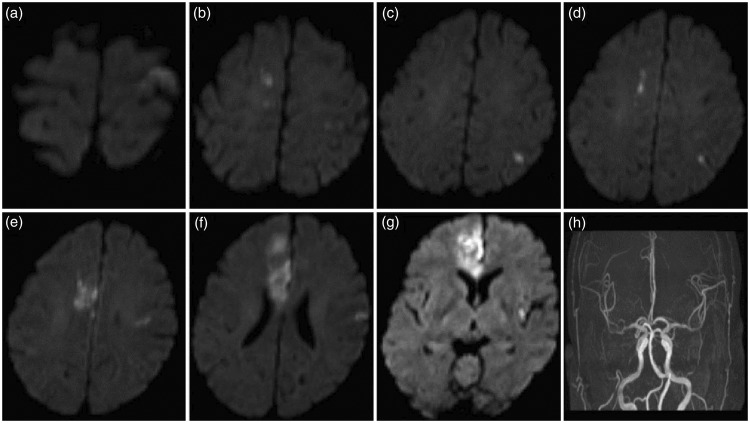
None of the patients with LCAS had any obvious stroke risk factors, such as smoking, hypertension, diabetes or hyperlipidaemia. There was no evidence of atrial fibrillation on electrocardiography, and echocardiography showed no signs of congenital heart disease or valvular disease. Carotid ultrasound, magnetic resonance angiography and whole-brain digital subtraction angiography showed no stenotic lesion in the carotid or vertebral basilar arteries. The clinical presentation in these patients was similar to that in patients with stroke alone, including hemiplegia or single-limb paralysis, partial-body sensory disturbances, slurring of speech and other focal neurologic deficits. However, some unique characteristics were noted in these patients, including cryptogenic cerebral infarction, multiple lesions involving multiple cerebral artery territories on MRI imaging, low mRS score, and high mortality within 30 days of the occurrence of cerebral infarction (Table 1 and Figure 2).
Table 1.
Clinical characteristics of patients with LCAS and patients with stroke alone.
| Variable | LCASG (n = 102) | SG (n = 102) | P value |
|---|---|---|---|
| Demographic variable | |||
| Age | 52.74 ± 10.49 | (51.21 ± 9.74) | <0.001a |
| Males | 84 (82.3%) | 84 (82.3%) | NSa |
| Females | 18 (17.6%) | 18 (17.6%) | NSa |
| Blood examination | |||
| RBC, ×1012/l | 4.20 ± 0.83 | 4.38 ± 0.62 | NSa |
| HGB, g/l | 120.24 ± 23.07 | 123.6 ± 18.5 | NSa |
| PLT, ×109/l | 207.8 ± 54.9 | 201.9 ± 67.4 | NSa |
| TT, s | 13.42 ± 2.22 | 13.11 ± 1.74 | NS |
| PT, s | 12.16 ± 1.53 | 11.75 ± 1.95 | NS a |
| APTT, s | 32.33 ± 3.82 | 32.24 ± 4.50 | NSa |
| INR | 1.04 ± 0.13 | 1.08 ± 0.61 | NSa |
| FIB, g/l | 4.81 ± 1.17 | 4.53 ± 0.93 | NSa |
| D-dimer, ng/ml | 478.1 ± 285.0 | 339.6 ± 256.9 | <0.001 |
| Multiple lesionsc | 55 (53.9%) | 8 (7.8%) | <0.001a |
| NHISS score | 8.86 ± 4.63 | 7.92 ± 3.68 | NSa |
| Prognosis at 30 days | |||
| NHISS score | 7.75 ± 5.62 | 4.47 ± 3.58 | <0.001a |
| mRS score | 2.79 ± 1.92 | 1.66 ± 1.22 | <0.001a |
| BI score | 65.2 ± 37.8 | 85.6 ± 22.2 | <0.001a |
| Death | 19 (18.63%) | 1 (0.98%) | <0.001b |
a = t-test, b = χ2 test
LCASG, lung cancer-associated stroke group; SG, stroke-alone group; HGB, hemoglobin; PLT, platelet; TT, thromboplastin time; PT, prothrombin time; APPT, activated partial thromboplastin time; FIB, fibrinogen NHISS, National Institutes of Health Stroke Scale, mRS, modified Rankin Scale; BI, Barthel Index.
NS, no statistically significant between-group differences (P ≥ 0.05).
Figure 2.
Diffusion-weighted imaging and magnetic resonance angiography findings in a typical case of cryptogenic stroke with active lung cancer.
Clinical characterization of lung cancer in patients with LCAS
The clinical presentation of patients with LCAS included cough, haemoptysis, chest pain, dyspnoea and laboured breathing, similar to that of patients with lung cancer alone. There were no differences in the histological characteristics of the lung cancer between the two groups. However, LCAS patients had a higher frequency of metastasis, and higher levels of D-dimer, CA125 and CA199, suggesting a more aggressive disease course (Table 2).
Table 2.
Clinical characteristics of patients with LCAS and patients with lung cancer alone.
| Variable | LCASG (n = 102) | LG (n = 102) | P value |
|---|---|---|---|
| General information | |||
| Age | 52.74 ± 10.49 | 53.04 ± 11.02 | 0.000a |
| Males | 84 (82.3%) | 84 (82.3%) | NSa |
| Females | 18 (17.6) | 18 (17.6) | NSa |
| Blood examination | |||
| RBC, ×1012/l | 4.20 ± 0.83 | 4.25 ± 0.68 | NSa |
| HGB, g/l | 120.2 ± 23.0 | 122.5 ± 16.0 | NSa |
| PLT, ×109/l | 207.8 ± 54.9 | 193.7 ± 54.9 | NSa |
| TT, s | 13.42 ± 2.22 | 14.77 ± 10.94 | NSa |
| PT, s | 12.16 ± 1.53 | 12.02 ± 1.50 | NSa |
| APTT, s | 32.33 ± 3.82 | 32.18 ± 4.68 | NSa |
| INR | 1.04 ± 0.13 | 1.07 ± 0.17 | NSa |
| FIB, g/l | 4.81 ± 1.17 | 4.60 ± 1.16 | NSa |
| D-dimer, ng/ml | 478.1 ± 285.0 | 277.4 ± 200.1 | 0.000a |
| CA125, U/ml | 233.3 ± 174.0 | 80.7 ± 46.9 | <0.001 |
| CA153, U/ml | 85.5 ± 32.0 | 77.3 ± 33.6 | NSa |
| CA199, U/ml | 212.4 ± 133.9 | 108.6 ± 73.6 | <0.000a |
| Lung cancer histology | NSb | ||
| Adenocarcinoma, n, % | 50 (49.0%) | 44 (43.1%) | |
| Squamous cell carcinoma, n, % | 42 (41.1%) | 46 (45.1%) | |
| Others, n, % | 10 (9.8%) | 12 (11.7%) | |
| Metastasis | <0.001b | ||
| Yes, n, % | 49 (48.0%) | 24 (23.5%) | |
| No, n, % | 53 (51.9 %) | 78 (76.4%) | |
| Cancer treatment | 0.005b | ||
| Non-surgical treatment, n, % | 38 (37.2%) | 58 (56.8%) | |
| Surgical treatment, n, % | 64 (62.7%) | 44 (43.1%) |
a= t-test, b = χ2 test
LCASG, lung cancer-associated stroke group; LG, lung cancer-alone group; TT, thromboplastin time; PT, prothrombin time; APPT, activated partial thromboplastin time; NHISS, National Institutes of Health Stroke Scale, mRS, modified Rankin Scale; BI, Barthel Index.
NS, no statistically significant between-group differences (P ≥ 0.05).
Risk factors for stroke in patients with lung cancer
To identify the potential risk factors for stroke in patients with lung cancer, we analysed seven significant variables, including platelets, D-dimer, CA125, CA155, CA199, metastasis, and surgical therapy, by multivariate logistic regression analysis. High blood levels of D-dimer, CA155 and CA199 were independently associated with stroke risk. We also examined the level-dependent risks. The risk of cerebral infarction in patients with lung cancer increased by 0.2% (odds ratio [OR] 1.002; 95% confidence interval [CI] 1.000, 1.004; P = 0.017) with an increase in D-dimer level of 1 ng/ml. The risk of cerebral infarction increased by 0.6% (OR 1.006; 95% CI 1.001, 1.010; P = 0.017) with an increase in CA125 of 1 U/mL, and by 2.1% (OR 1.021; 95% CI 1.011, 1.024; P = 0.000) with an increase in CA199 of 1 U/mL (Table 3). Spearman correlation analysis revealed positive associations between D-dimer levels and CA125 (Rs = 0.413, P < 0.05) and CA199 levels (Rs = 0.527, P < 0.05).
Table 3.
Multivariate logistic regression analysis of independent predictors of stroke risk in lung cancer patients.
| Risk factor | Β | SE(βi) | Wals | df | P value | Odds ratio | 95% CI |
|---|---|---|---|---|---|---|---|
| D-dimer, X2 | 0.002 | 0.001 | 5.745 | 1 | 0.017 | 1.002 | 1.000, 1.004 |
| CA125, X3 | 0.006 | 0.002 | 5.738 | 1 | 0.017 | 1.006 | 1.001, 1.010 |
| CA199, X5 | 0.021 | 0.004 | 30.040 | 1 | 0.000 | 1.021 | 1.011, 1.024 |
| Constant value | −3.818 | 0.536 | 50.710 | 1 | 0.000 | 0.022 |
df, degrees of freedom; SE, standard error; CI, confidence interval
Discussion
Cerebral infarction is a potentially fatal condition with a very short therapeutic window,24,25 and identification of its aetiology is critical to the successful management of this disease.26 The short therapeutic window means that the early detection of cerebral infarction is of critical importance,27 especially when it occurs in a patient with a malignancy.28 This situation is not rare, and several studies have clearly demonstrated a higher incidence of stroke in cancer patients compared with individuals without cancer.29 The optimal management of these patients is critical to their survival. Cancer-associated stroke is thought to differ from stroke occurring in the general population, though the differences have not been well-characterized. The current retrospective study aimed to fill this knowledge gap, based on data from a relatively large sample of patients with LCAS.
Most patients in the present study suffered from acute stroke within 4 months of the diagnosis of lung cancer, consistent with the findings of other studies. Bengt et al. found that 1.6% of cancer patients developed stroke within 6 months of the diagnosis of cancer,16 and the incidences of stroke in cancer patients within 6–12 months and within 1–5 years of cancer diagnosis were 1.1% and 1.2%, respectively.16 Chen et al.19 also reported a higher incidence of stroke in cancer patients in the first 6 months after the diagnosis of cancer. Collectively, these results suggest that a diagnosis of cancer should prompt consideration of stroke-prevention therapy in addition to chemotherapy, especially within 6 months of cancer diagnosis.
Previous studies revealed that most patients with cancer-associated stroke had no other risk factors,20,30–35 and patients with LCAS in the current study were defined as having active lung cancer and acute stroke, but without any other stroke risks factors.
In the present study, patients with LCAS had elevated levels of plasma D-dimer and multiple lesions involving multiple arterial territories in the brain. Similar clinical features have been found in patients with cancer-associated stroke in several previous studies.13–14,17 However, the present study also found elevated plasma CA125 and CA199 levels in LCAS patients, suggesting that patients with cryptogenic stroke and elevated plasma CA125 and CA199 levels should undergo appropriate investigations to rule out lung cancer. Further analysis showed that elevated levels of plasma D-dimer, plasma CA125 and CA199 were all independent risk factors for stroke in patients with active lung cancer.
The pathogenesis of stroke in cancer patients has been a focus of attention. Elevated D-dimer levels and multiple lesions in multiple artery territories in patients with cancer-associated stroke suggest that the hypercoagulable state in cancer patients may be an underlying reason for this association. However, the mechanisms responsible for the development of a hypercoagulable state in cancer patients remain unclear. Previous studies investigating this topic usually included patients with multiple anatomic types of cancer, along with stroke.
The present study showed that elevated plasma levels of D-dimer, CD125 and CA199 were independent risk factors for stroke in patients with active lung cancer, and that D-dimer levels were positively associated with CA125 and CA199 levels. We therefore concluded that elevated plasma levels of CA125 and CA199 not only act as biomarkers of lung cancer, but may also play a role in increasing the hypercoagulable state in lung cancer patients. Significantly increased CA125 levels have also been reported to be related to recurrent stroke.36 However, the mechanisms whereby elevated plasma levels of CA125 and CA199 enhance the coagulation state in lung cancer patients, leading to stroke, need further investigation.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant funding from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Krishnamurthi RV, Moran AE, Feigin VL, et al. Stroke prevalence, mortality and disability-adjusted life years in adults aged 20-64 years in 1990-2013: data from the global burden of disease 2013 study. Neuroepidemiology 2015; 45: 190–202. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay P, Furie KL, Davis SM, et al. World stroke organization global stroke services guidelines and action plan. Int J Stroke 2014; 9(Suppl A100): 4–13. [DOI] [PubMed] [Google Scholar]
- 3.Sherzai AZ, Elkind MS. Advances in stroke prevention. Ann N Y Acad Sci 2015; 1338: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderazi YJ, Grotta JC. Acute antithrombotic treatment of ischemic stroke. Curr Vasc Pharmacol 2014; 12: 353–364. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Krishnamurthi RV, Barker-Collo S, et al. The global burden of ischemic stroke: findings of the GBD 2010 study. Glob Heart 2014; 9: 107–112. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado NJ, Kazmi SO, Suarez JI. Update in the management of acute ischemic stroke. Crit Care Clin 2014; 30: 673–697. [DOI] [PubMed] [Google Scholar]
- 7.Silver B, Wulf Silver R. Stroke: subacute/inpatient management of acute ischemic stroke. FP Essent 2014; 420: 23–27. [PubMed] [Google Scholar]
- 8.Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 2008; 25: 2097–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics. CA Cancer J Clin 2015; 65(2): 87–108. [DOI] [PubMed] [Google Scholar]
- 10.Etgen T, Steinich I, Gsottschneider L. Thrombolysis for ischemic stroke in patients with brain tumors. J Stroke Cerebrovasc Dis 2014; 23: 361–366. [DOI] [PubMed] [Google Scholar]
- 11.Grisold W, Oberndorfer S, Struhal W. Stroke and cancer: a review. Acta Neurol Scand 2009; 119: 1–16. [DOI] [PubMed] [Google Scholar]
- 12.Plummer C, Henderson RD, O’Sullivan JD, et al. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke 2011; 42: 2410–2418. [DOI] [PubMed] [Google Scholar]
- 13.Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 2012; 43: 3029–3034. [DOI] [PubMed] [Google Scholar]
- 14.Guo YJ, Chang MH, Chen PL, et al. Predictive value of plasma (D)-dimer levels for cancer-related stroke: a 3-year retrospective study. J Stroke Cerebrovasc Dis 2014; 23: e249–e254. [DOI] [PubMed] [Google Scholar]
- 15.Tsai SJ, Huang YS, Tung CH, et al. Increased risk of ischemic stroke in cervical cancer patients: a nationwide population-based study. Radiat Oncol 2013; 8: 41–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zöller B, Ji J, Sundquist J, et al. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer 2012; 48: 1875–1883. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Jung KH, Park KH, et al. Clinical manifestation of cancer related stroke: retrospective case-control study. J Neurooncol 2013; 111: 295–301. [DOI] [PubMed] [Google Scholar]
- 18.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 2008; 5: e185–e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen PC, Muo CH, Lee YT, et al. Lung cancer and incidence of stroke: a population-based cohort study. Stroke 2011; 42: 3034–3039. [DOI] [PubMed] [Google Scholar]
- 20.Navi BB, DeAngelis LM, Segal AZ. Multifocal strokes as the presentation of occult lung cancer. J Neurooncol 2007; 85: 307–309. [DOI] [PubMed] [Google Scholar]
- 21.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 22.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 23.Kono T, Ohtsuki T, Hosomi N, et al. Cancer-associated ischemic stroke is associated with elevated D-dimer and fibrin degradation product levels in acute ischemic stroke with advanced cancer. Geriatr Gerontol Int 2012; 12: 468–474. [DOI] [PubMed] [Google Scholar]
- 24.Cumbler E. In-hospital ischemic stroke. Neurohospitalist 2015; 5: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George PM, Steinberg GK. Novel stroke therapeutics: unraveling stroke Pathophysiology and its impact on clinical treatments. Neuron 2015; 87: 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balasubramaian A, Mitchell P, Dowling R, et al. Evolution of endovascular therapy in acute stroke: implications of device development. J Stroke 2015; 17: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keach JW, Bradley SM, Turakhia MP, et al. Early detection of occult atrial fibrillation and stroke prevention. Heart 2015; 101: 1097–1102. [DOI] [PubMed] [Google Scholar]
- 28.Elyamany G, Alzahrani AM, Bukhary E. Cancer-associated thrombosis: an overview. Clin Med Insights Oncol 2014; 8: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seok JM, Kim SG, Kim JW, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol 2010; 68: 213–219. [DOI] [PubMed] [Google Scholar]
- 30.Borowski A, Ghodsizad A, Gams E. Stroke as a first manifestation of ovarian cancer. J Neurooncol 2005; 71: 267–269. [DOI] [PubMed] [Google Scholar]
- 31.Kim SJ, Park JH, Lee MJ, et al. Clues to occult cancer in patients with ischemic stroke. PLoS One 2012; 7: e44959–e44959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon HM, Kang BS, Yoon BW. Stroke as the first manifestation of concealed cancer. J Neurol Sci 2007; 258: 80–83. [DOI] [PubMed] [Google Scholar]
- 33.Mai H, Xia J, Wu Y, et al. Clinical presentation and imaging characteristics of occult lung cancer associated ischemic stroke. J Clin Neurosci 2015; 22: 296–302. [DOI] [PubMed] [Google Scholar]
- 34.Mirza HZ, Zuberi BJ, Zein TM, et al. Ischaemic stroke as the first presentation of occult squamous cell cancer. J Coll Physicians Surg Pak 2013; 23: 437–439. [PubMed] [Google Scholar]
- 35.Taccone FS, Jeangette SM, Blecic SA. First-ever stroke as initial presentation of systemic cancer. J Stroke Cerebrovasc Dis 2008; 17: 169–174. [DOI] [PubMed] [Google Scholar]
- 36.Jovin TG, Boosupalli V, Zivkovic SA, et al. High titers of CA-125 may be associated with recurrent ischemic strokes in patients with cancer. Neurology 2005; 64: 1944–1945. [DOI] [PubMed] [Google Scholar]