Abstract
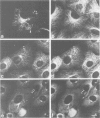
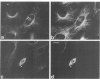
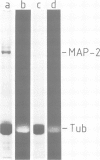
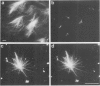
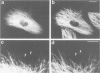
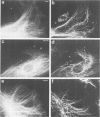
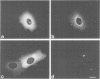
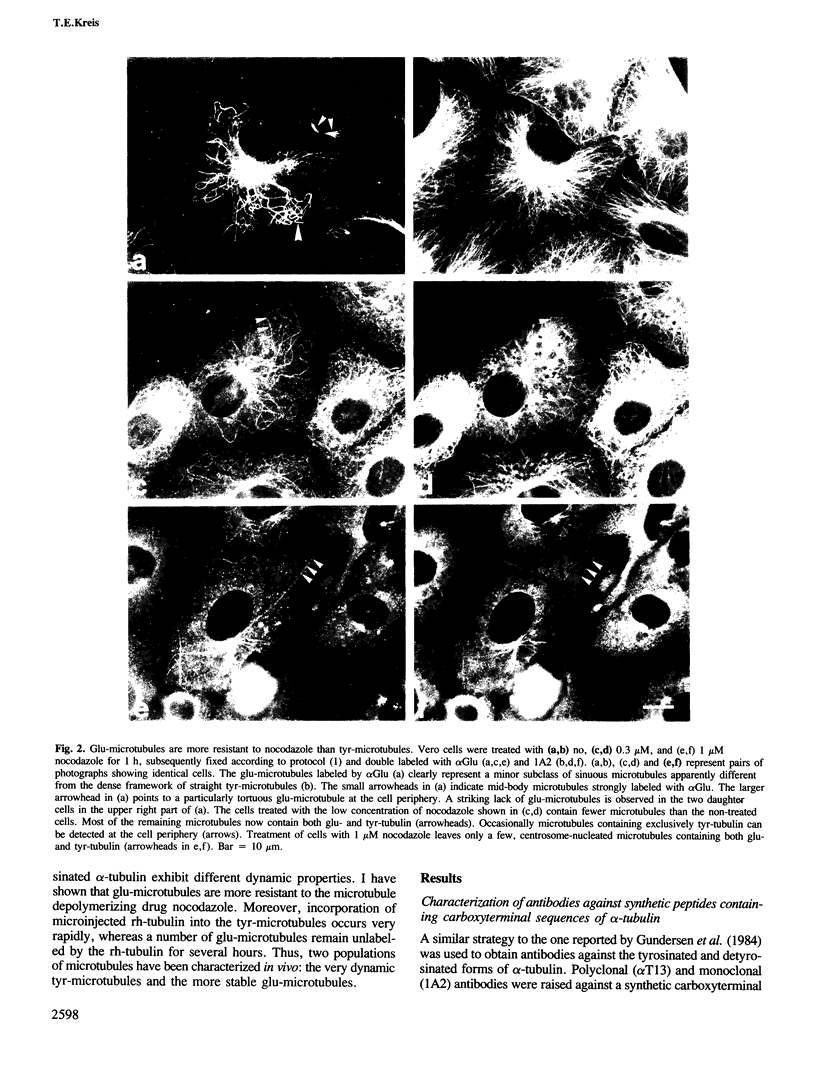
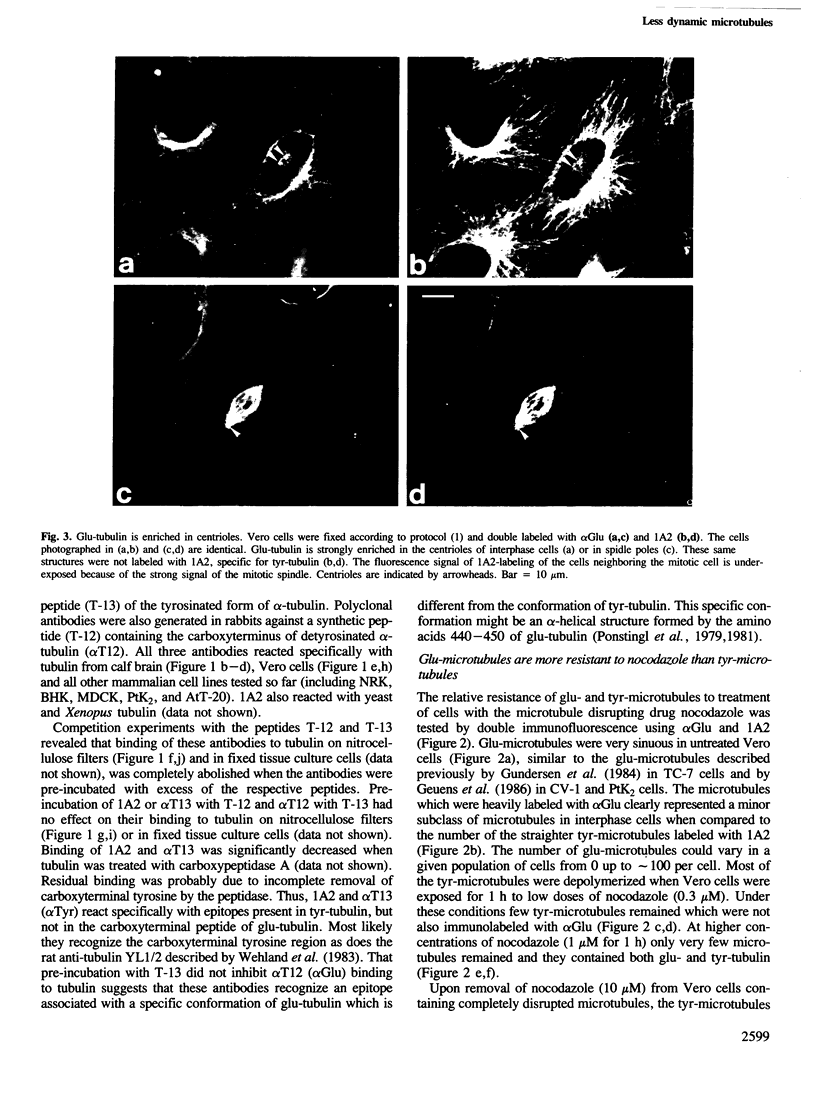
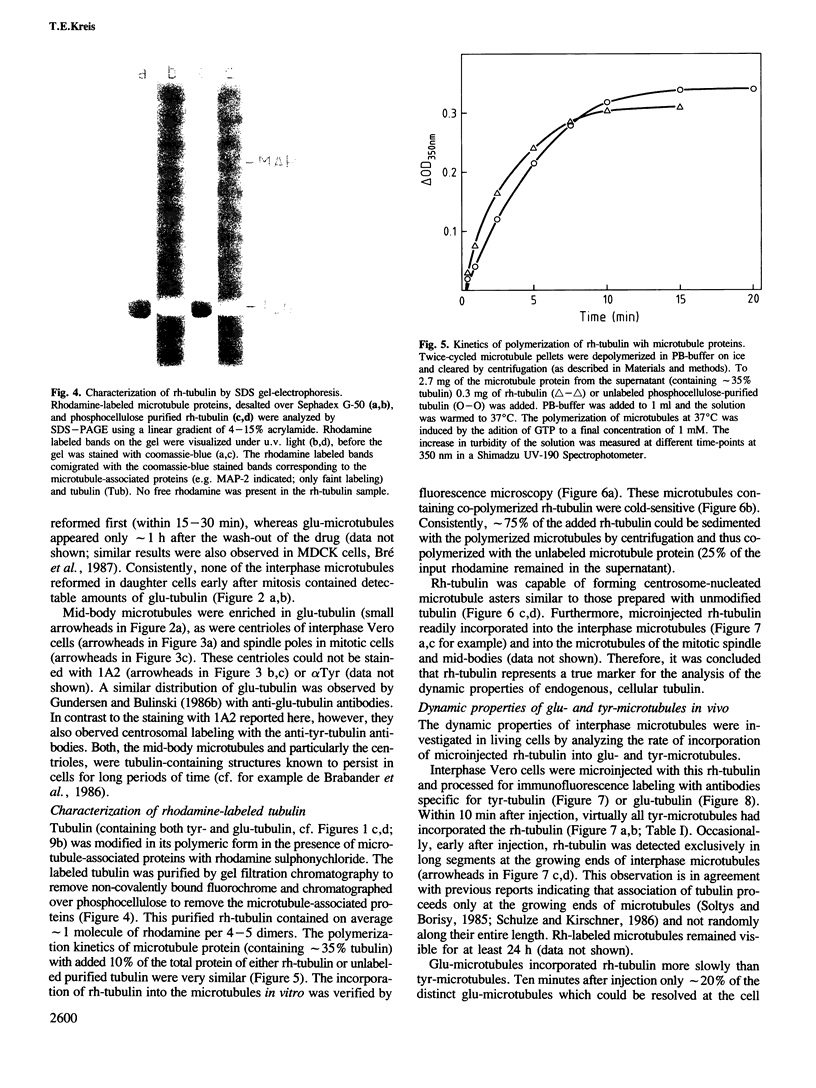
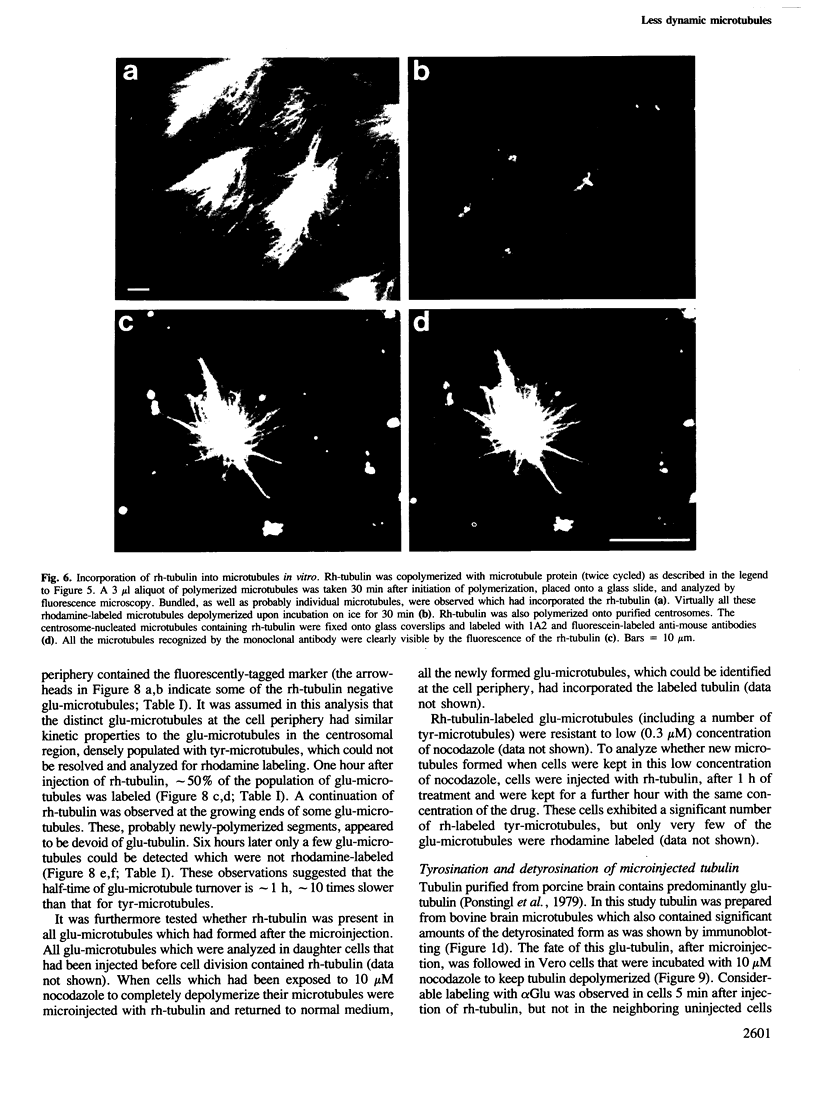
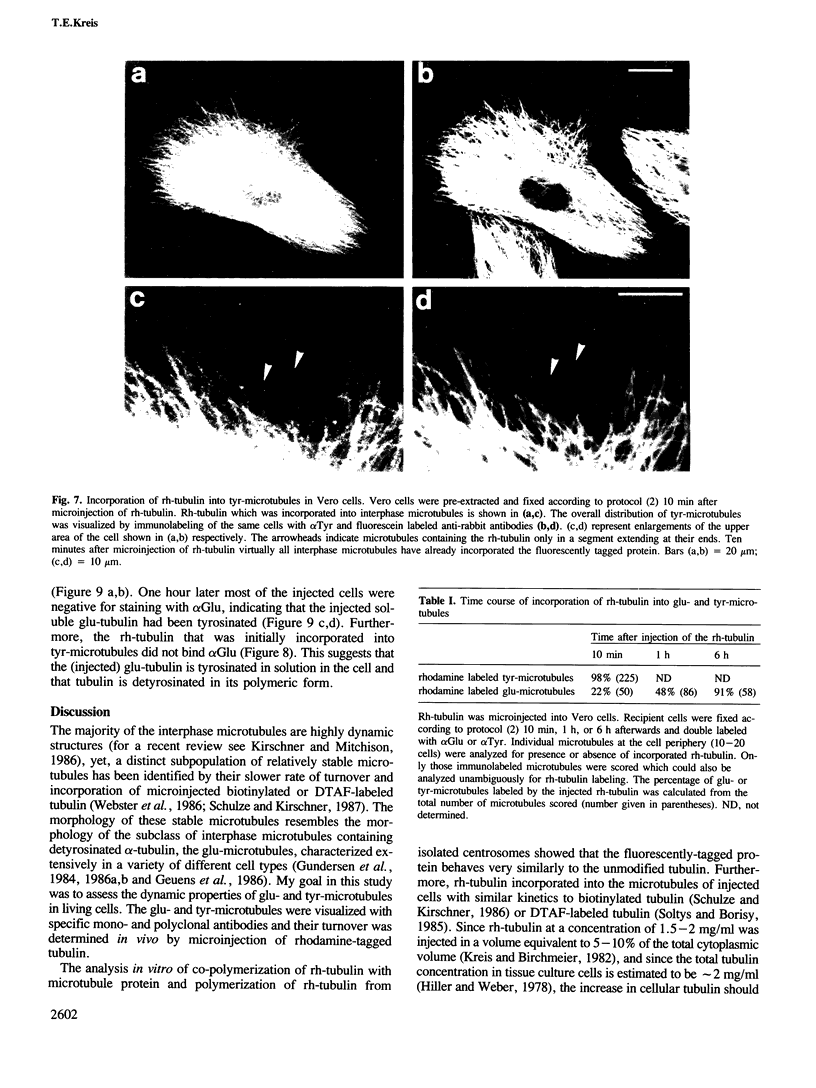
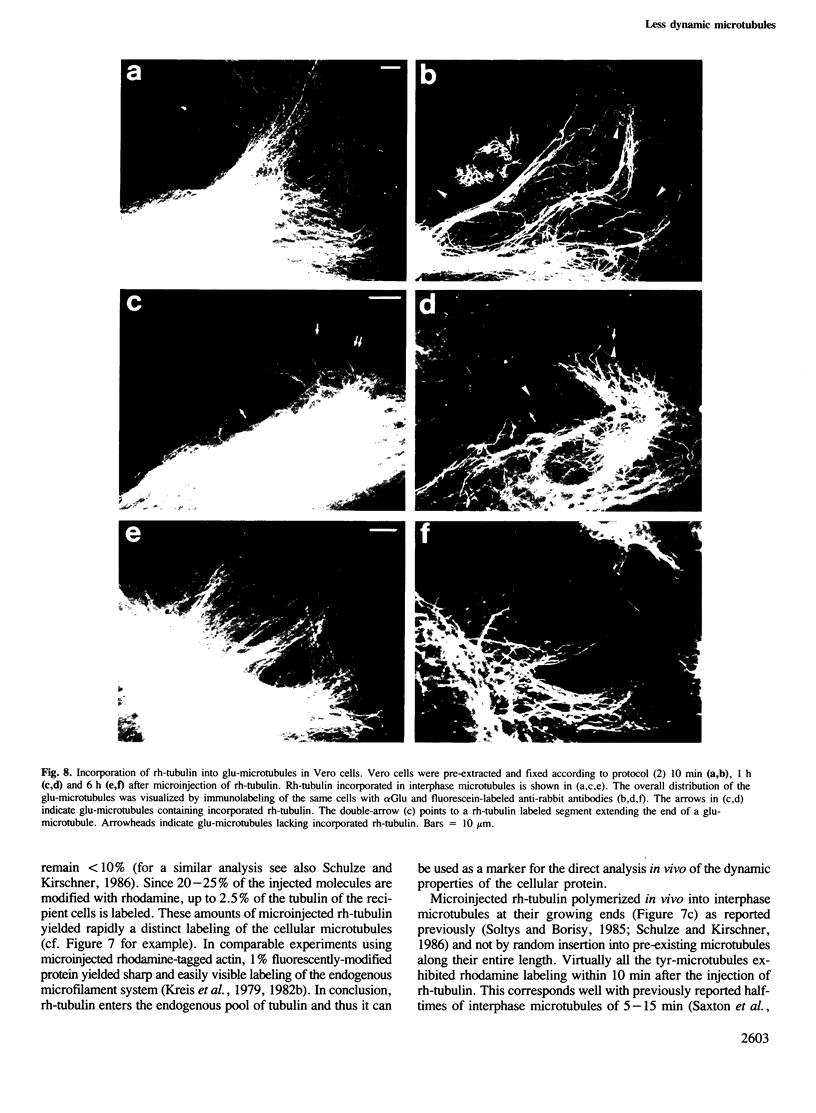
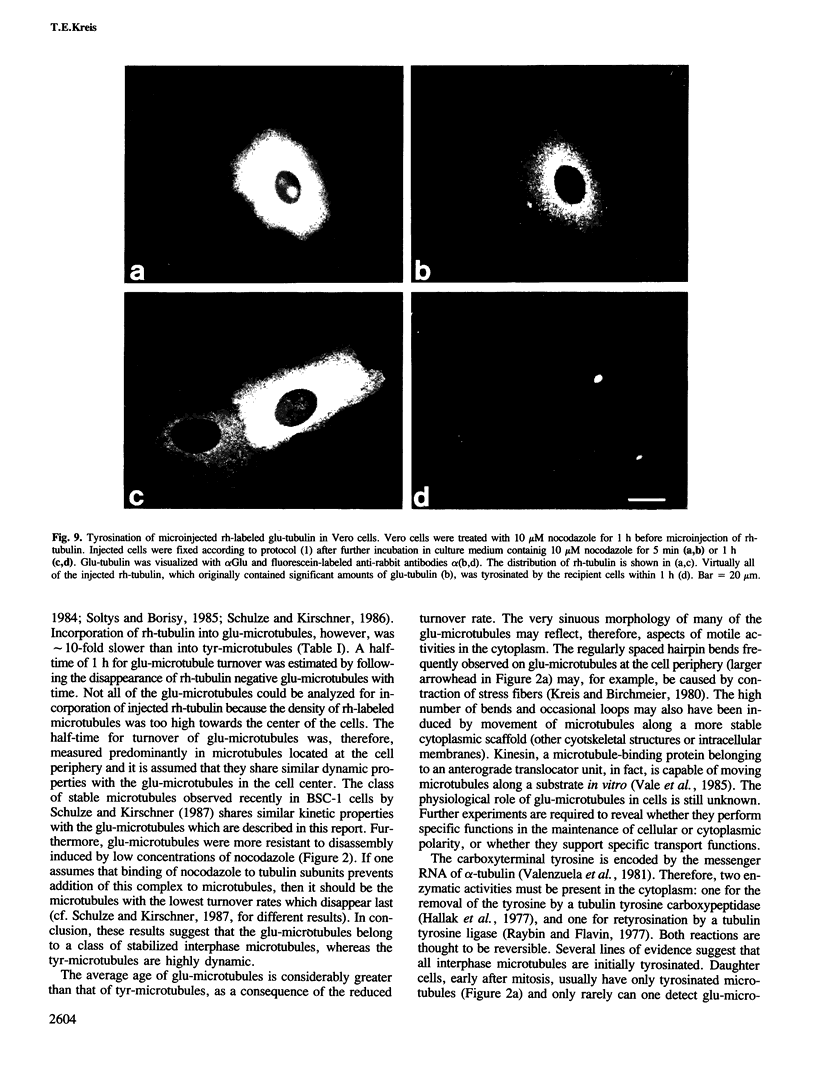
Peptide antibodies specific for tyrosinated (tyr-tubulin) or detyrosinated alpha-tubulin (glu-tubulin) have been generated for studying the relative stability of microtubules enriched in either form of alpha-tubulin. Treatment of Vero cells with nocodazole has revealed that interphase microtubules rich in glu-tubulin (glu-microtubules) are resistant to higher concentrations of the microtubule-disrupting drug than the microtubules containing only tyr-tubulin (tyr-microtubules). Glu-tubulin is enriched in centrioles and mid-bodies, but absent from the first interphase microtubules that have repolymerized in late telophase. Tubulin (including both forms) has been labeled with rhodamine (rh-tubulin) and microinjected into Vero cells to study in vivo the dynamic properties and incorporation rates of tubulin into microtubules rich in either glu- or tyr-tubulin. Tyr-microtubules are significantly more rapidly labeled by the microinjected rh-tubulin than glu-microtubules. Ten minutes after injection, rh-tubulin is present in virtually all tyr-microtubules. The half-time of turnover of glu-microtubules is approximately 1 h. Even several hours after microinjection, some of the glu-microtubules have consistently not incorporated visible amounts of rh-tubulin. These results suggest that tyr- and glu-microtubules respectively represent relatively dynamic and stable subclasses of interphase microtubules.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Weiss D. G., Hayden J. H., Brown D. T., Fujiwake H., Simpson M. Gliding movement of and bidirectional transport along single native microtubules from squid axoplasm: evidence for an active role of microtubules in cytoplasmic transport. J Cell Biol. 1985 May;100(5):1736–1752. doi: 10.1083/jcb.100.5.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Conjugates of immunoglobulin G with different fluorochromes. I. Characterization by anionic-exchange chromatography. Scand J Immunol. 1973;2(3):273–290. doi: 10.1111/j.1365-3083.1973.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., Aerts F., De Mey J. Microtubule dynamics during the cell cycle: the effects of taxol and nocodazole on the microtubule system of Pt K2 cells at different stages of the mitotic cycle. Int Rev Cytol. 1986;101:215–274. doi: 10.1016/s0074-7696(08)60250-8. [DOI] [PubMed] [Google Scholar]
- Geuens G., Gundersen G. G., Nuydens R., Cornelissen F., Bulinski J. C., DeBrabander M. Ultrastructural colocalization of tyrosinated and detyrosinated alpha-tubulin in interphase and mitotic cells. J Cell Biol. 1986 Nov;103(5):1883–1893. doi: 10.1083/jcb.103.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. Distribution of tyrosinated and nontyrosinated alpha-tubulin during mitosis. J Cell Biol. 1986 Mar;102(3):1118–1126. doi: 10.1083/jcb.102.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur J Cell Biol. 1986 Dec;42(2):288–294. [PubMed] [Google Scholar]
- Gundersen G. G., Kalnoski M. H., Bulinski J. C. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984 Oct;38(3):779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Hallak M. E., Rodriguez J. A., Barra H. S., Caputto R. Release of tyrosine from tyrosinated tubulin. Some common factors that affect this process and the assembly of tubulin. FEBS Lett. 1977 Feb 1;73(2):147–150. doi: 10.1016/0014-5793(77)80968-x. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K. Radioimmunoassay for tubulin: a quantitative comparison of the tubulin content of different established tissue culture cells and tissues. Cell. 1978 Aug;14(4):795–804. doi: 10.1016/0092-8674(78)90335-5. [DOI] [PubMed] [Google Scholar]
- Keith C. H., Feramisco J. R., Shelanski M. Direct visualization of fluorescein-labeled microtubules in vitro and in microinjected fibroblasts. J Cell Biol. 1981 Jan;88(1):234–240. doi: 10.1083/jcb.88.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J. V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982 Jun;93(3):576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986 May 9;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Birchmeier W. Microinjection of fluorescently labeled proteins into living cells with emphasis on cytoskeletal proteins. Int Rev Cytol. 1982;75:209–214. doi: 10.1016/s0074-7696(08)61005-0. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Birchmeier W. Stress fiber sarcomeres of fibroblasts are contractile. Cell. 1980 Nov;22(2 Pt 2):555–561. doi: 10.1016/0092-8674(80)90365-7. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schlessinger J. Mobility of microinjected rhodamine actin within living chicken gizzard cells determined by fluorescence photobleaching recovery. Cell. 1982 Jul;29(3):835–845. doi: 10.1016/0092-8674(82)90445-7. [DOI] [PubMed] [Google Scholar]
- Kreis T. E. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO J. 1986 May;5(5):931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E. Preparation, assay, and microinjection of fluorescently labeled cytoskeletal proteins: actin, alpha-actinin, and vinculin. Methods Enzymol. 1986;134:507–519. doi: 10.1016/0076-6879(86)34116-8. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Winterhalter K. H., Birchmeier W. In vivo distribution and turnover of fluorescently labeled actin microinjected into human fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3814–3818. doi: 10.1073/pnas.76.8.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Flavin M. Modulation of some parameters of assembly of microtubules in vitro by tyrosinolation of tubulin. Eur J Biochem. 1982 Nov;128(1):215–222. doi: 10.1111/j.1432-1033.1982.tb06954.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984 Nov 15;312(5991):232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Murofushi H. Purification and characterization of tubulin-tyrosine ligase from porcine brain. J Biochem. 1980 Mar;87(3):979–984. doi: 10.1093/oxfordjournals.jbchem.a132828. [DOI] [PubMed] [Google Scholar]
- Piperno G., LeDizet M., Chang X. J. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987 Feb;104(2):289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Little M., Krauhs E., Kempf T. Carboxy-terminal amino acid sequence of alpha-tubulin from porcine brain. Nature. 1979 Nov 22;282(5737):423–425. doi: 10.1038/282423a0. [DOI] [PubMed] [Google Scholar]
- Raybin D., Flavin M. Enzyme which specifically adds tyrosine to the alpha chain of tubulin. Biochemistry. 1977 May 17;16(10):2189–2194. doi: 10.1021/bi00629a023. [DOI] [PubMed] [Google Scholar]
- Saxton W. M., Stemple D. L., Leslie R. J., Salmon E. D., Zavortink M., McIntosh J. R. Tubulin dynamics in cultured mammalian cells. J Cell Biol. 1984 Dec;99(6):2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherson T., Kreis T. E., Schlessinger J., Littauer U. Z., Borisy G. G., Geiger B. Dynamic interactions of fluorescently labeled microtubule-associated proteins in living cells. J Cell Biol. 1984 Aug;99(2):425–434. doi: 10.1083/jcb.99.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder H. C., Wehland J., Weber K. Purification of brain tubulin-tyrosine ligase by biochemical and immunological methods. J Cell Biol. 1985 Jan;100(1):276–281. doi: 10.1083/jcb.100.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Dynamic and stable populations of microtubules in cells. J Cell Biol. 1987 Feb;104(2):277–288. doi: 10.1083/jcb.104.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Microtubule dynamics in interphase cells. J Cell Biol. 1986 Mar;102(3):1020–1031. doi: 10.1083/jcb.102.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon F. Organizing the cytoplasm for motility. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):17–22. doi: 10.1101/sqb.1982.046.01.005. [DOI] [PubMed] [Google Scholar]
- Soltys B. J., Borisy G. G. Polymerization of tubulin in vivo: direct evidence for assembly onto microtubule ends and from centrosomes. J Cell Biol. 1985 May;100(5):1682–1689. doi: 10.1083/jcb.100.5.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg J., Moskalewski S. Microtubules and the organization of the Golgi complex. Exp Cell Res. 1985 Jul;159(1):1–16. doi: 10.1016/s0014-4827(85)80032-x. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Sandoval I. V. A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. I. Biochemical characterization, effects on microtubule polymerization in vitro, and microtubule polymerization and organization in vivo. J Cell Biol. 1983 Nov;97(5 Pt 1):1467–1475. doi: 10.1083/jcb.97.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]