Abstract
Supercompensated brain glycogen levels may contribute to the development of hypoglycemia-associated autonomic failure (HAAF) following recurrent hypoglycemia (RH) by providing energy for the brain during subsequent periods of hypoglycemia. To assess the role of glycogen supercompensation in the generation of HAAF, we estimated the level of brain glycogen following RH and acute hypoglycemia (AH). After undergoing 3 hyperinsulinemic, euglycemic and 3 hyperinsulinemic, hypoglycemic clamps (RH) on separate occasions at least 1 month apart, five healthy volunteers received [1-13C]glucose intravenously over 80+ h while maintaining euglycemia. 13C-glycogen levels in the occipital lobe were measured by 13C magnetic resonance spectroscopy at ∼8, 20, 32, 44, 56, 68 and 80 h at 4 T and glycogen levels estimated by fitting the data with a biophysical model that takes into account the tiered glycogen structure. Similarly, prior 13C-glycogen data obtained following a single hypoglycemic episode (AH) were fitted with the same model. Glycogen levels did not significantly increase after RH relative to after euglycemia, while they increased by ∼16% after AH relative to after euglycemia. These data suggest that glycogen supercompensation may be blunted with repeated hypoglycemic episodes. A causal relationship between glycogen supercompensation and generation of HAAF remains to be established.
Keywords: 13C magnetic resonance spectroscopy, biophysical modeling, glycogen, hypoglycemia-associated autonomic failure, supercompensation
Introduction
Hypoglycemia is a common event in the lives of patients with type 1 and advanced type 2 diabetes who receive insulin or insulin secretagoges as therapy for their disease. Patients who experience recurrent episodes of hypoglycemia often develop hypoglycemia-associated autonomic failure (HAAF), a clinical syndrome in which the first symptom of hypoglycemia is confusion or loss of consciousness.1,2 Approximately 20% of patients with type 1 diabetes have impaired awareness of hypoglycemia3 and the fear of HAAF prevents many patients with diabetes from achieving the glycemic goals known to reduce the risk of developing the microvascular complications of the disease.4,5
The mechanisms responsible for HAAF remain uncertain and may include alterations in glucose uptake or metabolism by the brain, the use of alternate fuels and changes in hypothalamic neurotransmitter release among others.6 Upregulated fuel availability to the brain, in the form of glucose or alternative fuels, can result in a failure to detect systemic hypoglycemia, i.e. the “unawareness” component of HAAF. Therefore glycogen, the sole glucose reservoir in the brain, is thought to be involved in the pathophysiology of HAAF. Specifically, increased brain glycogen content above normal levels, a phenomenon termed supercompensation, following recurrent hypoglycemia (RH) has been proposed to play a role in the development of HAAF by facilitating maintenance of cerebral substrate availability, which may contribute to the blunting of the counter-regulatory hormone response during subsequent episodes of hypoglycemia.7,8 Consistently, glycogen is mobilized to support cerebral energy metabolism during hypoglycemia in rodents,7,9–11 humans,8 and lower vertebrates.12 Furthermore, evidence indicating brain glycogen supercompensation was reported following systemic hypoglycemia in rodents,7,11 lower vertebrates,12 and humans,8 after recurrent 2-deoxy-D-glucose-induced neuroglucopenia in rats13 and after prolonged exercise in rats.14 Note however that not all studies confirmed glycogen supercompensation following acute and recurrent hypoglycemia.10
To assess the role of glycogen supercompensation in the generation of HAAF in humans, we have previously used 13C magnetic resonance spectroscopy (MRS) in conjunction with intravenous (IV) administration of 13C-glucose and demonstrated increased rates of glycogen synthesis after an acute hypoglycemic (AH) episode in healthy volunteers, suggesting glycogen supercompensation.8 We then used the same methodology to investigate cerebral glycogen metabolism in patients with type 1 diabetes and hypoglycemia unawareness and found that these patients do not have higher brain glycogen levels than healthy controls, suggesting that glycogen supercompensation does not contribute to the development of HAAF in the setting of type 1 diabetes.15 However, in that study, the presence of diabetes itself or the relative hyperinsulinemia seen in the control group relative to the group with diabetes may have altered glycogen content/metabolism, thereby confounding the findings regarding the response to RH.
Therefore, in the current study, we investigated glycogen supercompensation in healthy volunteers who were preconditioned with RH (a HAAF model) in order to distinguish the effects of HAAF alone from those of diabetes. We then estimated the level of brain glycogen supercompensation following RH using 13C MRS. As a control for insulin exposure, these volunteers were exposed to the same degree of hyperinsulinemia during hypoglycemia and euglycemia pre-conditioning and generated appropriate insulin secretory responses during the 13C-glucose infusion period. We further compared the level of brain glycogen supercompensation following RH to that following AH in healthy humans.8 To estimate glycogen content following RH and AH, data were fitted with a biophysical model that takes into account the tiered glycogen structure.16,17
Materials and methods
Subjects
Five healthy male subjects (age 41 ± 9 years, BMI 27 ± 2 kg/m2) were studied after giving informed consent using procedures approved by the University of Minnesota Institutional Review Board: Human Subjects Committee. All procedures performed were in accordance with the 1975 Helsinki declaration and its later amendments. Exclusion criteria included history of ischemic heart disease, arrhythmia, seizure disorder, and being on medications known to alter blood flow or carbohydrate metabolism in addition to MR safety criteria (claustrophobia, weight over 300 pounds, and presence of paramagnetic metal in the body). Subjects were asked to avoid strenuous exercise from the day before the start of the protocol through to the completion on day 5.
Experimental design
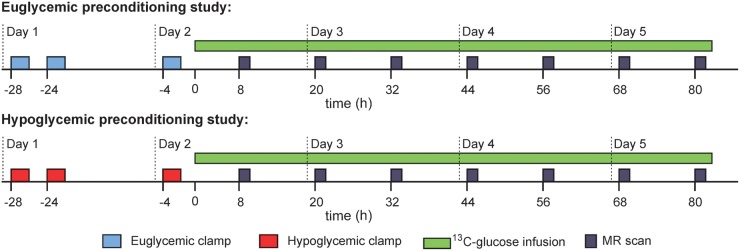
To induce HAAF in healthy volunteers (RH protocol), subjects underwent two hypoglycemic clamps in the morning and afternoon on day 1 and a third hypoglycemic clamp in the morning on day 2 (Figure 1), as described below. This protocol with three 2-h hypoglycemic, hyperinsulinemic clamps was shown to robustly induce HAAF in healthy subjects.18 Control experiments (euglycemic preconditioning) included three 2-h euglycemic clamps at the same times of day. The euglycemic preconditioning data from these subjects were previously utilized to optimize the fitting parameters of the novel biophysical model and to estimate glycogen content in the healthy human brain.17 All subjects underwent both euglycemic and hypoglycemic preconditioning experiments in random order, separated by at least 1 month, to allow paired comparisons between the protocols. Approximately 2 h after the end of the third clamp on day 2, [13C]glucose administration began with a 20 g bolus of [1-13C]glucose (Cambridge Isotope Laboratories, Andover, MA, USA, prepared as 20% weight/volume D-glucose in water with 50% enrichment), which was then followed by a continuous infusion of [1-13C]glucose (prepared as 20% weight/volume D-glucose in water with 25% enrichment) adjusted to maintain blood glucose at euglycemia (∼90 mg/dL) for 80+ h (Figure 1). Samples for blood glucose, insulin, and isotopic enrichment (IE) were obtained every 10–60 min. 13C glycogen levels in the occipital lobe were measured at ∼8, 20, 32, 44, 56, 68, and 80 h. The spectroscopist (GÖ) and modeler (MDN) were blinded to the preconditioning status of the subjects during data acquisition, quantification, and kinetic modeling. During the [13C]glucose infusion subjects received breakfast, lunch, and dinner with mid-morning, mid-afternoon, and bedtime snacks if desired. Each meal and snack had no more than 30 g of carbohydrates.
Figure 1.
Recurrent hypoglycemia study protocol. Timing of clamps and MR scans is approximate. Time = 0 corresponds to the IV administration of 13C-glucose bolus.
For the AH study, data obtained previously8 from healthy volunteers with similar age and BMI characteristics following single euglycemic and hypoglycemic clamps were used; procedures used for each type of clamp were the same for both AH and RH studies.
Euglycemic and hypoglycemic preconditioning
Preconditioning studies included 2-h clamps in the morning (8:00–10:00 AM) and afternoon (12:00–2:00 pm) of day 1 and a third clamp on the morning (8:00–10:00 AM) of day 2, as described previously.18 Briefly, subjects arrived at the Clinical and Translational Science Institute in the morning after an overnight fast. For the clamps, insulin was administered IV at a rate of 2.0 mU/kg min and potassium phosphate administered at a rate of 4 mEq/h. Arterialized blood samples were collected every 5 min for the measurement of plasma glucose. For hypoglycemic clamps, plasma glucose was allowed to fall to 50 mg/dL and then maintained at this level by a variable infusion of 20% dextrose. For euglycemic clamps, plasma glucose was maintained at ∼90 mg/dL. Approximately 2 h after starting insulin, the insulin and potassium were discontinued and the blood glucose was normalized with 20% dextrose infusion. Samples for glucagon, epinephrine, norepinephrine, and cortisol were drawn at baseline (30 min after the placement of the last IV catheter) and at minutes 60, 90, and 120 after starting insulin during the first and third clamps.
After the first and third clamps, hypoglycemia symptoms were quantified by using a previously validated questionnaire.19 Subjects were asked to score from 0 (none) to 6 (severe) each of 12 symptoms: six autonomic symptoms (heart pounding, shaky/tremulous, nervous/anxious, sweaty, hungry, tingling) and six neuroglycopenic symptoms (difficulty thinking, tired/drowsy, weak, warm, faint, dizzy).
Laboratory analyses
Plasma glucose concentration was measured in duplicates during the clamps and MR scanning using an Analox machine (Analox Instruments, Lunenburg, MA). Serum insulin concentrations were obtained by a chemiluminescent assay (Immulite, Diagnostic Products Corporation, Los Angeles, CA, USA), and the IE of the plasma glucose by gas-chromatography-mass spectrometry as described previously.20 Counterregulatory hormones were assayed at the Vanderbilt Diabetes Research and Training Center core laboratory. Plasma epinephrine and norepinephrine were measured by high-performance liquid chromatography (Dionex, formerly ESA, Inc.). Radioimmunoassay was used to measure plasma cortisol (Diagnostic Products Corporation, Inc) and glucagon (Millipore, Merck).
MR protocol
13C-glycogen levels in the brain were measured using methods described before.21 Briefly, measurements were performed on a 4 T magnet (Oxford Magnet Technology Inc., Witney, UK) interfaced to an Agilent DirectDrive console (Agilent Technologies, Santa Clara, CA, USA) using a quadrature 14 cm 1H surface coil combined with a 9 cm diameter linear 13C coil.22 Voxel placement was based on axial multi-slice RARE images (repetition time (TR) = 4 s, echo train length = 8, echo time (TE) = 60 ms). The [1-13C]glycogen signal was obtained from a 7 × 5 × 6 cm3 voxel in the occipital lobe by 3-D outer volume suppression combined with 1-D image-selected in vivo spectroscopy.21 Each data point presented was acquired over 30 min; the time points given for each data point are mid-way through the glycogen data acquisition. The amount of 13C label in the C1 position of glycogen was quantified by the external reference method.21,23 All 13C-glycogen levels were corrected for the cerebrospinal fluid (CSF) content of the voxel as described before.24 For the AH study data, % CSF was set to 12%, the average value obtained from the five subjects in the current study.
Modeling
To estimate glycogen concentration after RH and AH, 13C-glycogen data were fitted with a biophysical model that takes into account the tiered structure of the glycogen molecule.16 Details of how the model was fitted to data from the control (euglycemic preconditioning) studies was described in a prior article.17 Briefly, the model records the position of every glucosyl residue within an individual glycogen molecule. Addition and removal of each residue follows the established mechanisms underlying the action of glycogen synthase/glycogen branching enzyme and glycogen phosphorylase/glycogen debranching enzyme, respectively. The rate of glycogen synthesis and degradation vary non-linearly with the size of the glycogen molecule being subject to the available space for enzyme activity (steric effects) on the molecular surface. The model takes as input parameters the plasma glucose IE (to establish the probability of incorporation of labeled vs. unlabeled glucosyl residues), as well as the plasma glucose concentration (to determine the variations in the reaction rate of glycogen synthase).
Statistical analysis
Summary statistics and paired t-tests were used to compare mean plasma glucose and counterregulatory hormone levels and symptom scores between the first and third clamps during the preconditioning protocols. Total tissue glycogen content was determined by multiplying the overall number of residues in the model glycogen molecule by the coefficient obtained from least-square fitting between simulated and experimental data. Statistical errors on the glycogen estimate were calculated as standard deviation of the fit residuals. Comparisons of glycogen content between either RH or AH vs. euglycemic preconditioning groups were performed using paired one-tailed t-tests. Group data were shown as mean ± standard error (SEM) and threshold for statistical significance was set to p < 0.05.
Results
Euglycemic and hypoglycemic preconditioning
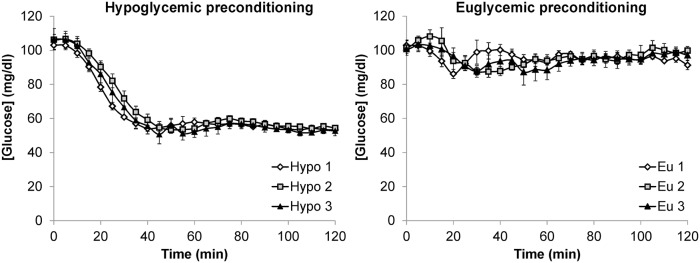
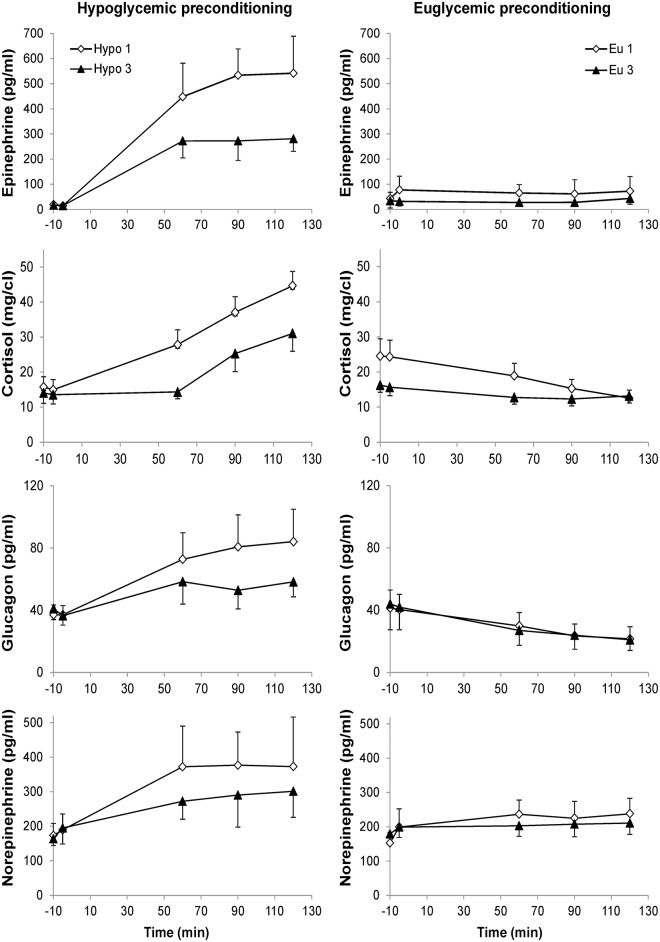
Mean plasma glucose concentrations during the three euglycemic clamps were well matched (Figure 2, Table 1). Similarly, mean plasma glucose concentrations achieved during the hypoglycemic plateaus were similar during all hypoglycemic clamps. The hypoglycemic preconditioning protocol induced HAAF in all volunteers, as evidenced by a blunted epinephrine and cortisol response during the third relative to the first hypoglycemic clamp (Table 1, Figure 3). During the hypoglycemic clamp studies, participants experienced symptoms that were blunted at the third vs. the first hypoglycemic clamp in three out of five subjects, insufficient for a statistically significant difference in this small cohort (Table 1).
Figure 2.
Plasma glucose levels (mean ± SEM) during the clamps in the euglycemic and hypoglycemic preconditioning studies.
Table 1.
Comparison of counterregulatory hormones and symptom response during final 30 min of first and third clamps during euglycemic and hypoglycemic preconditioning studies (mean ± SEM between subjects, N = 5 in each group).
| Hypoglycemic preconditioning |
Euglycemic preconditioning |
|||||
|---|---|---|---|---|---|---|
| Clamp #1 | Clamp #3 | p value* | Clamp #1 | Clamp #3 | p value* | |
| Plasma glucose (mg/dL) | 54 ± 2 | 53 ± 1 | 0.52 | 95 ± 1 | 97 ± 2 | 0.41 |
| Epinephrine (pg/mL) | 538 ± 119 | 277 ± 60 | 0.04 | 67 ± 57 | 36 ± 20 | 0.46 |
| Norepinephrine (pg/mL) | 375 ± 118 | 296 ± 82 | 0.23 | 231 ± 47 | 209 ± 34 | 0.36 |
| Glucagon (pg/mL) | 82 ± 21 | 56 ± 10 | 0.09 | 23 ± 8 | 22 ± 8 | 0.90 |
| Cortisol (mg/dL) | 40.9 ± 4.1 | 28.2 ± 4.9 | 0.02 | 13.9 ± 2.4 | 12.8 ± 1.7 | 0.46 |
| Total symptom score | 14.9 ± 4.2 | 12.9 ± 3.7 | 0.68 | 6.5 ± 2.8 | 6.1 ± 3.5 | 0.80 |
Plasma glucose data were averages from seven blood samples obtained from 90 to 120 min in each individual. Hormone and symptom score data represent an average of 90 and 120 min data.
Paired, two-tailed t-test.
Figure 3.
Plasma hormone concentrations during the first and third clamps in the hypoglycemic (left) and euglycemic (right) preconditioning studies. Error bars are SEM.
Glycogen supercompensation following recurrent hypoglycemia
Four of the five subjects completed the 5-day protocol (Figure 1) after both eu- and hypoglycemic preconditioning. The [1-13C]glucose infusion had to be stopped after 29 h due to complications with the IV line in the euglycemic preconditioning study of one subject. Plasma glucose and insulin levels and [1-13C]glucose enrichments were well matched between the eu- and hypoglycemic preconditioning studies; euglycemia and physiologic insulin levels were maintained throughout the 13C-glucose infusion in all studies (Figure 4). Time dependent plasma glucose concentrations and enrichments were incorporated in the modeling of the data and resulted in good correspondence between experimental and simulated time courses for label incorporation into glycogen at single subject level (Figure 5). Figure 5(a) shows the 13C-glycogen time courses for the subject in whom induction of HAAF was most robust, including blunting of all four hormones and symptom scores, at the third vs. first hypoglycemic clamp. These data demonstrate the similarity of 13C-labeling of glycogen following the eu- and hypoglycemic preconditioning protocols. Consistently, estimated glycogen level was not significantly higher following the RH protocol than the recurrent euglycemia protocol based on a paired comparison in all subjects who completed both protocols (Figure 5(a)).
Figure 4.

Time courses of average (±SEM) plasma glucose, serum insulin and plasma 13C isotopic enrichment (IE) levels during IV infusions of [1-13C]glucose following recurrent euglycemic (blue) and recurrent hypoglycemic (red) preconditioning in five healthy volunteers.
Figure 5.
13C-glycogen time courses and glycogen content estimates in the (a) recurrent hypoglycemia (current data) and (b) acute hypoglycemia (data from Öz et al.8) studies. Least-square fitting between experimental data and molecular-level simulations are shown in data from individual subjects. The error bars on experimental data have been estimated at 0.15 µmol/g. Glycogen content estimate (mean ± SEM) from N = 4 in (a) and from N = 5 in (b). *p < 0.05, paired, one-sided t-test.
Glycogen supercompensation following acute hypoglycemia
Data obtained after a single euglycemic or hypoglycemic clamp in five other subjects from a prior article8 were fitted using the same biophysical model. Consistent with the higher 13C-labeling of glycogen following the hypoglycemic than the euglycemic clamp, estimated glycogen concentration was 16 ± 4% (mean ± SEM) higher following a single hypoglycemic episode than a euglycemic episode (Figure 5(b)). This result was consistent with the conclusion in the previous study,8 where we had compared the 13C glycogen levels without using a biophysical model.
Discussion
Here we demonstrated that glycogen levels in the healthy human brain increase after single, but not recurrent, episodes of systemic hypoglycemia. These data show that the level of cerebral glycogen supercompensation is affected by the number of hypoglycemic episodes encountered and hence suggest that glycogen supercompensation is blunted with repeated hypoglycemic episodes.
Using similar 13C MRS methods in conjunction with IV 13C-glucose administration, we have previously shown that bulk brain glycogen turnover is slow both in healthy volunteers23,25 and in subjects with type 1 diabetes,15 necessitating several-day long infusions for reliable measurement of brain glycogen metabolism and estimation of its content in humans. The duration of 13C-glucose administration was even longer in the current study than our earlier studies8,15,25 and, together with the application of a novel biophysical model, revealed cerebral glycogen levels higher than previous estimates in control experiments.17 The demanding design of the studies, which required a 2-week commitment from volunteers to complete the paired experiments (Figure 1), limits the sample size. However, the repeated 13C-glycogen measurements in each individual and the fact that each volunteer serves as their own control allow the observation of statistically significant differences in glycogen labeling in response to euglycemic vs. hypoglycemic stimuli with this sample size.8
In the current study, the physiological status of the volunteers, including blood glucose levels during the preconditioning clamps (Figure 2) and blood glucose, insulin, and glucose IE during the 80+ h long infusions (Figure 4), was well matched between the eu- and hypoglycemic preconditioning studies of all participants, allowing a robust comparison of the paired experiments. HAAF was also induced successfully in all volunteers, based on the hormonal response during the third vs. the first clamp (Figure 3). Consistent with prior observations,18 epinephrine and cortisol responses were blunted the most with this RH protocol, while the norepinephrine and glucagon responses only showed trends. Furthermore, based on our previous observations, we expect that this blunting of the counterregulatory response to persist throughout the 13C-glucose infusion period.18
In comparing the 13C-gycogen labeling following RH and AH, the difference in outcomes was apparent by visual inspection of the raw data, even prior to modeling (Figure 5). Namely, 13C-gycogen levels were clearly higher following AH vs. euglycemia, as we had also previously reported,8 while there was no noticeable difference in glycogen labeling following RH vs. recurrent euglycemia. Glycogen supercompensation following AH, and not following RH, was then confirmed upon estimating glycogen content with a biophysical model.
There is extensive evidence for brain glycogen supercompensation following metabolic stressors, including hypoglycemia, from animal literature. Namely, brain glycogen was found to be supercompensated in rats following transient ischemia, transient hypoglycemic coma, and methionine sulfoximine induced seizures,26 in rats subjected to transient systemic hypoxia,27 in rats after transient neuroglucopenia upon intracerebroventricular injection of 2-deoxy-D-glucose,13 in mice recovering from hypoglycemia,11 in rats after exhaustive exercise,14 and in the rainbow trout after hypoglycemia induced by prolonged fasting.12 In addition, a 13C MRS study indicated glycogen supercompensation in the rat brain following insulin-induced hypoglycemia.7 In the one study where brain glycogen supercompensation was not detected after acute and recurrent hypoglycemia,10 brain glucose levels were not restored after insulin-induced hypoglycemia, as was done in all other studies that detected glycogen supercompensation in the brain. Therefore, brain glycogen supercompensates in animal brain after hypoglycemia as long as the blood and brain glucose are restored following the insult. Another common observation in these studies is the transient nature of glycogen supercompensation.11,12,14 Importantly, the level of glycogen supercompensation appears dependent on the intensity of the metabolic stress and how much glycogen is mobilized during the metabolic insult: Thus, glycogen supercompensation occurred only after prolonged, but not after short, fasting in the rainbow trout.12 Consistently, the percentage of brain glycogen supercompensation after exhaustive exercise positively correlated with the percentage of glycogen decrease noted during exercise across different brain regions in rats.14 Finally, repeated episodes of the metabolic stress may lead to a blunting of the glycogen supercompensation response: In exercise trained rats, glycogen levels were higher than controls only in two of five brain regions examined and to a much smaller degree than after acute exercise (7% in cortex and 9% in hippocampus after exercise training vs. 60% and 33%, respectively, following a single bout of exercise).14 Together these animal studies show that glycogen supercompensation transiently occurs in response to metabolic stressors, its extent is dependent on the intensity of the stress (e.g. duration and depth of hypoglycemia) and it dampens with recurrent episodes of the stressor. Our study showed that human brain glycogen supercompensation also occurs following an episode of hypoglycemia and is blunted with repeated episodes. This blunting effect is consistent with normal stress adaptation in healthy organisms.28 Perhaps glycogen supercompensation following the first one or two episodes provides sufficient extra fuel during the third episode, thereby reducing the need for further supercompensation at that time. Alternatively, glycogen supercompensation following acute hypoglycemia might support an adaptive response to the stress of hypoglycemia that allows a subsequent episode to be tolerated. Future experimentation is needed to address these possibilities and determine whether there is a causal relationship between glycogen supercompensation and generation of HAAF. It is possible that the primary metabolic adaptation to recurrent hypoglycemia that results in HAAF involves increased glucose uptake10,29,30 or lactate transport31,32 rather than glycogen supercompensation.
One limitation of our study is that the AH data were acquired several years prior to the RH data; however, identical MR methods were used and each study had its euglycemic control experiments. In the RH study, we maintained systemic euglycemia (95–100 mg/dL) during 13C-glucose infusions, while in the AH study, blood glucose during the 13C-glucose administration was slightly above euglycemia (115 mg/dL), a small difference insufficient to explain the substantial difference in the glycogen response in the two studies. We also estimated somewhat higher total glycogen levels in the RH study versus the AH study (Figure 5). Although the repeated insulin exposure during the three clamps in the RH study (vs. the one clamp in the AH study) prior to 13C-glucose administration (Figure 1) may have contributed to glycogen deposition in the brain, substantial glycogen synthesis due to insulin exposure during the two additional 2-h long clamps the day before 13C-glucose administration is unlikely. Indeed, while intracisternal administration of insulin increases brain glycogen levels,33,34 brain glucose or glycogen does not seem to increase after systemic administration of glucose and insulin,33 at least with short (1.5 h) administrations in rodents.35 Consistently, systemic insulin was reported to mainly increase brain glycogen turnover rather than concentration using 13C MRS.36 Finally, in conditions associated with glycogen deposition in the brain, the ratio of brain-to-plasma glucose was found to be increased35 and infusion of insulin during constant hyperglycemia does not affect the brain-to-plasma glucose ratio in humans.37 Therefore, the higher glycogen levels estimated in the RH study are likely related to other aspects of the studies, such as the length of the 13C-glucose infusions and the inclusion of wash-in and wash-out periods in the modeling (described below). In any case, this difference does not impact our main conclusions due to the availability of the euglycemic control data to compare with the hypoglycemic data of the same individuals who participated in each study.
The principal modeling-related limitation is that we assumed the same molecular turnover (i.e. 40 residues per minute17) during all conditions. Although repeated episodes of hypoglycemia might result in alterations of glycogen metabolism, we did not observe any difference in the goodness-of-fit (i.e. estimate of molecular turnover) between euglycemic and hypoglycemic conditions (data not shown). However, given our limited sample size, we emphasize that the modeling strategy we utilized needs further validation in animal studies as enzyme activities may be different in altered glycemic states.38,39 Another limitation relates to the different protocols of AH (wash-in/wash-out periods) and RH (only wash-in period) studies. Figure 5 shows that in the AH study simulated data match experimental measurements more consistently during the infusion (wash-in period) than after cessation of infusion (wash-out period). However, constraining the fit on the sole wash-in data did not result in statistically significant differences from the results obtained by fitting both wash-in and wash-out periods (data not shown). Namely, estimated glycogen supercompensation was 18 ± 3% after AH vs. euglycemia when only the wash-in data were fitted (p < 0.01). Note that even if the estimate of total glycogen content may be biased by the above-mentioned limitations, the main conclusions of the present work are unaffected. Indeed, they are based on % differences resulting from consistent simulations performed on euglycemic vs. hypoglycemic datasets (either for RH and AH studies). Finally, although this is currently the most detailed model of glycogen turnover, many factors, in particular during non-physiological conditions, can contribute to changing the label incorporation into the glycogen molecule.
In summary, we conclude that glycogen supercompensation occurs following moderate hypoglycemia in the human brain, and that repeated hypoglycemia exposure appears to blunt this response. This may also explain why glycogen levels are not higher in patients with type 1 diabetes and hypoglycemia unawareness than controls.15 Whether glycogen supercompensation following the first hypoglycemic episodes contributes to the generation of HAAF remains to be investigated in future studies.
Acknowledgments
We thank the staff of the Center for MR Research for maintaining and supporting the NMR system.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) grant R01 NS035192. The Center for Magnetic Resonance Research is supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant P41 EB015894, the Institutional Center Cores for Advanced Neuroimaging award P30 NS076408, and National Center for Research Resources (NCRR) grants S10 RR023730 and S10 RR027290. Mauro DiNuzzo is supported by the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 701635. Amir Moheet is supported by CTSA 5KL2TR000113. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
GÖ and ERS conceived the studies, and GÖ, ERS, and LEE designed the studies. GÖ, AKu, AM, AKh, and ERS performed the experiments. MDN performed all modeling and some statistical analyses. KK and LEE performed further statistical analyses. GÖ and MDN drafted the manuscript. All authors contributed to interpretation of the data and editing of the manuscript and gave final approval to the version to be submitted.
References
- 1.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2004; 350: 2272–2279. [DOI] [PubMed] [Google Scholar]
- 2.Dagogo-Jack SE, Craft S, Cryer PE. Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. J Clin Invest 1993; 91: 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geddes J, Schopman JE, Zammitt NN, et al. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med 2008; 25: 501–504. [DOI] [PubMed] [Google Scholar]
- 4.Prospective Diabetes Study Group UK. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 6.McCrimmon RJ, Öz G. Cerebral adaptation to recurrent hypoglycemia. Transl Endocrinol Metab 2012; 3: 89–114. [Google Scholar]
- 7.Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res 2003; 72: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Öz G, Kumar A, Rao JP, et al. Human brain glycogen metabolism during and after hypoglycemia. Diabetes 2009; 58: 1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh SW, Bergher JP, Anderson CM, et al. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmet hyl)propyl]-1H-indole-2-carboxamide). J Pharmacol Exp Ther 2007; 321: 45–50. [DOI] [PubMed] [Google Scholar]
- 10.Herzog RI, Chan O, Yu S, et al. Effect of acute and recurrent hypoglycemia on changes in brain glycogen concentration. Endocrinology 2008; 149: 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canada SE, Weaver SA, Sharpe SN, et al. Brain glycogen supercompensation in the mouse after recovery from insulin-induced hypoglycemia. J Neurosci Res 2011; 89: 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco AM, Gomez-Boronat M, Perez-Maceira J, et al. Brain glycogen supercompensation after different conditions of induced hypoglycemia and sustained swimming in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A Mol Integr Physiol 2015; 187: 55–60. [DOI] [PubMed] [Google Scholar]
- 13.Alquier T, Kawashima J, Tsuji Y, et al. Role of hypothalamic adenosine 5'-monophosphate-activated protein kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology 2007; 148: 1367–1375. [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Ishikawa T, Ito H, et al. Brain glycogen supercompensation following exhaustive exercise. J Physiol 2012; 590: 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Öz G, Tesfaye N, Kumar A, et al. Brain glycogen content and metabolism in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab 2012; 32: 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiNuzzo M. Kinetic analysis of glycogen turnover: relevance to human brain 13C-NMR spectroscopy. J Cereb Blood Flow Metab 2013; 33: 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Öz G, DiNuzzo M, Kumar A, et al. Revisiting glycogen content in the human brain. Neurochem Res 2015; 40: 2473–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moheet A, Kumar A, Eberly LE, et al. Hypoglycemia-associated autonomic failure in healthy humans: Comparison of two vs three periods of hypoglycemia on hypoglycemia-induced counterregulatory and symptom response 5 days later. J Clin Endocrinol Metab 2014; 99: 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Towler DA, Havlin CE, Craft S, et al. Mechanism of awareness of hypoglycemia. Perception of neurogenic (predominantly cholinergic) rather than neuroglycopenic symptoms. Diabetes 1993; 42: 1791–1798. [DOI] [PubMed] [Google Scholar]
- 20.Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab 2001; 281: E100–E112. [DOI] [PubMed] [Google Scholar]
- 21.Öz G, Henry PG, Tkáč I, et al. A localization method for the measurement of fast relaxing 13C NMR signals in humans at high magnetic fields. Appl Magn Reson 2005; 29: 159–169. [Google Scholar]
- 22.Adriany G, Gruetter R. A half-volume coil for efficient proton decoupling in humans at 4 Tesla. J Magn Reson 1997; 125: 178–184. [DOI] [PubMed] [Google Scholar]
- 23.Öz G, Henry PG, Seaquist ER, et al. Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochem Int 2003; 43: 323–329. [DOI] [PubMed] [Google Scholar]
- 24.Öz G, Hutter D, Tkáč I, et al. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord 2010; 25: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Öz G, Seaquist ER, Kumar A, et al. Human brain glycogen content and metabolism: implications on its role in brain energy metabolism. Am J Physiol Endocrinol Metab 2007; 292: E946–E951. [DOI] [PubMed] [Google Scholar]
- 26.Folbergrová J, Katsura KI, Siesjö BK. Glycogen accumulated in the brain following insults is not degraded during a subsequent period of ischemia. J Neurol Sci 1996; 137: 7–13. [DOI] [PubMed] [Google Scholar]
- 27.Brucklacher RM, Vannucci RC, Vannucci SJ. Hypoxic preconditioning increases brain glycogen and delays energy depletion from hypoxia-ischemia in the immature rat. Dev Neurosci 2002; 24: 411–417. [DOI] [PubMed] [Google Scholar]
- 28.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med 1998; 338: 171–179. [DOI] [PubMed] [Google Scholar]
- 29.Boyle PJ, Kempers SF, O'Connor AM, et al. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med 1995; 333: 1726–1731. [DOI] [PubMed] [Google Scholar]
- 30.Criego AB, Tkáč I, Kumar A, et al. Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J Neurosci Res 2005; 79: 42–47. [DOI] [PubMed] [Google Scholar]
- 31.Mason GF, Petersen KF, Lebon V, et al. Increased brain monocarboxylic acid transport and utilization in type 1 diabetes. Diabetes 2006; 55: 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herzog RI, Jiang L, Herman P, et al. Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. J Clin Invest 2013; 123: 1988–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellerup ET, Rafaelsen OJ. Brain glycogen after intracisternal insulin injection. J Neurochem 1969; 16: 777–781. [DOI] [PubMed] [Google Scholar]
- 34.Strang RH, Bachelard HS. Effect of insulin on levels and turnover of intermediates of brain carbohydrate metabolism in vivo. J Neurochem 1971; 18: 1799–1807. [DOI] [PubMed] [Google Scholar]
- 35.Nelson SR, Schulz DW, Passonneau JV, et al. Control of glycogen levels in brain. J Neurochem 1968; 15: 1271–1279. [DOI] [PubMed] [Google Scholar]
- 36.Morgenthaler FD, van Heeswijk RB, Xin L, et al. Non-invasive quantification of brain glycogen absolute concentration. J Neurochem 2008; 107: 1414–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seaquist ER, Damberg GS, Tkáč I, et al. The effect of insulin on in vivo cerebral glucose concentrations and rates of glucose transport/metabolism in humans. Diabetes 2001; 50: 2203–2209. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Chavez G, Hernandez-Berrones J, Luna-Ulloa LB, et al. Effect of diabetes on glycogen metabolism in rat retina. Neurochem Res 2008; 33: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 39.Sickmann HM, Waagepetersen HS, Schousboe A, et al. Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis. J Cereb Blood Flow Metab 2010; 30: 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]