Abstract
With-no-lysine kinase (WNK) and Na+-K+-2Cl− cotransporter 1 (NKCC1) are involved in the pathogenesis of hypertension. In this study, we investigated the WNK-NKCC1 signaling pathway in spontaneously hypertensive rats (SHR) and their associated susceptibility to stroke injury. Basal NKCC1 protein levels were higher in SHR than in normotensive Wistar Kyoto (WKY) rat brains. After inducing ischemic stroke, adult male WKY and SHR received either saline or NKCC1 inhibitor bumetanide (10 mg/kg/day, i.p.) starting at 3-h post-reperfusion. NKCC1 inhibition blunted the extent of ischemic infarction in SHR and improved their neurobehavioral functions. Interestingly, ischemia led to increased NKCC1 phosphorylation in SHR but not in WKY rats. Pronounced elevation of WNK1, WNK2 and WNK4 protein and downregulation of WNK3 were detected in ischemic SHR, but not in ischemic WKY rats. Upregulation of WNK-NKCC1 complex in ischemic SHR brain was associated with increased Ca2+-binding protein 39 (Cab39), without increases in Ste20-related proline alanine-rich kinase or oxidative stress-responsive kinase-1. Moreover, subacute middle cerebral artery stroke human brain autopsy exhibited increased expression of NKCC1 protein. We conclude that augmented WNK-Cab39-NKCC1 signaling in SHR is associated with an increased susceptibility to ischemic brain damage and may serve as a novel target for anti-hypertensive and anti-ischemic stroke therapy.
Keywords: Bumetanide, Cab39, hypertension, ischemic stroke, NKCC1, SHR, WNK kinase
Introduction
WNK (with-no-lysine (K)) kinases are serine (Ser)/threonine (Thr) kinases, which regulate electroneutral SLC12 cation-Cl− cotransporters (CCC) by phosphorylation.1 The Ste20-related proline alanine-rich kinase (SPAK) and oxidative stress-responsive kinase-1 (OSR1) are downstream substrates of WNK kinases acting as links between WNKs and ion transporters.1–3 The WNK signaling pathway plays an important role in renal NaCl and K+ handling and the pathogenesis of hypertension in humans, while SPAK is a hypertension susceptibility gene.4,5 WNK1 and WNK4 mutations lead to constitutive activation of the kidney-specific thiazide-sensitive Na+-Cl− cotransporter. This constitutive activation causes familial hyperkalemic hypertension (FHHt, also known as pseudohypoaldosteronism type 2), an autosomal dominant disorder characterized by hypertension, increased renal Na+ reabsorption, and impaired excretion of K+ and H+.6 However, mutations in kelch-like 3 (KLHL3) and cullin 3 (Cul3), components of an E3 ubiquitin ligase complex, can also cause FHHt via impaired regulation of WNK degradation.7,8 On the other hand, the WNK-SPAK/OSR1 signaling pathway is involved in maintaining arterial tone and blood pressure by directly stimulating Na+-K+-2Cl− cotransporter 1 (NKCC1) activity in resistance vessels. Global heterozygous knockdown of OSR1 or global knockout of SPAK or NKCC1 can lower blood pressure by extrarenal mechanisms.9–11
Developmental up-regulation of NKCC1 protein and mRNA was detected in aorta, heart, and kidney in spontaneously hypertensive rats (SHRs) at postnatal ages of 10–18 weeks.12 NKCC1 up-regulation in hypothalamus disrupts Cl− homeostasis and impairs GABAergic inhibition, providing a neurological basis for the diminished synaptic inhibition and elevated sympathetic vasomotor tone that accompany hypertension in SHRs.13 It is well documented that SHRs exhibit more severe ischemic brain damage than normotensive rats.14,15 Previous studies from our group and others showed that stimulation of NKCC1 activity is involved in ischemic cell damage through NKCC1-mediated Na+ and Cl− overload, cytotoxic edema as well as excitotoxicity.16–19 Interestingly, we have recently found that WNK3 and SPAK kinases are stimulated in cortical neurons and oligodendrocytes in mice after ischemic stroke.20 Knockout of WNK3 or SPAK in mice abolishes phosphoactivation of NKCC1 and reduces brain infarction and associated malignant edema after ischemic stroke.20,21 These findings led us to investigate the possibility that the WNK-SPAK/OSR1-NKCC1 signaling pathway is stimulated in the brains of SHRs, leading to increased susceptibility to ischemic damage.
Here, we report that SHR brain expressed higher levels of total and phosphorylated (activated) NKCC1 protein and exhibited larger cerebral infarct and hemispheric swelling than did normotensive Wistar Kyoto (WKY) rats. WNK1, WNK2, and WNK4 were up-regulated in ischemic SHR brains, but not in those of WKY. Pharmacological inhibition of NKCC1 function with bumetanide (BMT) nearly abolished the SHR-associated increment in post-ischemic infarct and hemispheric swelling volume. Together, our data suggest that an alternative WNK-Cab39-NKCC1 signaling pathway may play an important role in exacerbating cerebral ischemic damage in hypertensive SHRs.
Material and methods
Animal preparation
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The manuscript adheres to the ARRIVE guidelines for reporting animal experiments.
Male SHR and WKY rats of 10–13 weeks of age were purchased from Harlan Laboratory (Indianapolis, IN) and maintained in the University Laboratory Animal Facility until experimental use. All rats were housed in group (two per case) in a controlled temperature and humidity environment. They were maintained on a 12-h light/12-h dark cycle and provided with food and water ad libitum. At the time of experimental use, rats weighed 270–320 g.
Middle cerebral artery (MCA) occlusion model
Focal cerebral ischemia was induced by transient occlusion of the left MCA for 2 h as described previously.22 A detailed description of this method is provided in the Data Supplement.
Drug treatment
Either saline or bumetanide (10 mg/kg body weight/day in saline) was administered via intraperitoneal injection (Figure 1(a)). The initial dose of 5 mg/kg at 3 h and the second dose of 5 mg/kg at 8-h post-reperfusion were followed by two daily injections (b.i.d.).
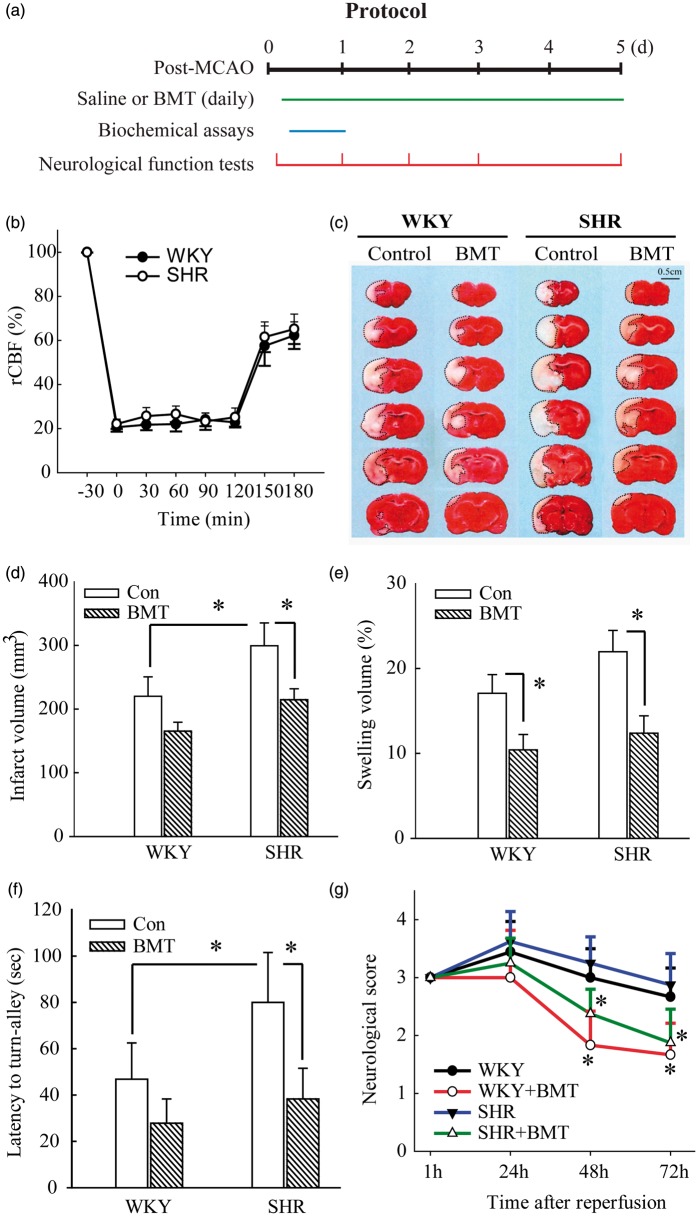
Figure 1.
NKCC1 inhibitor BMT attenuates ischemic stroke-mediated exacerbation of infarction and improves sensorimotor deficits in SHR. (a) Experimental protocol and data collection. BMT: bumetanide; tMCAO: transient middle cerebral artery occlusion. (b) rCBF measurements during and after 2-h tMCAO were indistinguishable in SHR and WKY rats. (c) Representative TTC staining images in WKY and SHRs at 24-h reperfusion, with quantitative analysis of infarct volume (d) and percent hemisphere swelling (e). Saline or bumetanide (10 mg/kg body weight/day in saline) was administered via intra-peritoneal injection, with an initial BMT dose (5 mg/kg) at 3 h and the second dose (5 mg/kg) at 8-h reperfusion and followed by two daily injections (bid). Data are mean ± SD, n = 4, *p < 0.05. (f) Turning-in-alley test latency. Data are mean ± SD, n = 8, *p < 0.05. (g) Neurological deficit scores in WKY and SHRs. Data are mean ± SD, n = 8, *p < 0.05 vs. respective control.
Calculation of infarct volume and hemispheric swelling
After 24 h of reperfusion, rats were anesthetized with 5% isoflurane vaporized in N2O and O2 (3:2) and decapitated. Coronal brain sections were stained with 1% 2,3,5-triphenyltetrazolium chloride monohydrate to measure infarct volume and hemispheric swelling as described before.23,24 A detailed description is provided in the Data Supplement.
Immunofluorescence and immunohistochemistry
De-identified, formalin-fixed, paraffin-embedded human brain autopsy tissue sections were obtained from the Neurodegenerative Brain Bank at the University of Pittsburgh with approval of the local Committee for Oversight of Research and Clinical Training Involving Decedents (CORID). Tissue sections of infarct tissue and separate sections of non-ischemic cortical tissue were obtained for all cases. All strokes were located within the MCA vascular territory and were classified by morphological criteria as ʻearly subacuteʼ.
In a blinded manner, immunohistochemical study was conducted on human autopsy brain samples. Brain sections were blocked for endogenous peroxidase and biotin before overnight application of primary antibodies (mouse anti-tNKCC1 1:100 or rabbit anti-MAP-2 1:200) at 4℃ followed by 60-min RT incubation with secondary antibodies. Subsequent immunodetection was with the Elite Vector Stain ABC System, using DAB as the chromogen substrate. Images were acquired with a Nikon TE-2000 brightfield microscope.
The immunofluorescence staining was conducted on human autopsy brain tissue sections and SHR brain sections as previously described.25,26 A detailed description is provided in the Data Supplement.
The online-only Data Supplement of the article is available for materials and other experimental methods used in the study.
Statistical analysis
Animal subjects were randomly assigned into different studies and surgical procedures, and data analyses were performed by investigators blinded to experimental conditions. The number of animals studied was 80% powered to detect 20% changes with α (two-sided) = 0.05. A total of 86 rats were used in the study, and no results were excluded from the analysis. Data were expressed as mean ±SD. Statistical significance was determined by Studentʼs t-test, or two-way ANOVA using the Tukey’s post-hoc test in case of multiple comparisons (GraphPad Prism 6.0, San Diego, CA, USA). Neurological deficit score was analyzed by the non-parametric Mann–Whitney test. The Pearson correlation coefficient was calculated using online statistics software (Office for Research Development and Education, version 1.1.23-r7). A probability value < 0.05 was considered statistically significant.
Results
Pharmacological inhibition of NKCC1 activity reduces infarction and severity of swelling and improves neurological function of SHR
NKCC1 activation during cerebral ischemia has been documented to disrupt cerebral ion homeostasis, leading to cellular damage in rat and mouse.16,18 We first tested the ability of pharmacological inhibition of NKCC1 with bumetanide (BMT) to attenuate the increased infarction severity in SHR. Regional cerebral blood flow was indistinguishable in WKY and SHR prior to, during, and after MCAO (Figure 1(b)). After 24-h reperfusion post-MCAO, cortical infarct volume in SHR brains (299.3 ± 35.7 mm3) was markedly larger than in WKY brains (220.0 ± 30.6 mm3, p < 0.05, Figure 1(c) and (d)). BMT administration significantly attenuated infarction size in SHR, reducing infarct volume to 214.5 ± 17.3 mm3 (p < 0.05). BMT treatment only moderately reduced infarct volume in WKY rats to 165.2 ± 14.2 mm3, a difference that did not reach statistical significance (p = 0.16). Figure 1(e) illustrates brain swelling in saline-treated WKY and SHR brains (17.0 ± 2.1 and 21.9 ± 2.5%, respectively). BMT treatment blunted brain swelling to indistinguishable lower levels in both SHR and WKY brains (12.3 ± 2.0% and 10.4 ± 1.8%, respectively).
BMT treatment also improved sensorimotor deficits in WKY and SHR. As shown in Figure 1(f), latency times in the turn-alley test (79.2 ± 21.5 s) were longer in saline-treated SHR than in WKY (46.8 ± 15.6 s, p < 0.05), reflecting the worsened sensory motor function deficits in SHR. BMT treatment significantly improved the sensory motor function deficits in SHR, with latency values indistinguishable from those of WKY. Faster improvement of neurological deficit scores was also detected in the BMT-treated WKY and SHR groups (Figure 1(g)). Taken together, these data strongly suggest that augmented NKCC1 expression and activation in ischemic SHR brains are associated with the latterʼs greater severity of ischemic injury.
Ischemic stroke-induced NKCC1 phosphorylation is elevated in SHR brain
Basal, non-ischemic levels of total NKCC1 protein (tNKCC1, 130 kDa and 170 kDa) in SHR brain exceeded those in WKY brain by 52.8 ± 9.1% in membrane fractions and by 39.1 ± 2.8% in whole brain lysates (Figure 2(a)). tNKCC1 protein levels in cytosol fractions of WKY and SHR brains did not differ. These data suggest that the increased tNKCC1 protein of SHR brain is enriched in membrane fractions, consistent with recent reports of elevated levels of glycosylated NKCC1 protein in SHR hypothalamus.13
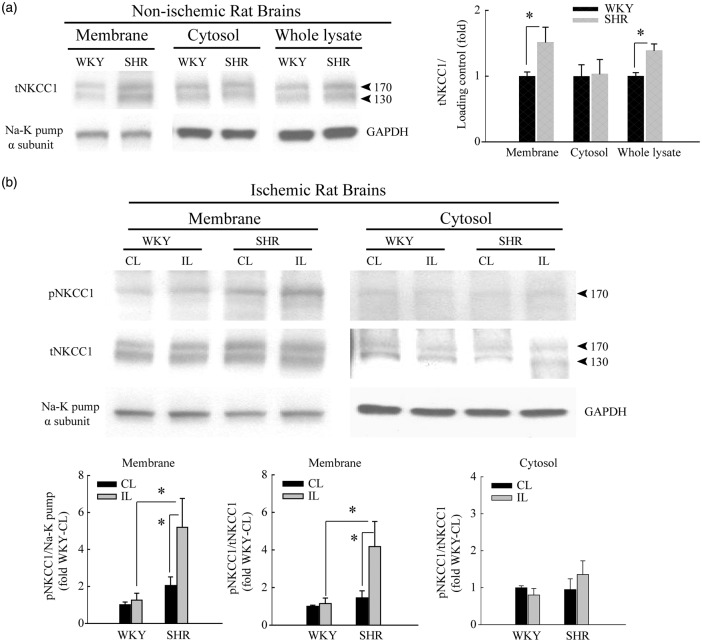
Figure 2.
Selective elevation of NKCC1 protein expression in the cerebral cortex of SHR brain after ischemic stroke. (a) Representative immunoblots showing higher baseline expression of total NKCC1 (tNKCC1) in whole homogenate and crude membrane fractions of brain cortices from normal control SHR than in those from WKY rats. α-subunit of Na+-K+ pump and GAPDH served as loading controls for membrane and cytosol fractions, respectively. Data are mean ± SD, n = 4, *p < 0.05 WKY vs. SHR. (b) Ischemic stroke selectively stimulates phosphorylated NKCC1 (pNKCC1) expression at 6-h post-tMCAO in SHR but not WKY rats. Cytosol and crude membrane protein fractions were prepared from contralateral (CL) and ipsilateral (IL) cortices of WKY and SHR. Data are mean ± SD, n = 4, *p < 0.05 WKY vs. SHR.
We investigated the possible contribution of elevated NKCC1 expression and function to the increased susceptibility of SHR brains to ischemic damage.15 The contralateral (CL) hemispheres of SHR brains revealed basal levels of phosphorylated NKCC1 (pNKCC1, 170 kDa band) 103.0 ± 19.5% higher than in WKY brains (Figure 2(b)). At 6-h post-reperfusion following tMCAO, no significant differences in expression of either tNKCC1 or pNKCC1 were detected in the membrane-enriched fraction from ipsilateral hemispheres of (IL) WKY brains. In contrast, ischemic stroke increased pNKCC1 expression by 2.5 ± 0.5 fold in the IL membrane-enriched fraction of SHR brains compared with CL membrane fractions of SHR. No differences in pNKCC1 expression were detected in cytosol fractions of either WKY or SHR brains. These findings further indicate that ischemic stroke triggers an earlier onset of stimulatory phosphorylation of NKCC1 in SHR brains than WKY brains. We hypothesized that the WNK kinase signaling pathway may be involved in this stimulation in SHR brains.
SHR brains exhibit pronounced upregulation of WNK1, WNK2, and WNK4 proteins after ischemic stroke
We investigated changes in the abundance of ion transporter kinases WNK1, WNK2, WNK3, and WNK4 in WKY and SHR brains after ischemic stroke. Figure 3(a) shows that WKY brains had low basal WNK1 protein expression (250 kDa) and did not exhibit significant changes after ischemic stroke. In contrast, ischemic SHR brains showed 4.2 ± 1.2 fold increase and 2.8 ± 0.8 fold increase of WNK1 protein (250 kDa) in membrane and cytosol fractions, respectively. A lower molecular weight WNK1 band (∼210 kDa) was detected in WKY and SHR brains, which was not detected in kidney tissue (Supplemental Figure 1). Ischemic stroke increased WKY brain abundance of the 210 kDa WNK1 protein by 2.9 ± 1.1 fold (cytosol) and 3.1 ± 1.0 fold (membrane fractions), respectively. Ischemic SHR cortical tissues exhibited far more dramatic increases of the 210 kDa WNK1 protein in the membrane fraction (8.1 ± 2.5 fold) and cytosol fraction (14.5 ± 4.5 fold).
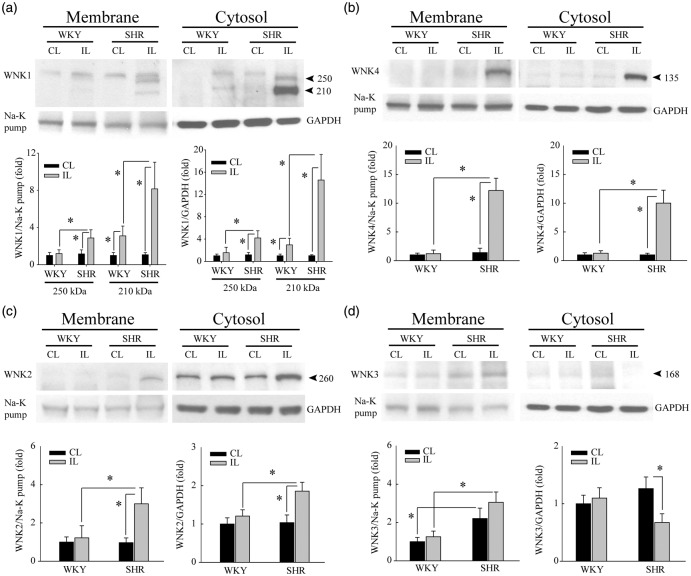
Figure 3.
SHR brains exhibit pronounced upregulation of WNK1, WNK2, and WNK4 proteins after ischemic stroke. (a) Upregulation of full length WNK1 protein (250 kDa) was detected at 6-h reperfusion post-tMCAO only in IL cortices of SHR but not WKY rats. Cytosol and crude membrane protein fractions were prepared from contralateral (CL) and ipsilateral (IL) cortices of WKY and SHR. Data are mean ± SD, n = 4, *p < 0.05. (b) Upregulation of WNK4 protein in the same samples as in (a). Data are mean ± SD, n = 4, *p < 0.05. (c) Upregulation of WNK2 protein in the same samples as in (a). Data are mean ± SD, n = 4, *p < 0.05. (d) Ischemic stroke did not cause significant changes of WNK3 protein expression in either WKY or SHR brains but reduced WNK3 expression in the IL cytosol fraction of SHR brains. Data are mean ± SD, n = 4, *p < 0.05.
WNK4 protein upregulation (135 kDa) was detected in ischemic SHR brains, with a 12.2 ± 2.1 fold increase in the IL membrane fraction and a 10.0 ± 2.2 fold increase in the IL cytosol fraction (Figure 3(b)). In the case of WNK2 protein (260 kDa), SHR brains exhibited only a moderate upregulation of WNK2 (2–3 fold) in the IL cortex in response to ischemic stroke (Figure 3(c)). No such change was detected in ischemic WKY brains. Last, higher basal expression of WNK3 protein (168 kDa) was detected in the membrane fractions of SHR brains than of WKY brains under both control and ischemic conditions. Interestingly, ischemic stroke did not further elevate WNK3 levels in the membrane fraction, but instead selectively reduced expression of WNK3 in the cytosol fraction of SHR brains (Figure 3(d)). Taken together, these data demonstrate differential regulation of WNK kinase proteins in WKY and SHR brains in response to ischemic stroke.
Ischemic stroke triggers inhibition of SPAK/OSR1 phosphorylation and degradation of SPAK in SHR brains
We hypothesized that up-regulation of WNK1, WNK2, and WNK4 may stimulate the intermediate kinases SPAK/OSR1.2,3 High basal levels of pSPAK protein (∼68 kDa) were detected in WKY and SHR brains. Ischemic stroke did not significantly alter pSPAK protein expression in WKY brains; however, we unexpectedly observed decreased levels of pSPAK protein (∼68 kDa) in both membrane and cytosol fractions of SHR brains (Figure 4(a) and (b)). tSPAK/tOSR1 (68 kDa, 55 kDa) were also drastically reduced in the cytosol fraction of IL cortex of SHR. This decrease was accompanied by emergence in both membrane and cytosol fractions of IL cortex from SHR brains of a novel 45 kDa polypeptide, not recognized by specific antibodies to OSR1 (Supplemental Figure 2) or to pSPAK (pSer383) (Figure 4(a) and (b)). Therefore, the 45 kDa band may be a truncated form of SPAK protein. On the other hand, pOSR1 levels in IL cortices of ischemic SHR and WKY brains did not change. Therefore, increased NKCC1 phosphorylation in ischemic SHR brains may be independent of SPAK/OSR1 complex activation.
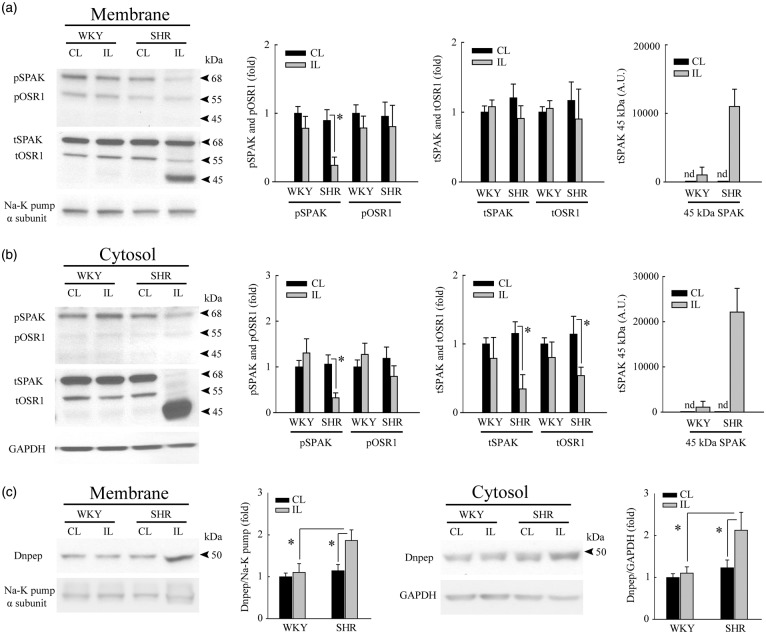
Figure 4.
Ischemic stroke selectively triggers inhibition of SPAK/OSR1 phosphorylation and degradation of SPAK in SHR brains. (a) Plasma membrane fraction of the IL cerebral cortex of SHR brains exhibits selective reduction of pSPAK (68 kDa) expression at 6-h reperfusion post-tMCAO. A protein band of ∼45 kDa was detected in the membrane fraction of SHR brains by anti-tSPAK antibody but not by the anti-pSPAK/pOSR1 antibody (arrowhead). The band was interpreted as a putative, proteolytic cleavage product of pSPAK. Data are mean ± SD, n = 4, *p < 0.05. (b) Cytosol fractions from IL cerebral cortex of SHR brains show reduced expression of pSPAK, tSPAK, tOSR1, and increased accumulation of the putative cleaved SPAK band (∼45 kDa) undetected by the anti-pSPAK/pOSR1 antibody (arrowhead). Data are mean ± SD, n = 4, *p < 0.05. (c) Upregulation of Dnpep protease (aspartyl aminopeptidase, ∼50 kDa) was detected only in IL cortices of SHR but not in WKY rats at 6-h reperfusion post-tMCAO. Cytosol and crude plasma membrane protein fractions were prepared from the contralateral (CL) and ipsilateral (IL) cortices of WKY and SHR. Data are mean ± SD, n = 4, *p < 0.05.
The ischemia-induced expression of the lower molecular weight 45 kDa SPAK-immunoreactive polypeptide in ischemic SHR brain prompted us to assess the expression of a SPAK-specific protease, DNPEP (aspartyl aminopeptidase).27 DNPEP levels in ischemic SHR brains were 1.9 ± 0.2 fold higher in the membrane fraction and 2.1 ± 0.4 fold higher in the cytosol fraction than those of ischemic WKY brain (Figure 4(c)). These results strongly suggest that the 45 kDa SPAK-immunoreactive band of ischemic SHR brain is a proteolytic product of SPAK produced by DNPEP protease activation.
We also examined expression of KLHL2/3 and CUL3 proteins, critical components of the canonical E3 ubiquitin ligase complex, which regulates WNK abundance (Supplemental Figure 3(A) and (B)).28,29 KLHL2/3 was down-regulated in the membrane fraction of ischemic SHR brains, but increased in the cytosol fraction (Supplemental Figure 3(A)). These results indicate that ischemic stroke-induced net increases of WNK proteins are unlikely due to compromised function of CUL3 and KLHL2/3 proteins. However, changes of other CUL and KLHL proteins in ischemic brains remain to be determined.
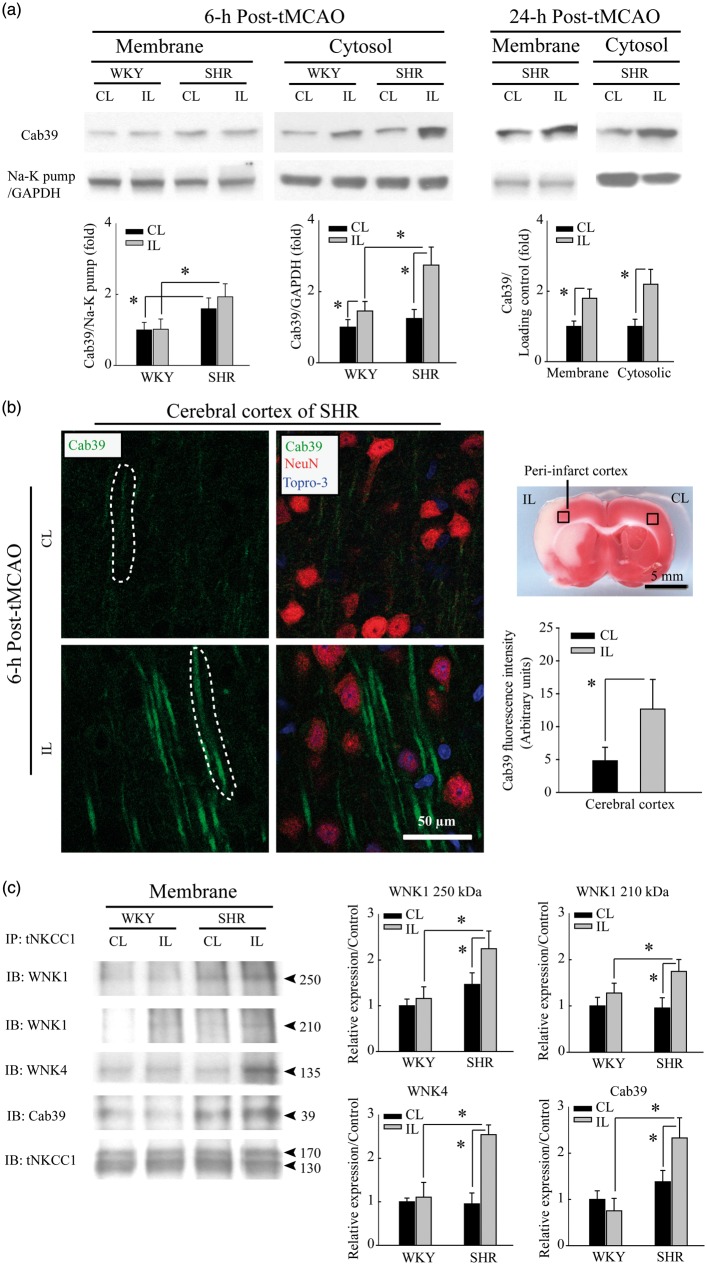
Ischemic stroke-mediated up-regulation of scaffolding protein Cab39 in SHR brains
The lack of SPAK/OSR1 activation in ischemic SHR brains led us to hypothesize that stimulation of the WNK-NKCC1 complex may involve intermediaries that do not require SPAK or OSR1. One candidate is the scaffolding protein Cab39 (or MO25 for mouse protein 25), which facilitates SPAK/OSR1-independent phosphorylation of NKCC1.30 Figure 5(a) shows that trends of Cab39 (∼39 kDa) levels were higher (although not statistically significant) in both CL and IL membrane fractions from SHR than from WKY brains. Ischemic stroke further increased Cab39 protein levels in the cytosol fraction of SHR brains (2.7 ± 0.5 fold) at 6-h post-tMCAO, in contrast to a moderate Cab39 increase (1.5 ± 0.3 fold) in the cytosol fraction of ischemic WKY brains. By 24-h post-tMCAO, SHR brains showed significantly increased Cab39 protein expression in the membrane fraction (1.8 ± 0.2 fold) and sustained elevation in the cytosolic fractions (2.0 ± 0.4 fold). We speculate that the apparent lack of Cab39 protein upregulation at 6-h post-ischemia as detected by immunoblotting of membrane fractions reflects a regional increase of limited magnitude. This speculation is supported by immunofluorescence staining (Figure 5(b)). The low basal level of Cab39 protein expression observed in the non-ischemic SHR cortex contrasted with clearly elevated Cab39 in neuronal processes of SHR cortical tissues 6-h post-ischemia. Increased Cab39 expression in neuronal processes co-localized with neuronal dendrite marker protein MAP-2 in SHR brains (Supplemental Figure 4). These results suggest that upregulation of Cab39 expression may facilitate WNK-NKCC1 interactions. To test this possibility, we conducted a co-immunoprecipitation study. As shown in Figure 5(c), WNK1, WNK4, and Cab39 proteins were co-immunoprecipitated along with NKCC1 protein using the anti-tNKCC1 T4 monoclonal antibody. WNK1, WNK4, and Cab39 proteins were specifically enriched in tNKCC1 immunoprecipitations from ischemic SHR brains. Taken together, these findings suggest that WNK1/4, Cab39, and NKCC1 protein contribute to a multiprotein complex.
Figure 5.
Ischemic stroke selectively triggers upregulation of Cab39 protein expression in SHR brains. (a) SHR brain membrane fractions exhibit greater Cab39 protein abundance than do WKY brain membrane fractions. Ischemic stroke increases Cab39 levels in SHR cytosol fractions to a greater degree than in WKY brain cytosol fractions at 6-h reperfusion post-tMCAO. At 24-h reperfusion post-tMCAO, Cab39 expression was further increased in both membrane and cytosolic fractions in SHR brain. Data are mean ± SD, n = 4, *p < 0.05. (b) Immunofluorescence analysis showing Cab39 upregulation in peri-infarct cortex of SHR brain at 6-h post-tMCAO. Data are mean ± SD, n = 3, *p < 0.05. (c) Increased levels of WNK1, WNK4, and Cab39 proteins were detected in anti-tNKCC1 immunoprecipitates from ischemic IL cerebral cortices of SHR at 6-h reperfusion post-tMCAO compared with CL cortex of WKY. Data are mean ± SD, n = 4, *p < 0.05.
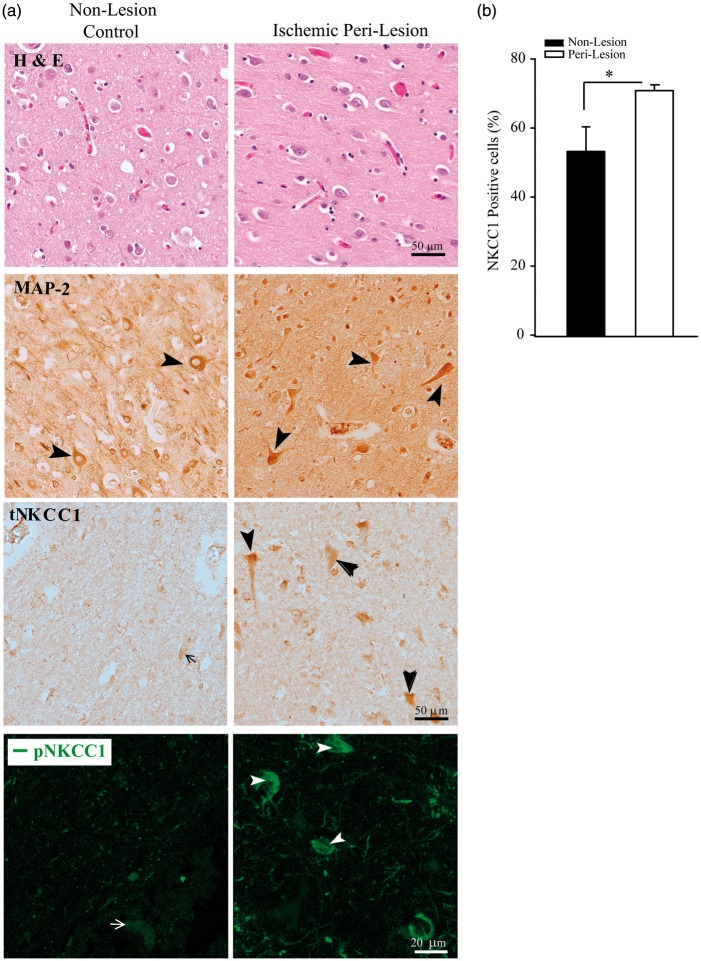
Human subacute MCA stroke brain autopsy exhibited elevated NKCC1 protein expression
To test the possible role of NKCC1 in human ischemic stroke, we examined expression of tNKCC1 protein in brain autopsy specimens from three subacute MCA ischemic stroke subjects (Supplemental table I). Non-lesion and peri-lesion penumbra regions were identified with H & E staining (Figure 6(a), upper panel). In the non-lesion region, neurons exhibited abundant MAP-2 expression, in contrast to the low intensity of tNKCC1 immunoreactivity (arrow). The latter represents non-specific signals because a similar low immunoreactive background of tNKCC1 staining was detected in the brain sections of NKCC1−/− global knockout mice (data not shown). However, in the peri-lesion penumbra region, many neurons with larger soma showed significantly increased expression of tNKCC1 protein (arrowhead, Figure 6(a), middle panel) as compared with the non-lesion regions (Figure 6(b)). Most tNKCC1-positive cells exhibited neuron-like morphology, similar to that of MAP-2-positive cells. Increased expression of pNKCC1 protein was also detected in the peri-lesion penumbra region (Figure 6(a), the bottom panel). However, positive immunoreactive signal of pNKCC1 was detected in three out of six subjects. Taken together, these data demonstrate that human ischemic stroke upregulated tNKCC1 and pNKCC1 protein expression in neurons in the ischemic penumbra, possibly contributing to ischemic neuronal damage. The data suggest that targeting the WNK-NKCC1 signaling pathway may help rescue at risk peri-infarct neurons and so improve therapy of ischemic stroke.
Figure 6.
Elevated tNKCC1 and pNKCC1 protein expression in subacute human middle cerebral artery ischemic stroke brain autopsy. (a) Non-lesion and peri-lesion regions in human ischemic stroke brain autopsy samples were identified with H & E staining. tNKCC1 expression in neuronal cells was shown in representative immunohistochemical images. Arrow: low NKCC1 expression. Arrowhead: high NKCC1 expression. Scale bar: 50 µm. Representative immunofluorescence images of pNKCC1 expression in brain cells (bottom panel). Arrow: low pNKCC1 expression. Arrowhead: high pNKCC1 expression. Scale bar: 20 µm. (b) Percentage of tNKCC1-positive cells among total To-pro3-positive cells. Numerical data are mean ± SD, n = 3 (one male, two female), *p < 0.05.
Discussion
Elevated NKCC1 protein expression and activation in SHR brains
SHR exhibit developmental upregulation of NKCC1 protein and mRNA between 10 and 18 weeks of age12 and excessive NKCC1 activity underlies in large part the diminished synaptic inhibition and elevated sympathetic vasomotor tone contributing to the hypertension of SHR.13 In agreement with these reports, we detected elevated basal expression of tNKCC1 and pNKCC1 protein in cerebral cortex of SHR brains, as compared with normotensive WKY brains. We found that ischemic stroke triggered a significant upregulation of pNKCC1 in the membrane fraction of ischemic SHR brains but not in WKY brains at 6-h post-ischemia. This suggests NKCC1 phosphoactivation is differentially regulated in SHR and WKY in response to ischemic stroke. This finding led us to investigate changes in the WNK-SPAK/OSR1 signaling pathway, a key regulator of NKCC1 activity, in SHR and WKY rats.
Normotensive WKY rats were less sensitive to ischemic stroke and exhibited a smaller infarct size (<20% hemispheric volume) after 24–48 h following permanent MCA occlusion.14,15 In our study, similar basal levels of pNKCC1 protein were detected in WKY and SHR brains. No ischemia-induced elevation of pNKCC1 was detected in ischemic WKY brains at 6-h post-ischemia. However, BMT treatment effectively decreased brain swelling at 24-h post-ischemia. We speculate that the onset of ischemic stroke-mediated stimulation of pNKCC1 in WKY rats is slower than in SHRs, explaining the lack of elevation in pNKCC1 expression in WKY rats at 6-h post-stroke. pNKCC1 levels at 6-h post-ischemia also failed to increase in normotensive WNK3 wild-type (WNK3 WT) mice, but increased significantly between 6 - and 24-h post-ischemia.20 Therefore, the moderate protective effects of BMT at 24-h post-ischemia in WKY rats may result from blocking stimulation of NKCC1 during the 24-h post-tMCAO period. This view is further supported by previous reports of BMT-mediated protection of ischemic brain injury in normotensive Sprague Dawley rats and C57BL/6 mice.20,31 Alternatively, regulatory mechanisms other than protein phosphorylation are involved in regulation of NKCC1 activity. N-linked glycosylation of NKCC1 represents another important post-translational mechanism that governs the functional expression of NKCC1 by affecting its intrinsic activity and trafficking into the plasma membrane.13 The possible differences in changes of N-linked glycosylation of NKCC1 in WKY and SHR brains after ischemic stroke remain to be examined in the future studies.
Increased Wnk1, Wnk2, and Wnk4 are likely responsible for NKCC1 activation in ischemic SHR brains
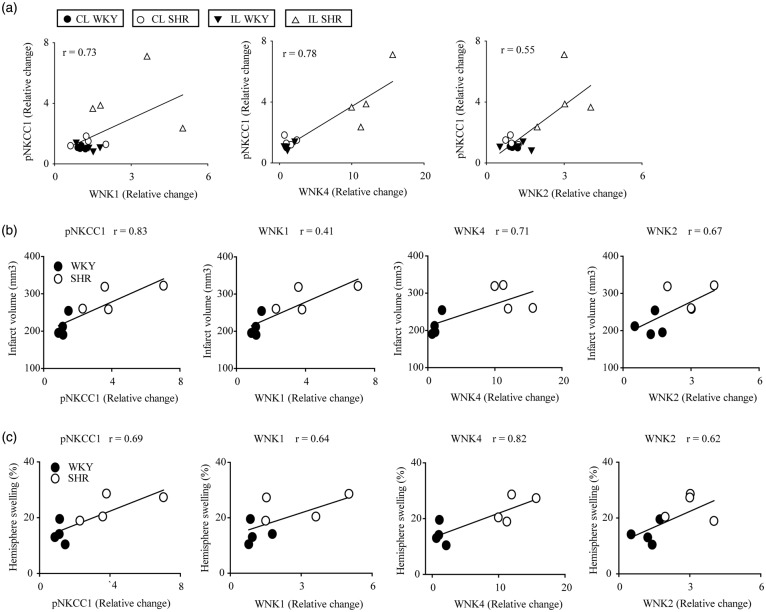
WNK kinases and SPAK/OSR1 regulate the phosphorylation state of ion transporter proteins and play important roles in renal salt handling and in the pathogenesis of hypertension,2,4 the most important modifiable risk factor for stroke.32 However, despite the importance of WNK-SPAK/OSR1 signaling in hypertension, our understanding of the function and regulation of these kinases in the ischemic brain injury remains limited. Here, we found that upregulation of WNK protein expression in the IL cortex of ischemic SHR brains (in the order WNK1 > WNK4 > WNK2) was associated with increased NKCC1 phosphorylation (Figure 7). The co-immunoprecipitation of WNK1 and WNK4 with NKCC1 suggests that WNK kinases might directly phosphorylate NKCC1 in ischemic SHR brains.
Figure 7.
Correlation of expression and activation of NKCC1/WNK pathway signaling proteins, infarct volume and hemisphere swelling in ischemic SHR and WKY brains. (a) Correlation between phosphorylation of NKCC1 and expression of WNK kinases at 6 h after MCAO in WKY and SHRs (p < 0.05, Pearson’s correlation). (b) Correlation between phosphorylation of NKCC1 and expression of WNK kinases versus infarct volume (p < 0.05, Pearson’s correlation). (c) Correlation between NKCC1 phosphorylation, and expression of WNK1/2/4 kinases versus hemispheric swelling (p < 0.05, Pearson correlation). These correlation analyses used the same cohort of data from Figures 1–3.
We recently reported that WNK3, an isoform highly expressed in the normotensive mouse and rat brains,33 acts as a dominant upstream kinase of SPAK/OSR1-NKCC1 phospho-activation in ischemic stroke.20 Knockout of WNK3 in normotensive mice abolished phosphorylation of both SPAK/OSR1 and NKCC1 in ischemic brains and reduced infarction and cerebral edema.20 However, the ischemic stroke-associated WNK3 protein expression in SHR brains was not as pronounced as that of WNK1 and WNK4 in the membrane fraction, and was decreased ∼50% in the cytosol fraction. These findings suggest that the four mammalian WNK kinase orthologs are differentially regulated in hypertensive and normotensive brains in response to ischemic stroke. Hypertension-dependent transcriptional or post-translational changes in the expression of specific WNK kinases might account for the observed differences between the two models, a hypothesis that warrants further testing in future studies.
Supplemental Figure 5 indicates that NKCC1 activation is not up-stream of the WNK-Cab39 function. The figure shows that cerebral ischemia in SHR triggered irreversible changes of WNK1, WNK4, and Cab39 protein that were insensitive to NKCC1 inhibition by BMT.
We detected a large increase of a 210 kDa WNK1 protein in the cytosolic fraction of ischemic cortex of SHR, but pNKCC1 in the respective cytosolic fraction did not change. This implies that the 210 kDa WNK1 variant is either inactive or NKCC1 may need to be at the plasma membrane to undergo phosphorylation.34 As shown in Supplemental Figure 1, 24-h post-ischemic brain tissue of SHRs expressed both 250 kDa and 210 kDa bands of WNK1, the latter more prominent in the cytosolic fraction. Kidney tissues, in contrast, displayed a different complement of WNK1 proteins, suggesting that the WNK1 expression pattern in brain was tissue-specific and a consequence of cerebral ischemia. Since downstream SPAK/OSR1 phosphorylation was decreased in the subcellular fraction where the 210 kDa brain WNK1 protein was expressed, we speculate that this shorter WNK1 species may be itself inactive, perhaps functioning in brain as kinase-inactive kidney-specific WNK1 isoforms in kidney.35
Scaffolding protein Cab39, but not SPAK/OSR1, may serve as a link between WNK-NKCC1 interactions
Phosphorylation of SPAK (68 kDa) was decreased but phosphorylation of OSR1 (55 kDa) was unchanged in ischemic SHR brains. Moreover, expression of a truncated 45 kDa SPAK-immunoreactive polypeptide emerged only in the ischemic SHR brains. Since the anti-tSPAK antibody36 is directed against C-terminal SPAK amino acid residues 362–374, the truncated form of SPAK is likely missing an N-terminal fragment. The antibody to pSPAK directed at pS383 of SPAK9 revealed no increase in phosphorylation of the 45 kDa SPAK band. The lack of S383 phosphorylation in the 45 kDa truncated SPAK may indicate that it is a poor substrate for WNK-mediated phosphorylation. This speculation warrants further investigation.
The concurrent increase in Dnpep expression and the 45 kDa truncated SPAK in ischemic SHR brains suggests that this truncated form of SPAK might be a previously recognized cleavage product of Dnpep.27 Previous studies indicate that truncated SPAK isoforms can exert inhibitory effects on the kinase activity of full length SPAK and OSR1.37,38 We propose that Dnpep-mediated proteolytic processing of SPAK into its inhibitory form might dampen stimulatory effects of WNK1, WNK2, or WNK4 on NKCC1 activity following ischemic stroke. However, we cannot rule out a possibility that the 45 kDa protein may represent a short form of SPAK as previously identified in kidney, such as SPAK2, which is inactive and generated from an alternative translation start in the full-length transcript.37–39 Interestingly, in contrast to a dramatic decrease of tSPAK in the IL cytosolic fraction of SHR cortex, tSPAK in the membrane fraction remains largely unchanged. This suggests that membrane-bound tSPAK may be protected from proteolytic cleavage by DNPEP, a predominantly cytosolic protease/peptidase.40 Moreover, though tOSR1 abundance was decreased in cytosolic fractions of IL cortex of SHR brains, the 45 kDa band is not an OSR1-cleavage product, as it was not recognized by an OSR1 specific antibody. This suggests that changes in OSR1 protein abundance in the cytosolic fraction of SHR ischemic cortex may reflect transcriptional downregulation of OSR1 upon ischemic stroke.
Taken together, our data suggest that SHR brains may utilize a SPAK/OSR1-independent pathway in regulating the WNK-NKCC1 signaling complex, as summarized in the schematic of Supplemental Figure 6. WNK kinases can activate NKCC1 through phosphorylation via a SPAK/OSR1-independent pathway.30 The scaffolding protein Cab39 was recently demonstrated to enhance WNK4-mediated phosphorylation of NCC and NKCC1 overexpressed in Xenopus laevis oocytes.30 The mechanism requires the PASK/FRAY homology 2(PF2) domain of WNK4, which is homologous to a domain in SPAK and OSR1 that binds RFX[V/I] motifs present in Na-coupled cation chloride cotransporters.30,41 Thus, the PF2 domain facilitates anchoring of WNK4 to an N-terminal RFX[V/I] motif in NKCC1. This interaction is likely quiescent, but in the presence of the Ste20 kinase activator Cab39, WNK4 can stimulate NKCC1 phosphoactivation via a kinase-dependent mechanism.30 In the current study, increased Cab39 protein expression in ischemic SHR brains was co-localized with NKCC1, WNK1, and WNK4 in the same co-immunoprecipitation complex. These findings further suggest that elevated expression of Cab39 may serve as an intermediate link to facilitate WNK-NKCC1 signaling in ischemic SHR brains (Supplemental Figure 6). Cab39 activation depends on elevation of intracellular Ca2+.42 Excessive Ca2+ influx and/or Ca2+ release from intracellular Ca2+ stores are the hallmark of ischemic brain damage.43,44 In fact, increased intracellular Ca2+ secondary to activation of ionotropic or metabotropic glutamate receptors stimulated NKCC1 activity in nasal epithelia45 and neurons.46,47 Moreover, striatal levels of phospho-GluR1 and of calcium/calmodulin kinase-IIα were higher in SHR than in WKY rats.48 Therefore, excessive Ca2+ signaling in ischemic SHR brains may favor activation of the WNK-Cab39-NKCC1 complex.
It is likely that WNK-Cab39 signaling could also affect other cation chloride cotransporters. K+-Cl− cotransporter 2 (KCC2) and KCC3 are expressed in the brain and are required along with NKCC1 for physiological regulation of cell volume and ionic homeostasis in the central nervous system.49 Therefore, the roles of WNK-Cab39-KCC signaling in the context of ischemic stroke merit further study.
Pharmacological inhibition of NKCC1 reduces infarction severity and improves the sensorimotor deficits in SHR after ischemic stroke
Multiple in vivo studies have shown that NKCC1 plays a role in ischemic and hypoxic cell damage, and that its inhibition is neuroprotective.16,17,31 We detected a positive correlation between elevated NKCC1 protein expression, NKCC1 phosphorylation, ischemic infarct size, and the magnitude of brain swelling in WKY and SHR brains (Figure 7, Pearson’s correlation, p < 0.05). Interestingly, we found that the neuroprotective effect of NKCC1 inhibition by BMT was greater in SHR brains than in normotensive WKY brains. Daily BMT administration begun 3-h post-MCAO nearly abolished the incremental severity of ischemia-induced infarction and the increased brain swelling observed in SHR. We previously showed that BMT administration either prior to ischemia induction or during ischemia significantly reduced brain edema and infarction in SHR.16,22 The current study shows that post-ischemic administration of BMT is equally effective in reducing ischemic damage and neurological deficits in SHR. This finding further supports targeting of WNK-NKCC1 signaling pathways as novel strategies for ischemic stroke therapy.
To date, there are no specific WNK inhibitors available to block WNK kinase activity or interrupt the WNK-Cab39-NKCC1 cascade. Therefore, development of a blood–brain barrier-permeable WNK kinase inhibitor will be beneficial for the development of anti-hypertension and anti-ischemic stroke therapies.
Conclusions
In summary, we report here that unilateral ischemic stroke triggers more robust stimulation of the WNK-NKCC1 signaling pathway in SHRs than in normotensive WKY rats. Ischemic SHR brains show concurrent upregulation of WNK1, WNK4, and WNK2 protein expression and elevated NKCC1 phosphorylation, associated with increased infarction size and increased ipsilateral hemispheric swelling in ischemic SHR brains. The conventional SPAK/OSR1 effector kinases were not stimulated in ischemic SHR brains. In contrast, the kinase scaffolding protein Cab39 was upregulated and associated with NKCC1 and WNK1/WNK4 proteins in the same immunoprecipitation complex. Moreover, inhibition of NKCC1 with BMT attenuated the increased severity of infarction and brain swelling in SHR and improved post-ischemic neurological function. These findings suggest that increased WNK-Cab39-NKCC1 signaling is involved in the pathogenesis of ischemic brain damage in SHR. Our study reveals a pathophysiological role for non-canonical WNK-Cab39 signaling in a disease model of ischemic stroke, and identifies the WNK-Cab39-NKCC1 signaling complex as a novel target for treatment of hypertension and ischemic stroke.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH Grant R01 NS38118 (DS), VA I01BX002891-01A1 (DS), NIH R01 DK098145 (ARS), and NIA Grant P50 AG005133 (JK).
Supplementary Material
Acknowledgements
We are grateful to Dr. Clayton A Wiley (Department of Pathology, University of Pittsburgh) for his assistance in the stroke autopsy study and reading of the manuscript.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
MIHB and HY performed surgery procedures, TTC staining, and data analysis. MIHB, SS, and HY performed behavioral tests and data analysis. MIHB and SS conducted and quantified immunoblotting and immunoprecipitation studies. MIHB, SS, GB, and JK performed immunohistology and immunofluorescent staining studies. MIHB, KTK, SSY, SHL, SLA, ARS, and DS designed the experiments. MIHB, JK, KTK, SLA, ARS, and DS completed the manuscript writing.
Supplementary material
Supplementary material for this paper can be found at http://jcb.sagepub.com/content/by/supplemental-data
References
- 1.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the wnk-spak/osr1 signalling pathway. J Cell Sci 2008; 121: 3293–3304. [DOI] [PubMed] [Google Scholar]
- 2.Vitari AC, Thastrup J, Rafiqi FH, et al. Functional interactions of the spak/osr1 kinases with their upstream activator wnk1 and downstream substrate nkcc1. Biochem J 2006; 397: 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vitari AC, Deak M, Morrice NA, et al. The wnk1 and wnk4 protein kinases that are mutated in gordon's hypertension syndrome phosphorylate and activate spak and osr1 protein kinases. Biochem J 2005; 391: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson FH, Disse-Nicodeme S, Choate KA, et al. Human hypertension caused by mutations in wnk kinases. Science 2001; 293: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, O'Connell JR, McArdle PF, et al. From the cover: Whole-genome association study identifies stk39 as a hypertension susceptibility gene. Proc Natl Acad Sci USA 2009; 106: 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Healy JK. Pseudohypoaldosteronism type ii: History, arguments, answers, and still some questions. Hypertension 2014; 63: 648–654. [DOI] [PubMed] [Google Scholar]
- 7.Boyden LM, Choi M, Choate KA, et al. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature 2012; 482: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis-Dit-Picard H, Barc J, Trujillano D, et al. Klhl3 mutations cause familial hyperkalemic hypertension by impairing ion transport in the distal nephron. Nat Genet 2012; 44: 456–460, S451–S453. [DOI] [PubMed] [Google Scholar]
- 9.Yang SS, Lo YF, Wu CC, et al. Spak-knockout mice manifest gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 2010; 21: 1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin SH, Yu IS, Jiang ST, et al. Impaired phosphorylation of na(+)-k(+)-2cl(-) cotransporter by oxidative stress-responsive kinase-1 deficiency manifests hypotension and bartter-like syndrome. Proc Natl Acad Sci USA 2011; 108: 17538–17543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer JW, Flagella M, Sutliff RL, et al. Decreased blood pressure and vascular smooth muscle tone in mice lacking basolateral na(+)-k(+)-2cl(-) cotransporter. Am J Physiol Heart Circ Physiol 2002; 283: H1846–H1855. [DOI] [PubMed] [Google Scholar]
- 12.Cho HM, Lee HA, Kim HY, et al. Expression of Na+-K+-2Cl- cotransporter 1 is epigenetically regulated during postnatal development of hypertension. Am J Hypertens 2011; 24: 1286–1293. [DOI] [PubMed] [Google Scholar]
- 13.Ye ZY, Li DP, Byun HS, et al. Nkcc1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J Neurosci 2012; 32: 8560–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratton JA, Sauter A, Rudin M, et al. Susceptibility to cerebral infarction in the stroke-prone spontaneously hypertensive rat is inherited as a dominant trait. Stroke 1998; 29: 690–694. [DOI] [PubMed] [Google Scholar]
- 15.Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: Influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab 1988; 8: 449–461. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y, Dempsey RJ, Flemmer A, et al. Inhibition of na(+)-k(+)-cl(-) cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Res 2003; 961: 22–31. [DOI] [PubMed] [Google Scholar]
- 17.Lenart B, Kintner DB, Shull GE, et al. Na-K-Cl cotransporter-mediated intracellular Na+ accumulation affects ca2+ signaling in astrocytes in an in vitro ischemic model. J Neurosci 2004; 24: 9585–9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Luo J, Kintner DB, et al. Na(+)-dependent chloride transporter (nkcc1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab 2005; 25: 54–66. [DOI] [PubMed] [Google Scholar]
- 19.O'Donnell ME, Lam TI, Tran L, et al. The role of the blood-brain barrier na-k-2cl cotransporter in stroke. Adv Exp Med Biol 2004; 559: 67–75. [DOI] [PubMed] [Google Scholar]
- 20.Begum G, Yuan H, Kahle KT, et al. Inhibition of wnk3 kinase signaling reduces brain damage and accelerates neurological recovery after stroke. Stroke 2015; 46: 1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H, Nepomuceno R, Gao X, et al. Deletion of the wnk3-spak kinase complex in mice improves radiographic and clinical outcomes in malignant cerebral edema after ischemic stroke. J Cereb Blood Flow Metab 2016. Epub ahead of print 9 February 2016. DOI: 10.1177/0271678X16631561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Y, Dempsey RJ, Sun D. Na+-K+-Cl- cotransporter in rat focal cerebral ischemia. J Cereb Blood Flow Metab 2001; 21: 711–721. [DOI] [PubMed] [Google Scholar]
- 23.Kim E, Tolhurst AT, Cho S. Deregulation of inflammatory response in the diabetic condition is associated with increased ischemic brain injury. J Neuroinflammation 2014. DOI: 10.1186/1742-2094-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swanson RA, Morton MT, Tsao-Wu G, et al. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 1990; 10: 290–293. [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Begum G, Pointer K, et al. Wnk1-osr1 kinase-mediated phospho-activation of Na+-K+-2Cl− cotransporter facilitates glioma migration. Mol Cancer 2014. DOI: 10.1186/1476-4598-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhuiyan MIH, Kim JC, Hwang SN, et al. Ischemic tolerance is associated with vegf-c and vegfr-3 signaling in the mouse hippocampus. Neuroscience 2015; 290: 90–102. [DOI] [PubMed] [Google Scholar]
- 27.Markadieu N, Rios K, Spiller BW, et al. Short forms of ste20-related proline/alanine-rich kinase (spak) in the kidney are created by aspartyl aminopeptidase (dnpep)-mediated proteolytic cleavage. J Biol Chem 2014; 289: 29273–29284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick JA, Yang CL, Zhang C, et al. Hyperkalemic hypertension-associated cullin 3 promotes wnk signaling by degrading klhl3. J Clin Invest 2014; 124: 4723–4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susa K, Sohara E, Rai T, et al. Impaired degradation of wnk1 and wnk4 kinases causes phaii in mutant klhl3 knock-in mice. Hum Mol Genet 2014; 23: 5052–5060. [DOI] [PubMed] [Google Scholar]
- 30.Ponce-Coria J, Markadieu N, Austin TM, et al. A novel ste20-related proline/alanine-rich kinase (spak)-independent pathway involving calcium-binding protein 39 (cab39) and serine threonine kinase with no lysine member 4 (wnk4) in the activation of na-k-cl cotransporters. J Biol Chem 2014; 289: 17680–17688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Donnell ME, Chen YJ, Lam TI, et al. Intravenous hoe-642 reduces brain edema and na uptake in the rat permanent middle cerebral artery occlusion model of stroke: Evidence for participation of the blood-brain barrier na/h exchanger. J Cereb Blood Flow Metab 2013; 33: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rundek T, Sacco RL. Risk factor management to prevent first stroke. Neurol Clin 2008; 26: 1007–1045, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahle KT, Rinehart J, de Los Heros P, et al. Wnk3 modulates transport of Cl- in and out of cells: Implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci USA 2005; 102: 16783–16788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaek LL, Kortenoeven ML, Aroankins TS, et al. Phosphorylation decreases ubiquitylation of the thiazide-sensitive cotransporter ncc and subsequent clathrin-mediated endocytosis. J Biol Chem 2014; 289: 13347–13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanya AR, Yang CL, Zhu X, et al. Dominant-negative regulation of wnk1 by its kidney-specific kinase-defective isoform. Am J Physiol Renal Physiol 2006; 290: F619–F624. [DOI] [PubMed] [Google Scholar]
- 36.Moriguchi T, Urushiyama S, Hisamoto N, et al. Wnk1 regulates phosphorylation of cation-chloride-coupled cotransporters via the ste20-related kinases, spak and osr1. J Biol Chem 2005; 280: 42685–42693. [DOI] [PubMed] [Google Scholar]
- 37.McCormick JA, Mutig K, Nelson JH, et al. A spak isoform switch modulates renal salt transport and blood pressure. Cell Metab 2011; 14: 352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm PR, Taneja TK, Liu J, et al. Spak isoforms and osr1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 2012; 287: 37673–37690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piechotta K, Garbarini N, England R, et al. Characterization of the interaction of the stress kinase spak with the Na+-K+-2Cl− cotransporter in the nervous system: Evidence for a scaffolding role of the kinase. J Biol Chem 2003; 278: 52848–52856. [DOI] [PubMed] [Google Scholar]
- 40.Wilk S, Wilk E, Magnusson RP. Purification, characterization, and cloning of a cytosolic aspartyl aminopeptidase. J Biol Chem 1998; 273: 15961–15970. [DOI] [PubMed] [Google Scholar]
- 41.Moon TM, Correa F, Kinch LN, et al. Solution structure of the wnk1 autoinhibitory domain, a wnk-specific pf2 domain. J Mol Biol 2013; 425: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto H, Matsushiro A, Nozaki M. Molecular cloning of a novel mrna sequence expressed in cleavage stage mouse embryos. Mol Reprod Dev 1993; 34: 1–7. [DOI] [PubMed] [Google Scholar]
- 43.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature 1999; 399: A7–A14. [DOI] [PubMed] [Google Scholar]
- 44.Deshpande JK, Siesjo BK, Wieloch T. Calcium accumulation and neuronal damage in the rat hippocampus following cerebral ischemia. J Cereb Blood Flow Metab 1987; 7: 89–95. [DOI] [PubMed] [Google Scholar]
- 45.Shin JH, Namkung W, Choi JY, et al. Purinergic stimulation induces ca2+-dependent activation of Na+-K+-2Cl− cotransporter in human nasal epithelia. J Biol Chem 2004; 279: 18567–18574. [DOI] [PubMed] [Google Scholar]
- 46.Sun D, Murali SG. Stimulation of Na+-K+-2Cl− cotransporter in neuronal cells by excitatory neurotransmitter glutamate. Am J Physiol 1998; 275: C772–C779. [DOI] [PubMed] [Google Scholar]
- 47.Schomberg SL, Su G, Haworth RA, et al. Stimulation of na-k-2cl cotransporter in neurons by activation of non-nmda ionotropic receptor and group-i mglurs. J Neurophysiol 2001; 85: 2563–2575. [DOI] [PubMed] [Google Scholar]
- 48.Lecrux C, Nicole O, Chazalviel L, et al. Spontaneously hypertensive rats are highly vulnerable to ampa-induced brain lesions. Stroke 2007; 38: 3007–3015. [DOI] [PubMed] [Google Scholar]
- 49.Kahle KT, Rinehart J, Lifton RP. Phosphoregulation of the na-k-2cl and k-cl cotransporters by the wnk kinases. Biochim Biophys Acta 2010; 1802: 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.