Abstract
Liver dysfunction is common in individuals receiving parenteral nutrition (PN) and particularly in neonates and infants. Abnormalities of liver function tests in patients receiving short term PN are usually transient but in individuals receiving long term PN, substantial liver damage and ultimately end stage liver disease may occur. The aetiology is complex, involving a large number of patient related and nutrition related factors. The terminology intestinal failure associated liver disease (IFALD) is therefore more appropriate than PN associated liver disease. Effort should be made to prevent liver dysfunction by managing sepsis, avoiding parenteral overfeeding, employing cyclical parenteral feeding and encouraging enteral nutrition where possible. Intake of soybean based parenteral lipid emulsions should be reduced in individuals with established IFALD, possibly to be replaced by lipid emulsions containing medium chain triacylglycerol, monounsaturated fatty acids or fish oil although larger clinical studies are needed. Similarly, evidence supporting the widespread use of parenteral choline and taurine supplementation in the prevention or treatment of IFALD remains limited. There are more data to support the use of oral antibiotics to treat bacterial overgrowth and oral ursodeoxycholic acid in neonates. Ultimately, severe IFALD may necessitate referral for small intestine and/or liver transplantation.
Introduction
Since it was developed in the second half of the 20th century, parenteral nutrition (PN) has become established as a life saving treatment for patients with intestinal failure. However, patients receiving PN are at risk of developing hepatic complications. PN associated liver disease was first described in the early 1970s.1 The incidence of hepatic dysfunction, possible aetiologies and strategies to avoid and manage these complications will be discussed. The term intestinal failure associated liver disease (IFALD) is used in place of PN associated liver disease in this review as the liver dysfunction that occurs in patients on PN may be caused by factors other than PN.
Incidence of liver dysfunction in patients receiving PN
Short term home parenteral nutrition
Abnormalities of liver function tests (LFTs) are common in adults with acute intestinal failure receiving short term PN. Early reports2 describe the development of abnormal LFTs occurring after only 2 weeks of a lipid free PN with a high glucose content (elevated aspartate aminotransferase, alkaline phosphatase and bilirubin concentrations in 68%, 54% and 21% of patients, respectively). More recent reports in which more balanced parenteral regimens are used3 describe less frequently abnormal LFTs although this is still clearly a common phenomenon (after 4 weeks of PN, aspartate aminotransferase, alkaline phosphatase and bilirubin concentrations were elevated in 27%, 32% and 31% of patients, respectively). In general, these elevations are mild, often normalise even if PN is continued and usually resolve fully once it is discontinued,4 possibly also due to the initiation of enteral feeding. A more recent audit of patients revealed that 34% patients had abnormal LFTs before PN was started.5 In those patients, LFTs worsened in 60% and resolved in 30% while receiving PN. Only 9% of patients developed abnormal LFTs during PN. For patients who developed abnormal LFTs or worsening LFTs on PN, the underlying cause was thought to be sepsis in 46% and the underlying liver disease in 24%.
Abnormalities of LFTs observed in patients receiving acute PN are therefore significantly influenced by factors relating to underlying disease, especially ongoing sepsis and pre-existing liver disease.
Long term PN
The incidence of abnormal LFTs, abnormal liver histology and more advanced liver disease in adults receiving long term home parenteral nutrition varies between studies (table 1).
Table 1.
Abnormal liver function in patients on long term parenteral nutrition
| Reference | Liver function abnormality | Rate of abnormality in HPN patients (%) | Note |
|---|---|---|---|
| Luman12 | Abnormal LFTs | 48 | ↑Alkaline phosphatase commonest abnormality |
| Salvino63 | Abnormal LFTs | 95 | |
| Cavicchi10 | Chronic biochemical cholestasis* | 65 after 6 months | High lipid content in PN |
| Lloyd13 | Chronic biochemical cholestasis* | 24 | Point prevalence |
| Ito64 | Advanced liver disease† | 19 | |
| Chan65 | Advanced liver disease† | 14 | |
| Cavicchi10 | Complicated liver disease† | 26 at 2 years | High lipid content in PN |
| 50 at 6 years | |||
| Salvino63 | Severe liver disease‡ | 4 |
Persistent elevation to >1.5 times the upper limit of the normal range for >6 months of two of three biochemical variables (alkaline phosphatase, γ-glutamyl transferase and conjugated bilirubin).
Fibrosis or cirrhosis.
Combination of total bilirubin >3 mg/dl, albumin <3.2 g/dl and prothrombin time >3 s prolonged.
HPN, home parenteral nutrition; LFTs, liver function tests; PN, parenteral nutrition.
IFALD is very common in neonates and infants. Unlike adults, intrahepatic cholestasis rather than steatosis is the commonest finding, perhaps reflecting the immaturity of the biliary excretion system in neonates. Neonatal cholestasis relates closely to birth weight, prematurity and duration of PN.6 7 Ninety per cent of neonates who are receiving PN for >3 months develop cholestasis.6 Neonatal cholestasis is also related to bacterial and fungal sepsis and is often rapidly progressive with hepatic failure occurring in 17% of patients.8
Liver histopathology in patients receiving PN
Abnormal LFTs in adults receiving short term PN are characterised by hepatic steatosis, with accumulation of macro- and microvesicular fat within the hepatocytes, which may be accompanied by an extent of steatohepatitis.4 These abnormalities are strongly influenced by the underlying disease, state especially ongoing sepsis. Abnormal liver histology correlates more closely with the presence of intra-abdominal sepsis, renal failure and pre-existing liver disease than with the use of PN.9
For patients who develop abnormal LFTs receiving long term PN, the commonest histopathological features include intracellular and intracanalicular cholestasis, macrovesicular steatosis, microvesicular steatosis and periportal fibrosis. Other features include hepatocellular injury, multinucleated giant cells, phospholipidosis, portal inflammation, acute cholangitis, extramedullary haematopoiesis, bile duct proliferation, varying degrees of fibrosis and cirrhosis10 11
Aetiology of IFALD
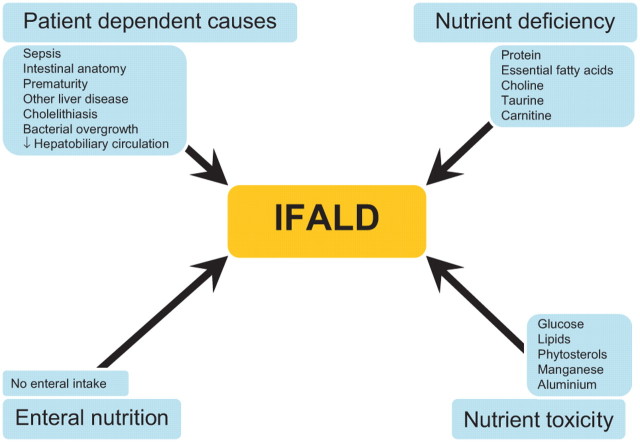
The aetiology of hepatic dysfunction in both adults and children receiving PN is complex and multifactorial. The possible aetiological factors can be divided into four groups (figure 1).
Figure 1.
Factors implicated in the aetiology of intestinal failure associated liver disease (IFALD).
Patient dependent causes
Abnormalities of LFTs may relate to underlying liver disease in patients receiving PN rather than the effects of the PN itself. Other causes include the following.
Sepsis
The presence of sepsis is an important precipitant of cholestasis in neonates and of abnormal LFTs in adults receiving short term PN. In addition, bacterial overgrowth can lead to cholestasis via generation of cholestatic secondary bile acids from the bacterial dehydroxylation of chenodeoxycholic acid,4 endotoxaemia and bacterial translocation.
Intestinal anatomy
IFALD has been shown to be related to small intestinal length in a number of studies. One study has reported that a small intestinal length of <1 m is associated with abnormal LFTs in adults receiving long term PN12 while another study has reported an increased risk of chronic cholestasis in adults with a small intestinal length of <0.5 m.10 The presence or absence of colon in continuity with the small intestine has also recently been associated with the development of IFALD.13 Some authors have postulated that short bowel predisposes to liver dysfunction as a result of impairment of enterohepatic bile salt circulation and abnormal bile acid metabolism.10 Others have argued that bowel length may simply be a surrogate marker of parenteral energy requirement.12 13
Infant prematurity
IFALD is common in neonates with the smallest premature infants being the most susceptible.6 7 This may be due to immaturity of the hepatic transport mechanisms and metabolism of bile acids. Abnormalities have been demonstrated in neonates affecting bile acid synthesis and conjugation,14 hepatic uptake and secretion,15 and intestinal uptake and recirculation of bile acids.16
Lack of enteral nutrition
Reduced enteral nutrition is associated with an increased reliance on PN. It is difficult to know if the increased risk of liver dysfunction in individuals with very little oral intake is a result of the lack of enteral nutrition or the effects of parenteral feeding. Fasting coupled with total PN reduces the secretion of a number of gastrointestinal hormones, including gastrin, motilin, pancreatic polypeptide, insulinotropic polypeptide and glucagon.17 This reduction may reduce intestinal motility, promoting bacterial overgrowth, and may predispose to biliary stasis.
Nutrient deficiency
Hepatic steatosis develops in kwashiorkor as there is insufficient protein for the manufacture of very low density lipoprotein (VLDL), which is needed for hepatic triacylglycerol (TAG) export.18 However, patients receiving PN should receive adequate amino acids for VLDL synthesis. Steatosis also occurs in essential fatty acid deficiency19 but this occurs rarely in patients on PN, unless fat free parenteral regimens are used in individuals with little or no enteral lipid intake.
It has been proposed recently that deficiencies of a number of methionine metabolites, such as carnitine, choline and taurine, may be responsible for both steatosis and cholestasis in patients receiving PN. Orally ingested methionine can be converted to these metabolites via hepatic trans-sulphuration pathways although these pathways are underdeveloped in premature infants.20 Methionine administered parenterally to the systemic circulation rather than to the portal circulation is also transaminated to mercaptans, hence reducing the synthesis of carnitine, choline and taurine.21
Carnitine
Carnitine is involved in the transport of long chain fatty acids across the mitochondrial membrane so that they can undergo oxidation. In deficiency states in which carnitine levels are very low (<10% normal levels), hepatic steatosis can develop.22 Plasma carnitine is about 50% normal levels in patients receiving PN, which is considerably higher than in patients with a congenital or acquired deficiency.23 24 Intervention studies have failed to show any benefit of parenteral carnitine supplementation in patients receiving long term PN in relation to hepatic abnormalities.25
Choline
Choline is required for the synthesis of VLDL and hence hepatic TAG export. Plasma choline concentrations are low in >90% of patients receiving PN26 and deficiency results in impaired hepatic TAG secretion and subsequent steatosis.26 Choline deficiency in patients receiving PN has been shown to correlate with elevated transaminase levels and steatosis in adults and children.26 27 Small studies have shown that these abnormalities can be reversed by either oral or parenteral choline supplementation.28 29
Taurine
Taurine is important for bile salt conjugation, particularly in preterm infants. It promotes bile flow and attenuates the cholestatic effects of secondary bile salts such as lithocholate.30 Taurine deficiency in neonates is associated with cholestatic liver disease31 and this can be prevented by parenteral taurine supplementation.32 Plasma and biliary taurine concentrations are also low in adults with a short bowel33 and supplementation may improve plasma taurine but not biliary taurine concentrations.33 Intervention studies have not demonstrated a significant benefit of parenteral taurine in adults.
Antioxidants
Individuals receiving PN may be deficient in antioxidants, in particular vitamin E and selenium.34 Increased oxidative stress might predispose to lipid peroxidation of hepatic lipid stores resulting in inflammation and steatohepatitis.35 However, studies in children suggest that the occurrence of IFALD is independent of oxidant load.36 In addition, there is little evidence that antioxidant levels are depleted in patients receiving PN, and vitamin E is commonly added to intravenous lipid emulsions.35
Nutrient toxicity
Glucose
Early PN formulations contained large amounts of energy supplied as glucose, and infusion rates often exceeded the maximum glucose oxidation rate. Glucose infusion at rates of >5 mg/kg/min result in steatosis37 by stimulating insulin release (stimulating hepatic lipogenesis and acylglycerol production) and inhibiting mitochondrial fatty acid oxidation,38 resulting in a build up of TAG within hepatocytes. The adverse effects of insulin hypersecretion may also explain why continuous PN infusions are associated with a greater extent of hepatic dysfunction than cyclic infusion. Allowing ≥8 h each day without parenteral glucose infusion has been shown to lower insulin levels and improve LFTs.39
Lipid
The replacement of a proportion of glucose energy with parenteral lipid has been shown to reduce the incidence of steatosis.40 However, excess lipid may also increase hepatic complications. Very high parenteral lipid intakes of >4 g/kg/day may result in lipid overload due to the inability of the reticuloendothelial system to clear large amounts of polyunsaturated fatty acids and phospholipids.41 More modest parenteral lipid intake is also associated with IFALD. The parenteral intake of soybean based lipid emulsions of >1 g/kg/day is associated with a 2.3-fold increased risk of chronic cholestasis and a 5.5-fold increased risk of advanced liver disease (fibrosis or cirrhosis).10 Postulated mechanisms include impaired phospholipid excretion,10 inhibition of hepatic TAG release12 or the accumulation of phytosterols.42
Second generation (medium chain triglyceride/long chain triglyceride mixtures and monounsaturated fatty acids) and third generation (fish oil, multiple combination lipids) lipid emulsions are associated with fewer hepatic complications43–45 although more definitive studies are required before these become routine practice.
Other components
A number of other components of parenteral infusions have been suggested to cause IFALD. High amino acid content is suggested to promote cholestasis in neonates.46 Manganese and copper are both excreted via the biliary route and can accumulate in cholestasis, exacerbating hepatic dysfunction.47 48
Management of IFALD
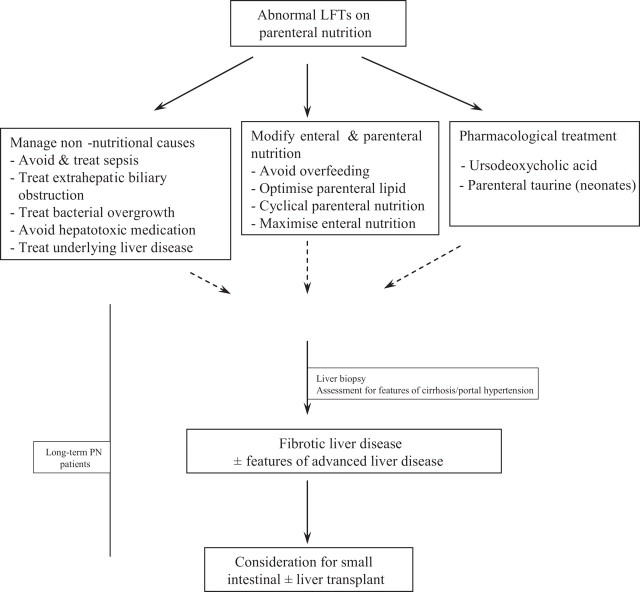
The strategies used in the management of IFALD are illustrated in figure 2.
Figure 2.
Management of intestinal failure associated liver disease. LFTs, liver function tests; PN, parenteral nutrition.
Treatment of non-nutritional causes
Given the importance of sepsis as a causative factor for IFALD, it is vital that every effort is made to reduce its incidence. Intra-abdominal sepsis should be treated using a procedure that is clinically appropriate, usually with a combination of antibiotics and/or minimally invasive drainage procedures. It is also important to avoid central venous catheter associated sepsis. Efforts to reduce bacterial overgrowth have been more successful in neonates than in adults, suggesting a greater pathophysiological role in the former. Several small studies have suggested that antibiotics such as metronidazole and gentamicin may reduce the incidence of cholestasis in both neonates and adults receiving PN.49–51
Cholelithiasis is common in both adults and children receiving PN due to a combination of factors, including reduced oral intake, ileal resection, weight loss and drug treatment.34 The incidence of biliary sludge in adults receiving PN has been estimated to approach 100% after >6 weeks of treatment52 and gallstones have been demonstrated to form in 45% of patients with short bowel syndrome.53
Medications, especially antibiotics, are a common cause of abnormal LFTs and should always be considered in a patient receiving PN with abnormal LFTs. All hepatotoxic medication should be minimised.
Adjustments to parenteral and enteral nutrition
It is important not to overfeed patients receiving PN, given the deleterious effects of infusion of excess parenteral glucose and lipid described earlier. Both USA and UK guidelines suggest a total daily energy intake of 105–146 kJ (25–35 kcal)/kg and a daily protein intake of 0.8–1.5 g/kg,54 55 although more accurate assessment of the basal metabolic rate and energy requirements can be made by performing indirect calorimetry. Given that the majority of patients receiving long term PN will continue to eat, it is also necessary to estimate enteral energy and protein absorption and reduce parenteral provision accordingly. It is important to try to maximise either oral or enteral nutrition as this will counteract the adverse effects of fasting, reduce parenteral requirements and promote intestinal adaptation.
The optimal dose and type of parenteral lipid that should be provided to minimise hepatic dysfunction remains unclear. In patients who have no enteral intake, some parenteral lipid is needed to prevent essential fatty acid deficiency.54 A linoleic acid intake of 2–4% total energy intake is adequate for this purpose56 and, given the very high linoleic acid content of soybean based lipid emulsions, this level of intake is easily achievable. US guidelines suggest that parenteral lipid should supply 20–30% total energy and the daily intake should be <2.5 g/kg and ideally <1.5 g/kg.54 Evidence suggests that the intake of soybean based lipid should be <1.0 g/kg/day.10 Second and third generation lipid emulsions may be considered for patients with IFALD. However, generally it is sufficient to decrease the lipid prescribed rather than switching to a different lipid emulsion.
Finally, cyclical rather than continuous PN should be administered to minimise the adverse effects of prolonged insulin hypersecretion. Allowing an 8 h break from PN has been shown to improve LFTs and reduce insulin levels.39 Cyclical PN also allows greater patient freedom, which may be associated with an improved quality of life in patients receiving long term PN.55 At a practical level, this is achieved by slowly increasing the PN infusion rate and checking for hyperglycaemia. In diabetic patients it is more important to achieve tight glucose control and this may limit or preclude cyclical PN.
Pharmacological treatment
Ursodeoxycholic acid (UDCA) is an exogenous bile acid which promotes bile flow and is used in primary biliary cirrhosis and cholelithiasis. Several studies in neonates with short bowel and IFALD have shown a benefit of 10–30 mg/kg/day UDCA on LFTs.57 58 Evidence of a benefit of UDCA in adults is limited to a single study showing that treatment with oral UDCA reduced γ-glutamyl transferase and alanine aminotransferase concentrations.59 UDCA may also worsen diarrhoea in some individuals.
Plasma choline and taurine concentrations are reduced in patients receiving PN. Multivariate analysis of data collected as part of a larger multicentre trial suggests that parenteral taurine reduces the incidence of cholestasis in severely premature infants and those with necrotising enterocolitis.32 There is considerably less evidence of a benefit of taurine supplementation in adults, with a reduction in aspartate aminotransferase levels being reported in a single small study of adults receiving long term PN.33 Choline supplementation in adults has been shown to be associated with a reduction in hepatic steatosis and improvements in LFTs.29 However, availability and stability issues prevent its use at the moment.
Small intestinal and liver transplantation
Impending or overt liver failure associated with IFALD is recognised as an indication for small intestinal transplantation,54 and IFALD is one of the commonest reasons for performing intestinal transplantation.60 UK transplant units have suggested consideration of transplantation only if there is portal hypertension, cirrhosis or bridging fibrosis.61 In neonates and infants, for whom there is the possibility of an intestinal adaptation with weaning from PN, transplantation of liver alone, which has a considerably better outcome than combined small intestinal and liver transplantation, may be possible as a bridging measure until enteral nutrition is established. In the meantime, the selection of patients for intestinal transplantation (with or without a liver transplant) presents a considerable challenge and recent consensus guidance is now published.62
Footnotes
Competing interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Peden VH, Witzleben CL, Skelton MA. Total parenteral nutrition. J Pediatr 1971;78:180–1. [DOI] [PubMed] [Google Scholar]
- 2.Lindor KD, Fleming CR, Abrams A, et al. Liver function values in adults receiving total parenteral nutrition. JAMA 1979;241:2398–400. [PubMed] [Google Scholar]
- 3.Clarke PJ, Ball MJ, Kettlewell MG. Liver function tests in patients receiving parenteral nutrition. JPEN J Parenter Enteral Nutr 1991;15:54–9. [DOI] [PubMed] [Google Scholar]
- 4.Quigley EM, Marsh MN, Shaffer JL, et al. Hepatobiliary complications of total parenteral nutrition. Gastroenterology 1993;104:286–301. [DOI] [PubMed] [Google Scholar]
- 5.Baker ML, Nightingale JMD. Abnormal liver function tests and parenteral nutrition. Clin Nutr 2004;23:864–5. [Google Scholar]
- 6.Beale EF, Nelson RM, Bucciarelli RL, et al. Intrahepatic cholestasis associated with parenteral nutrition in premature infants. Pediatrics 1979;64:342–7. [PubMed] [Google Scholar]
- 7.Beath SV, Davies P, Papadopoulou A, et al. Parenteral nutrition-related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg 1996;31:604–6. [DOI] [PubMed] [Google Scholar]
- 8.Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr 1998;27:131–7. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe BM, Walker BK, Shaul DB, et al. Effect of total parenteral nutrition on hepatic histology. Arch Surg 1988;123:1084–90. [DOI] [PubMed] [Google Scholar]
- 10.Cavicchi M, Beau P, Crenn P, et al. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med 2000;132:525–32. [DOI] [PubMed] [Google Scholar]
- 11.Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol 2007;4:277–87. [DOI] [PubMed] [Google Scholar]
- 12.Luman W, Shaffer JL. Prevalence, outcome and associated factors of deranged liver function tests in patients on home parenteral nutrition. Clin Nutr 2002;21:337–43. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd DA, Zabron AA, Gabe SM. Chronic biochemical cholestasis in patients receiving home parenteral nutrition: prevalence and predisposing factors. Aliment Pharmacol Ther 2008;27:552–60. [DOI] [PubMed] [Google Scholar]
- 14.Balistreri WF, Heubi JE, Suchy FJ. Immaturity of the enterohepatic circulation in early life: factors predisposing to “physiologic” maldigestion and cholestasis. J Pediatr Gastroenterol Nutr 1983;2:346–54. [PubMed] [Google Scholar]
- 15.Suchy FJ, Bucuvalas JC, Goodrich AL, et al. Taurocholate transport and Na+-K+-ATPase activity in fetal and neonatal rat liver plasma membrane vesicles. Am J Physiol 1986;251:G665–73. [DOI] [PubMed] [Google Scholar]
- 16.de Belle RC, Vaupshas V, Vitullo BB, et al. Intestinal absorption of bile salts: immature development in the neonate. J Pediatr 1979;94:472–6. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg GR, Wolman SL, Christofides ND, et al. Effect of total parenteral nutrition on gut hormone release in humans. Gastroenterology 1981;80:988–93. [PubMed] [Google Scholar]
- 18.Cook GC, Hutt MS. The liver after kwashiorkor. BMJ 1967;3:454–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson TJ, Sgoutas D. Essential fatty acid deficiency in four adult patients during total parenteral nutrition. Am J Clin Nutr 1975;28:258–63. [DOI] [PubMed] [Google Scholar]
- 20.Viña J, Vento M, García-Sala F, et al. L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am J Clin Nutr 1995;61:1067–9. [DOI] [PubMed] [Google Scholar]
- 21.Chawla RK, Berry CJ, Kutner MH, et al. Plasma concentrations of transsulfuration pathway products during nasoenteral and intravenous hyperalimentation of malnourished patients. Am J Clin Nutr 1985;42:577–84. [DOI] [PubMed] [Google Scholar]
- 22.Karpati G, Carpenter S, Engel AG, et al. The syndrome of systemic carnitine deficiency. Clinical, morphologic, biochemical, and pathophysiologic features. Neurology 1975;25:16–24. [DOI] [PubMed] [Google Scholar]
- 23.Bowyer BA, Fleming CR, Ilstrup D, et al. Plasma carnitine levels in patients receiving home parenteral nutrition. Am J Clin Nutr 1986;43:85–91. [DOI] [PubMed] [Google Scholar]
- 24.Moukarzel AA, Dahlstrom KA, Buchman AL, et al. Carnitine status of children receiving long-term total parenteral nutrition: a longitudinal prospective study. J Pediatr 1992;120:759–62. [DOI] [PubMed] [Google Scholar]
- 25.Bowyer BA, Miles JM, Haymond MW, et al. L-carnitine therapy in home parenteral nutrition patients with abnormal liver tests and low plasma carnitine concentrations. Gastroenterology 1988;94:434–8. [DOI] [PubMed] [Google Scholar]
- 26.Buchman AL, Moukarzel A, Jenden DJ, et al. Low plasma free choline is prevalent in patients receiving long term parenteral nutrition and is associated with hepatic aminotransferase abnormalities. Clin Nutr 1993;12:33–7. [DOI] [PubMed] [Google Scholar]
- 27.Buchman AL, Sohel M, Moukarzel A, et al. Plasma choline in normal newborns, infants, toddlers, and in very-low-birth-weight neonates requiring total parenteral nutrition. Nutrition 2001;17:18–21. [DOI] [PubMed] [Google Scholar]
- 28.Buchman AL, Dubin M, Jenden D, et al. Lecithin increases plasma free choline and decreases hepatic steatosis in long-term total parenteral nutrition patients. Gastroenterology 1992;102:1363–70. [PubMed] [Google Scholar]
- 29.Buchman AL, Ament ME, Sohel M, et al. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial. JPEN J Parenter Enteral Nutr 2001;25:260–8. [DOI] [PubMed] [Google Scholar]
- 30.Belli DC, Roy CC, Fournier LA, et al. The effect of taurine on the cholestatic potential of sulfated lithocholate and its conjugates. Liver 1991;11:162–9. [DOI] [PubMed] [Google Scholar]
- 31.Cooper A, Betts JM, Pereira GR, et al. Taurine deficiency in the severe hepatic dysfunction complicating total parenteral nutrition. J Pediatr Surg 1984;19:462–6. [DOI] [PubMed] [Google Scholar]
- 32.Spencer AU, Yu S, Tracy TF, et al. Parenteral nutrition-associated cholestasis in neonates: multivariate analysis of the potential protective effect of taurine. JPEN J Parenter Enteral Nutr 2005;29:337–43. [DOI] [PubMed] [Google Scholar]
- 33.Schneider SM, Joly F, Gehrardt MF, et al. Taurine status and response to intravenous taurine supplementation in adults with short-bowel syndrome undergoing long-term parenteral nutrition: a pilot study. Br J Nutr 2006;96:365–70. [DOI] [PubMed] [Google Scholar]
- 34.Nightingale JM. Hepatobiliary, renal and bone complications of intestinal failure. Best Pract Res Clin Gastroenterol 2003;17:907–29. [DOI] [PubMed] [Google Scholar]
- 35.Buchman AL, Iyer K, Fryer J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology 2006;43:9–19. [DOI] [PubMed] [Google Scholar]
- 36.Lavoie JC, Chessex P, Gauthier C, et al. Reduced bile flow associated with parenteral nutrition is independent of oxidant load and parenteral multivitamins. J Pediatr Gastroenterol Nutr 2005;41:108–14. [PubMed] [Google Scholar]
- 37.Burke JF, Wolfe RR, Mullany CJ, et al. Glucose requirements following burn injury. Parameters of optimal glucose infusion and possible hepatic and respiratory abnormalities following excessive glucose intake. Ann Surg 1979;190:274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, Nussbaum MS, Teague D, et al. Increasing dextrose concentrations in total parenteral nutrition (TPN) causes alterations in hepatic morphology and plasma levels of insulin and glucagon in rats. J Surg Res 1988;44:639–48. [DOI] [PubMed] [Google Scholar]
- 39.Hwang TL, Lue MC, Chen LL. Early use of cyclic TPN prevents further deterioration of liver functions for the TPN patients with impaired liver function. Hepatogastroenterology 2000;47:1347–50. [PubMed] [Google Scholar]
- 40.Meguid MM, Akahoshi MP, Jeffers S, et al. Amelioration of metabolic complications of conventional total parenteral nutrition. A prospective randomized study. Arch Surg 1984;119:1294–8. [DOI] [PubMed] [Google Scholar]
- 41.Bigorgne C, Le Tourneau A, Vahedi K, et al. Sea-blue histiocyte syndrome in bone marrow secondary to total parenteral nutrition. Leuk Lymphoma 1998;28:523–9. [DOI] [PubMed] [Google Scholar]
- 42.Clayton PT, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition 1998;14:158–64. [DOI] [PubMed] [Google Scholar]
- 43.Carpentier YA, Richelle M, Haumont D, et al. New developments in fat emulsions. Proc Nutr Soc 1990;49: 375–80. [DOI] [PubMed] [Google Scholar]
- 44.Pálová S, Charvat J, Kvapil M. Comparison of soybean oil- and olive oil-based lipid emulsions on hepatobiliary function and serum triacylglycerols level during realimentation. J Int Med Res 2008;36:587–93. [DOI] [PubMed] [Google Scholar]
- 45.Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 2008;121:e678–86. [DOI] [PubMed] [Google Scholar]
- 46.Vileisis RA, Inwood RJ, Hunt CE. Prospective controlled study of parenteral nutrition-associated cholestatic jaundice: effect of protein intake. J Pediatr 1980;96:893–7. [DOI] [PubMed] [Google Scholar]
- 47.Fell JM, Reynolds AP, Meadows N, et al. Manganese toxicity in children receiving long-term parenteral nutrition. Lancet 1996;347:1218–21. [DOI] [PubMed] [Google Scholar]
- 48.Blaszyk H, Wild PJ, Oliveira A, et al. Hepatic copper in patients receiving long-term total parenteral nutrition. J Clin Gastroenterol 2005;39:318–20. [DOI] [PubMed] [Google Scholar]
- 49.Spurr SG, Grylack LJ, Mehta NR. Hyperalimentation-associated neonatal cholestasis: effect of oral gentamicin. JPEN J Parenter Enteral Nutr 1989;13:633–6. [DOI] [PubMed] [Google Scholar]
- 50.Kubota A, Okada A, Imura K, et al. The effect of metronidazole on TPN-associated liver dysfunction in neonates. J Pediatr Surg 1990;25:618–21. [DOI] [PubMed] [Google Scholar]
- 51.Capron JP, Gineston JL, Herve MA, et al. Metronidazole in prevention of cholestasis associated with total parenteral nutrition. Lancet 1983;1:446–7. [DOI] [PubMed] [Google Scholar]
- 52.Messing B, Bories C, Kunstlinger F, et al. Does total parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology 1983;84:1012–19. [PubMed] [Google Scholar]
- 53.Nightingale JM, Lennard-Jones JE, Gertner DJ, et al. Colonic preservation reduces need for parenteral therapy, increases incidence of renal stones, but does not change high prevalence of gall stones in patients with a short bowel. Gut 1992;33:1493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buchman AL, Scolapio J, Fryer J. AGA technical review on short bowel syndrome and intestinal transplantation. Gastroenterology 2003;124:1111–34. [DOI] [PubMed] [Google Scholar]
- 55.National Institute for Health and Clinical Excellence. Nutrition Support in Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. NICE Clinical Guideline 32. London: NICE, 2006.
- 56.Food and Agriculture Organization/World Health Organization/United Nations University. Fats and Oils in Human Nutrition. Report of a Joint Expert Consultation. FAO Food and Nutrition Paper no. 57. Geneva: WHO, 1993. [PubMed]
- 57.Chen CY, Tsao PN, Chen HL, et al. Ursodeoxycholic acid (UDCA) therapy in very-low-birth-weight infants with parenteral nutrition-associated cholestasis. J Pediatr 2004;145:317–21. [DOI] [PubMed] [Google Scholar]
- 58.De Marco G, Sordino D, Bruzzese E, et al. Early treatment with ursodeoxycholic acid for cholestasis in children on parenteral nutrition because of primary intestinal failure. Aliment Pharmacol Ther 2006;24:387–94. [DOI] [PubMed] [Google Scholar]
- 59.Beau P, Labat-Labourdette J, Ingrand P, et al. Is ursodeoxycholic acid an effective therapy for total parenteral nutrition-related liver disease? J Hepatol 1994;20:240–4. [DOI] [PubMed] [Google Scholar]
- 60.Grant D, Abu-Elmagd K, Reyes J, et al. 2003 Report of the intestine transplant registry: a new era has dawned. Ann Surg 2005;241:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Middleton SJ, Jamieson NV. The current status of small bowel transplantation in the UK and internationally. Gut 2005;54:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beath S, Pironi L, Gabe S, et al. Collaborative strategies to reduce mortality and morbidity in patients with chronic intestinal failure including those who are referred for small bowel transplantation. Transplantation 2008;85:1378–84. [DOI] [PubMed] [Google Scholar]
- 63.Salvino R, Ghanta R, Seidner DL, et al. Liver failure is uncommon in adults receiving long-term parenteral nutrition. JPEN J Parenter Enteral Nutr 2006;30:202–8. [DOI] [PubMed] [Google Scholar]
- 64.Ito Y, Shils ME. Liver dysfunction associated with long-term total parenteral nutrition in patients with massive bowel resection. JPEN J Parenter Enteral Nutr 1991;15:271–6. [DOI] [PubMed] [Google Scholar]
- 65.Chan S, McCowen KC, Bistrian BR, et al. Incidence, prognosis, and etiology of end-stage liver disease in patients receiving home total parenteral nutrition. Surgery 1999;126:28–34. [DOI] [PubMed] [Google Scholar]