Abstract
Transcranial Doppler (TCD) sonography is a frequently employed technique for quantifying cerebral blood flow by assuming a constant arterial diameter. Given that exercise increases arterial pressure by sympathetic activation, we hypothesized that exercise might induce a change in the diameter of large cerebral arteries. Middle cerebral artery (MCA) cross-sectional area was assessed in response to handgrip exercise by direct magnetic resonance imaging (MRI) observations. Twenty healthy subjects (11 female) performed three 5 min bouts of rhythmic handgrip exercise at 60% maximum voluntary contraction, alternated with 5 min of rest. High-resolution 7 T MRI scans were acquired perpendicular to the MCA. Two blinded observers manually determined the MCA cross-sectional area. Sufficient image quality was obtained in 101 MCA-scans of 19 subjects (age-range 20–59 years). Mixed effects modelling showed that the MCA cross-sectional area decreased by 2.1 ± 0.8% (p = 0.01) during handgrip, while the heart rate increased by 11 ± 2% (p < 0.001) at constant end-tidal CO2 (p = 0.10). In conclusion, the present study showed a 2% decrease in MCA cross-sectional area during rhythmic handgrip exercise. This further strengthens the current concept of sympathetic control of large cerebral arteries, showing in vivo vasoconstriction during exercise-induced sympathetic activation. Moreover, care must be taken when interpreting TCD exercise studies as diameter constancy cannot be assumed.
Keywords: Transcranial Doppler, exercise, cerebral blood flow, magnetic resonance imaging, magnetic resonance angiography, cerebral blood flow measurement
Introduction
The regulation of cerebral blood flow (CBF) is critical for the maintenance of oxygen and nutrient supply to the metabolically active brain. CBF control is multifactorial, influenced largely by the partial pressure of arterial carbon dioxide, mean arterial pressure and cerebral metabolism.1 Despite the abundance of sympathetic nerve fibres emanating from the cervical and stellate ganglia that innervate the cerebral arteries, the role of the autonomic nervous system in the control of cerebral blood flow (CBF) in humans has remained contentious for decades.2–10
In peripheral circulations, arterioles rather than large arteries are the main site of vascular resistance. However, for the brain, large vessels contribute ∼50% to vascular resistance.11 This noticeable contribution of large arteries in vascular resistance is most likely of importance in defending the cerebral microcirculation against surges in blood pressure by limiting fluctuations in microcirculatory pressure and cerebral perfusion.12–14 Several observations have been put forward as an argument for sympathetic influence on the human cerebral vasculature.6,15 At first, blocking sympathetic outflow to the human cerebral vasculature increases CBF, suggesting a sympathetically mediated restraint of cerebrovascular perfusion.15 Second, systemic agents that impair sympathetic signalling (alpha-adrenergic) reduce measures of cerebral pressure autoregulation.15,16 Finally, in healthy subjects, spillover of sympathetic neurotransmitters in the jugular vein is increased during sympathetic activation independently from neurotransmitters released in the brain.17 These observations suggest that the cerebral vasculature is under sympathetic control, leaving the challenge to identify changes in human brain artery diameter during sympathetic activation, especially when these would impact interpretation of physiological measurements, such as Transcranial Doppler (TCD).
In humans, changes in CBF are commonly evaluated non-invasively by measuring the CBF-velocity (CBFv) by TCD sonography in the large cerebral arteries with real-time resolution.18 A limitation of the majority of studies is that a constant diameter of the insonated brain artery is assumed to directly link changes in CBFv to changes in regional CBF. This assumption of constancy of brain artery diameter has been evidenced to be violated by high levels of PaCO219,20 and pressure pulsatility,21,22 but it is as yet unknown whether the assumed constancy of brain artery diameter holds true during exercise. Given that functional activation increases CBF and that sympathetic activation and arterial pressure increase with exercise, we hypothesized that exercise affects the diameter of large cerebral arteries. To that purpose, we set out to quantify the middle cerebral artery (MCA) cross-sectional area in rest and in response to rhythmic handgrip exercise using high resolution 7 T magnetic resonance imaging (MRI).
Materials and methods
Subjects
Twenty healthy, non-smoking, non-diabetic, right-handed subjects (9 males, 11 females) without history of cardiovascular disease underwent MRI during rhythmic handgrip exercise. No information about the active or chronic use of vasoactive medication or painkillers was obtained. One subject used a beta-adrenergic blocking agent for mild hypertension, which was considered not to have an effect on the cardiovascular response to handgrip exercise.23 Written informed consent was obtained from all participants prior to examination. The protocol was performed in accordance with the Helsinki protocol, as approved by the institutional Medical Ethics Committee of the Leiden University Medical Center.
Measurements
MRI measurements were performed on a 7 T Philips MRI system. Image acquisition parameters have been described previously22 and are listed in Table 1. The MCA contralateral to the exercise hand was located on an orthogonally reconstructed axial Time-Of-Flight angiogram. Additional sagittal reconstruction was created along the course of the MCA to aid the planning procedure. Subsequently, the high resolution 2D scan based on the black-blood approach and with a voxel-size of 0.2 × 0.2 × 5 mm3 was positioned perpendicular to the MCA. This scan will be referred to as ‘MCA-scan’ for the remainder of this article. Care was taken to select a straight part of the proximal MCA and exclude small branches whenever possible. Image blurring due to variations in vessel lumen across the cardiac cycle was minimized by prospectively triggering the acquisition to the subject’s heart beat using the finger pulse-oximetry unit of the scanner.22 Acquisition duration was 2.5 to 3.5 min, depending on the heart rate (HR) of the subject. End-tidal CO2 (PetCO2) was continuously monitored via a nasal cannula connected to a capnograph (Capnomac Ultima, Datex), located in the control room adjacent to the scanner. HR was obtained from the finger pulse-oximetry unit of the MRI scanner. After 10–15 min of preparatory scans, a brachial blood pressure measurement was performed on the right arm (Invivo Magnitude, Orlando, FL).
Table 1.
MRI scan parameters.
| Time-of-flight angiogram | Black blood T2-weighted (MCA-scan) | |
|---|---|---|
| Planning orientation | 3D acquisition covering the circle of Willis | Single slice, perpendicular to the MCA |
| Scan technique | Fast field echo | Spin echo |
| Acceleration type | None | Turbo spin echo; factor 12 + 4 dummy |
| Repetition time/echo time | 12.6/3.7 ms | 2 heart beats/86 ms |
| Flip/refocusing angle | 20°/– | 110°/105° |
| Field of view | 180 × 170 × 40 mm3 | 240 × 180 × 5 mm3 |
| Acquisition matrix | 544 × 540 | 1200 × 900 |
| Scan duration | 4 min | 2.5–3.5 min, depending on heart rate |
| Additional parameters | – | Trigger delay 50 ms |
The time-of-flight angiogram was used to locate the MCA and additional reconstructions ensured perpendicular planning of the high-resolution black blood T2 MCA-scan. The MCA-scan was used to determine the cross-sectional area of the MCA.
Protocol
A graphical representation of the protocol is illustrated in Figure 1. Exercise consisted of 5 min of dynamic handgripping, i.e. repeated 2 s hand contractions alternated with 2 s of relaxation. Prior to scanning the subjects were familiarized with the protocol and handgrip-device (Gripforce 500N, Curdes, Philadelphia, PA, USA). Maximum voluntary contraction (MVC) of the dominant hand was assessed by taking the maximum of three attempts. To achieve and maintain a steady state, rhythmic handgrip started the first minute at 80% MVC to be directly followed by 60% MVC for 4 min. Pilot studies proofed that this approach results in a robust and steady response for the complete duration of the exercise bout. Graphical representations of the imposed exercise rhythm as well as the force applied by the volunteer were displayed onto a screen in the scanner to provide visual feedback to the subject. The exercise protocol started with 5 min of rest followed by 5 min of handgrip. This was repeated 3 times to facilitate the detection of small differences in cross-sectional area. Acquisition of the MCA-scan was started 90 s after start of the rest or exercise bout. A total of six MCA scans (three at rest and three during exercise) were obtained per subject.
Figure 1.

Graphical representation of the exercise protocol. Relative timing of the MCA-scan is indicated by the grey area and the relative force level by a dashed line.
Data processing
The MCA-scans were anonymized and viewed in proprietary Philips viewing software. Two observers blinded to subject-number and condition (JV, AGTB) manually drew elliptical regions of interests to determine the MCA cross-sectional area twice. Quality of the MCA-scans was visually assessed, and scans with visible motion artefacts or a badly discernable vessel wall were excluded from analysis. All PetCO2 recordings and HR data were visually inspected and artefacts were manually removed. Values during rest and exercise were averaged per subject. Relative (percentage) change was calculated by normalizing with respect to the resting condition: relative = 100% (handgrip – rest)/rest.
Statistics
The Intraclass Correlation Coefficient assessed the consistency within and between observers (ICC C-k, SPSS v23). The effect of handgrip was assessed with a mixed-effect model (Statistics Toolbox, Matlab v2015b) taking the percentage change in vessel cross-sectional area, HR and PetCO2 as dependent variable. A fixed intercept with subjects as random intercept was used to model the change in cross-sectional area. The possible effects of time, age and gender and change in PetCO2 on the cross-sectional area change were investigated in separate models, using a fixed effect analysis. Normality of the residuals was assessed using Lilliefors test. Based on a sample-variance of 8.19 ± 1.32 mm2 as measured during normocapnia in a previous study,19 the a priori power was set to 0.9 to detect a difference of 5% in cross-sectional area resulting in a sample size of n = 20. Significance threshold was set at 0.05. Values are mean ± SE except when stated otherwise.
Results
Sufficient image quality was achieved in 101 of 120 scans, such that 19 subjects entered final analysis. The median (range) age was 30 (20–59) years and resting systolic and diastolic arterial pressures were 112 ± 9 and 69 ± 10 mmHg (mean ± SD), respectively.
During exercise, HR increased 11.2 ± 1.7% (p < 0.001), without change in PetCO2 (−0.9 ± 0.5%, p < 0.10, see Table 2).
Table 2.
Average response to rhythmic handgrip exercise.
| Rest | Handgrip | Effect estimate | ||
|---|---|---|---|---|
| (mean ± SD) | (mean ± SD) | (mean ± SE) | ||
| Area (mm2) | 7.47 ± 1.18 | 7.31 ± 1.15 | −2.1 ± 0.81% | p = 0.01 |
| HR (bpm) | 62.0 ± 12.5 | 68.4 ± 12.5 | 11.2 ± 1.7% | p < 0.001 |
| PetCO2 (kPa) | 5.06 ± 0.36 | 5.02 ± 0.36 | −0.9 ± 0.5% | p = 0.10 |
Fixed effect estimates determined by corresponding Mixed Effects model. Area: cross-sectional area of the middle cerebral artery; HR: heart rate; PetCO2: end-tidal carbon dioxide.
Both the consistency of MCA area measurements within (ICC A-k = 0.978 and 0.977 for JV and AGTB, respectively) and between observers (ICC C-k = 0.981) were considered sufficient to accept the reliability of the average MCA cross-sectional area measurement. A representative example of the image quality of the MCA-scan is depicted in Figure 2.
Figure 2.
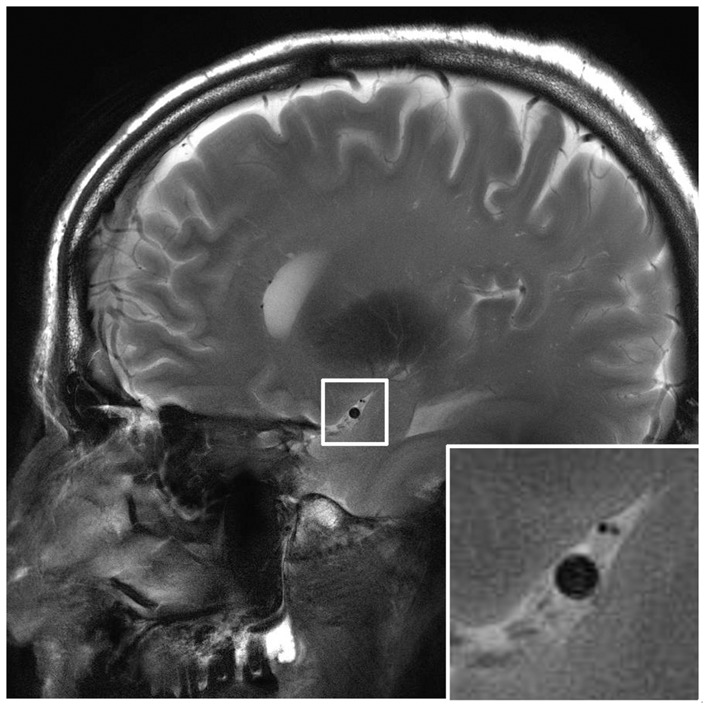
Example of a high resolution cardiac triggered MRI scan of the MCA; based on the black blood principle. The white box indicates the zoomed area showing the lumen of the MCA.
The mean cross-sectional area during rest was 7.47 ± 1.18 mm2 (diameter of 3.1 mm), which decreased during handgrip exercise by 0.16 mm2 to 7.31 ± 1.15 mm2, see Figure 3. The change in cross-sectional area was −2.11 ± 0.81% (95% confidence interval: −3.74%, −0.49%, p = 0.01), see Figure 4, corresponding to a decrease in diameter of 1.1 ± 0.4% (0.033 mm). Repeated exercise challenges (time-effect) did not have an effect on this relation (p = 0.07).
Figure 3.
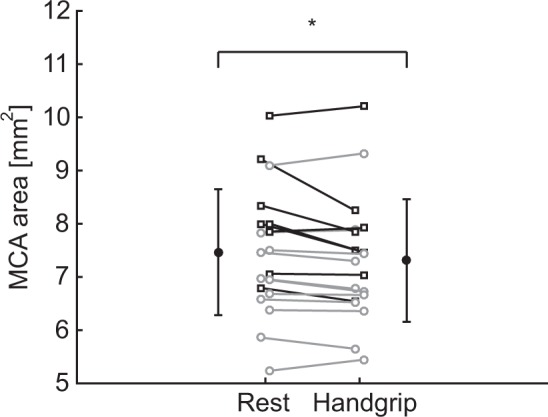
Effect of dynamic handgrip exercise on the average MCA cross-sectional area. Black error-bars indicate mean and standard deviation. Squares represent males and circles females. Corresponding measurements of a subject are connected by a line. *p < 0.05 rest vs. handgrip.
Figure 4.
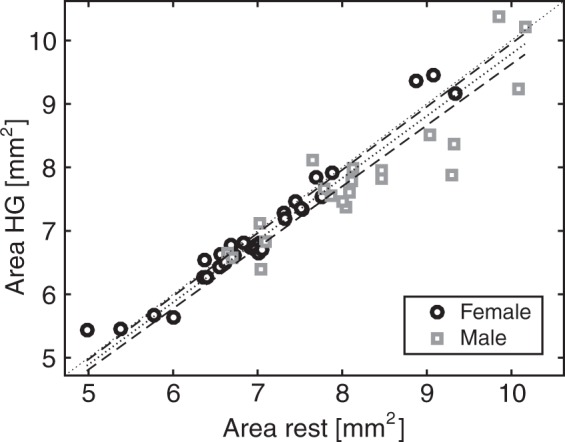
Scatterplot of the cross-sectional area during rest and handgrip. All 57 data pairs are plotted for male (square) and female (circle) subjects. The Mixed Model Estimate is indicated by the dotted line with 95% confidence intervals (dashed lines). The line of identify (dotted line) is added for visual reference.
The age of the participants was strongly clustered into two groups. Therefore, the effect of age was assessed by splitting the population into a group below (three males, eight females) and above 40 years of age (five males, three females). There was no relation between age and the change in cross-sectional area in response to exercise (p = 0.76). The change in PetCO2 did not have a relation to the change in cross-sectional area (p = 0.51). There was a larger reduction in cross-sectional area in males compared with females (βmale = −3.46 ± 1.47, p = 0.02).
Discussion
The main finding of the present study is that a 2% reduction in the contralateral MCA cross-sectional area in response to rhythmic handgrip. This suggests that vasoconstriction occurs in a large brain artery during exercise induced sympathetic activation. This falsifies the hitherto assumed constancy of cerebral artery diameter.
Regarding the evaluation of cerebral perfusion with TCD, the concern has been raised whether the diameter of the basal cerebral arteries remains constant during sympathetic activation, specifically exercise.8,9,24 To our knowledge, this is the first study to directly observe a MCA lumen change during exercise with high resolution MRI. To date, a single study estimated the change in MCA cross-sectional area specifically during rhythmic handgrip.8 Their estimate of 21% change was indirect, i.e. by comparing the regular TCD signal with the spectrally averaged Doppler signal, and therefore not easily comparable with the present findings. A study assessing the effect of sympathetic activation on arterial diameter did not find a change in a small peripheral artery of the foot as assessed with ultrasound.7 Moreover, direct MRI observations of the MCA during sympathetic stimulation by lower body negative pressure25 also did not find a diameter change. The absence of observable changes in these studies suggest that the magnitude of any changes can be considered minor. Moreover, changes in MCA cross-sectional during CO2 inhalation and pressure pulsatility can also be considered small.19,20,22 This is in line with the current finding of 2% vasoconstriction, which indicate that MCA cross-sectional area changes are small when induced by sympathetic activation by moderate exercise.
The extent in MCA diameter response to exercise and sympathetic activation could differ depending on factors such as age and gender. Findings of the present study suggest a larger change in MCA cross-sectional area in males compared with females in response to rhythmic handgrip. This finding coincides with the observation that premenopausal females exhibit lower sympathetic outflow in response to static handgrip exercise26 and lower cerebrovascular reactivity27,28 as a marker for brain vessel dilatory capacity. Contrary, with age, no relation with the degree of MCA vasoconstriction was observed in present study. This is in line with preserved sympathetic response to autonomic challenges29,30 and preserved carbon dioxide cerebrovascular reactivity31,32 in aging. We consider that the limited sample size of the gender and age groups precludes proper inference of possible differences.
Small changes in vessel lumen can have profound influence on the estimation of CBF by relative velocity measurements. A decrease in vessel lumen proportionally increases the flow velocity even at constant blood flow. However, with exercise both the lumen and blood flow and are changing. For example, during handgrip, the CBFv increases from 50 to 55 cm/s, corresponding to an apparent 10% increase in CBF. However, a concurrent decrease in cross-sectional area of 2.1% reduces the underlying CBF change to 7.9% rather than the 10%, as would be estimated from the velocity only. Moreover, the variation in the cross-sectional area change ranged (mean ± std) from −10.4 ± 4.7% (subject 3, male, n = 3) to 4.3 ± 5.3% (subject 16, female, n = 2), respectively, overestimating and underestimating the underlying CBF response to handgrip exercise by 10.5% and by 5.3% in these subjects. Given a measured 10% change in velocity, the corresponding change in CBF, corrected for the change in lumen area of these subjects, would be +0.5% and +15.3%, respectively. While the change in lumen seems slight, it constitutes a sizable part of the TCD determined cerebral haemodynamic response during handgrip exercise.
Measuring small changes in vessel lumen requires high precision measurements. The acquisition resolution of the employed MCA-scan was 0.2 × 0.2 mm2; therefore, the average change in MCA cross-sectional area of 0.16 mm2 constitutes ∼4 pixels, providing for sufficient statistical power in the current study to infer these changes. The determination of the cross-sectional area can be dependent on the effective contrast of the acquisition as well as on the observer accuracy. Reliability of the cross-sectional area assessments was augmented by excluding scans with poorly discernable MCA vessel wall (e.g. subject motion), as well as by averaging the observations of two observers, who were blinded to participant number and condition.
High resolution scanning was complemented with cardiac triggering that stabilized the imaging by timing the acquisition with respect to the cardiac cycle. Although this approach prolongs scan time, it mitigates blurring effects and imaging artefacts. In addition, the sensitivity of the MCA-scan to variations in blood flow has been minimized by the application of dummy echoes, thereby creating a clear signal void within the vessel lumen.19 Although some dependency of the intravascular signal on the blood flow velocity may persist, an increase in flow velocity near the vessel wall would make the lumen appear larger rather than smaller. This effect can therefore not explain the observed decrease in cross-sectional area as found in the current study, it might only have resulted in an underestimation of the actual decrease in MCA cross-sectional area.
A potential limitation of the present study is the application of concurrent visual feedback to maintain the desired exercise level. Stimulation of the visual cortex by the visual feedback is associated with regional increases in CBF. However, the visual cortex is supplied by the posterior cerebral circulation rather than by the MCA, rendering an effect of visual stimulation on the MCA cross-sectional area negligible. Another limitation of the present study is that the level of sympathetic activation was not directly determined. This could have contributed to the large between-subject variation. However, the exercise protocol was setup to provide a consistent response within- and between-subjects, by scaling the prescribed force to the individually maximal voluntary contraction. Currently, no data exist how the level of (rhythmic handgrip) exercise impacts sympathetic activation, and we can therefore not exclude an effect of the level of sympathetic activation on the large between-subject variation. In the present study, arterial pressure was not monitored during exercise, as brachial pressure measurements would occlude either the handgripping arm or disrupt blood flow in the finger used for triggering the MCA-scan. However, it has been well documented that the arterial pressure increases in response to rhythmic handgrip exercise.34,35 This increase in pressure is required to provide the work to counteract the higher vascular resistance and actually increase the blood flow velocity. Without an increase in pressure, the blood flow would decrease with a constant flow velocity. Therefore, given that the arterial pressure increases during handgrip exercise, then the observed vasoconstriction in the MCA can be considered to modulate the blood flow velocity and lead to an overestimation when using TCD to assess CBF changes.
Conclusion
In conclusion, the present study observed a 2% decrease in MCA cross-sectional area with MRI during rhythmic handgrip exercise in healthy subjects. This further strengthens the current concept of sympathetic control of large cerebral arteries, showing in vivo vasoconstriction during exercise induced sympathetic activation. Finally, the assumption of constancy vessel diameter is falsified. Therefore, care must be taken when interpreting TCD measurements of the cerebral circulation in exercise studies.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Rembrandt Institute of Cardiovascular Science.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
JV, AGTB, MJPvO and JJvL designed and JV performed the research; JV and AGTB analysed the data; JV, AGTB, JJvL and MJPvO wrote the article; JV, AGTB, MAB, MJAPD, JJvL and MJPvO approved the final version.
References
- 1.Ainslie PN, Bailey DM. Your ageing brain: the lows and highs of cerebral metabolism. J Physiol 2013; 591: 1591–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strandgaard S, Sigurdsson ST. Point: Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Counterpoint: Sympathetic nerve activity does not influence cerebral blood flow. J Appl Physiol 2008; 105: 1366–1367. [DOI] [PubMed] [Google Scholar]
- 3.Purkayastha S, Saxena A, Eubank WL, et al. α1-Adrenergic receptor control of the cerebral vasculature in humans at rest and during exercise. Exp Physiol 2013; 98: 451–461. [DOI] [PubMed] [Google Scholar]
- 4.van Lieshout JJ, Secher NH. Point: Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. J Appl Physiol 2008; 105: 1364–1366. [DOI] [PubMed] [Google Scholar]
- 5.Strandgaard S, MacKenzie ET, Sengupta D, et al. Upper limit of autoregulation of cerebral blood flow in the baboon. Circ Res 1974; 34: 435–440. [DOI] [PubMed] [Google Scholar]
- 6.Willie CK, Tzeng YC, Fisher JA, et al. Integrative regulation of human brain blood flow. J Physiol 2014; 592: 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pott F, Ray CA, Olesen HL, et al. Middle cerebral artery blood velocity, arterial diameter and muscle sympathetic nerve activity during post-exercise muscle ischaemia. Acta Physiol Scand 1997; 160: 43–47. [DOI] [PubMed] [Google Scholar]
- 8.Giller CA, Giller AM, Cooper CR, et al. Evaluation of the cerebral hemodynamic response to rhythmic handgrip. J Appl Physiol 2000; 88: 2205–2213. [DOI] [PubMed] [Google Scholar]
- 9.Giller CA. The Emperor has no clothes: velocity, flow, and the use of TCD. J Neuroimaging 2003; 13: 97–98. [PubMed] [Google Scholar]
- 10.Seifert T, Secher NH. Sympathetic influence on cerebral blood flow and metabolism during exercise in humans. Prog Neurobiol 2011; 95: 406–426. [DOI] [PubMed] [Google Scholar]
- 11.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res 1990; 66: 8–17. [DOI] [PubMed] [Google Scholar]
- 12.Cassaglia PA, Griffiths RI, Walker AM. Sympathetic nerve activity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1255–R1261. [DOI] [PubMed] [Google Scholar]
- 13.Ainslie PN, Brassard P. Why is the neural control of cerebral autoregulation so controversial? F1000Prime Rep 2014; 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 2006; 100: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 15.ter Laan M, van Dijk JM, Elting JW, et al. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth 2013; 111: 361–367. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Zuckerman JH, Iwasaki K, et al. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 2002; 106: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell DA, Lambert G, Secher NH, et al. Jugular venous overflow of noradrenaline from the brain: a neurochemical indicator of cerebrovascular sympathetic nerve activity in humans. J Physiol 2009; 587: 2589–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 1982; 57: 769–774. [DOI] [PubMed] [Google Scholar]
- 19.Verbree J, Bronzwaer AGT, Ghariq E, et al. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol 2014; 117: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 20.Coverdale NS, Gati JS, Opalevych O, et al. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol 2014; 117: 1090–1096. [DOI] [PubMed] [Google Scholar]
- 21.Kuroda J, Kinoshita M, Tanaka H, et al. Cardiac cycle-related volume change in unruptured cerebral aneurysms: a detailed volume quantification study using 4-dimensional CT angiography. Stroke 2012; 43: 61–66. [DOI] [PubMed] [Google Scholar]
- 22.Warnert EA, Verbree J, Wise RG, et al. Using high-field magnetic resonance imaging to estimate distensibility of the middle cerebral artery. Neurodegen Dis 2016; 16: 407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyberg G. Blood pressure and heart rate response to isometric exercise and mental arithmetic in normotensive and hypertensive subjects. Clin Sci Mol Med Suppl 1976; 3: 681s–685s. [DOI] [PubMed] [Google Scholar]
- 24.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol 2008; 104: 306–314. [DOI] [PubMed] [Google Scholar]
- 25.Serrador JM, Picot PA, Rutt BK, et al. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 2000; 31: 1672–1678. [DOI] [PubMed] [Google Scholar]
- 26.Ettinger SM, Silber DH, Collins BG, et al. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol 1996; 80: 245–251. [DOI] [PubMed] [Google Scholar]
- 27.Conijn MM, Hoogduin JM, van der Graaf Y, et al. Microbleeds, lacunar infarcts, white matter lesions and cerebrovascular reactivity – a 7 T study. Neuroimage 2012; 59: 950–956. [DOI] [PubMed] [Google Scholar]
- 28.Kassner A, Winter JD, Poublanc J, et al. Blood-oxygen level dependent MRI measures of cerebrovascular reactivity using a controlled respiratory challenge: reproducibility and gender differences. J Magn Reson Imaging 2010; 31: 298–304. [DOI] [PubMed] [Google Scholar]
- 29.Ng AV, Callister R, Johnson DG, et al. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol 1994; 267(Pt 2): H344–H353. [DOI] [PubMed] [Google Scholar]
- 30.Gagnon D, Schlader ZJ, Crandall CG. Sympathetic activity during passive heat stress in healthy aged humans. J Physiol 2015; 593: 2225–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kastrup A, Dichgans J, Niemeier M, et al. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke 1998; 29: 1311–1314. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Rodgers ZB, Kuo AH. Cerebrovascular reactivity measured with arterial spin labeling and blood oxygen level dependent techniques. Magn Reson Imaging 2015; 33: 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Aguirre GK, Kimberg DY, et al. Arterial spin labeling perfusion fMRI with very low task frequency. Magn Reson Med 2003; 49: 796–802. [DOI] [PubMed] [Google Scholar]
- 34.Hartwich D, Fowler KL, Wynn LJ, et al. Differential responses to sympathetic stimulation in the cerebral and brachial circulations during rhythmic handgrip exercise in humans. Exp Physiol 2010; 95: 1089–1097. [DOI] [PubMed] [Google Scholar]
- 35.Shoemaker JK, MacDonald MJ, Hughson RL. Time course of brachial artery diameter responses to rhythmic handgrip exercise in humans. Cardiovasc Res 1997; 35: 125–131. [DOI] [PubMed] [Google Scholar]


