Abstract
Blood–brain barrier (BBB) disruption plays an important role in pathophysiological progress of ischemic stroke. However, our knowledge of the dynamic change of BBB permeability and its mechanism remains limited. In the current study, we used a non-human primate (NHP) MCAO model and a serial CSF sampling method that allowed us to determine the dynamic change of BBB permeability by calculating the CSF/serum albumin ratio (AR). We showed that AR increased rapidly and significantly after ischemia, and the fold increase of AR is highly correlated with the infarction size during the subacute phase. Moreover, we determined the temporal change of MMP-1, MMP-2, MMP-3, MMP-9, MMP-10, MMP-13, TIMP-1, and TIMP-2 in CSF and serum. Each MMP and TIMP showed different change patterns when comparing their values in CSF and serum. Based on the longitudinal dataset, we showed that the fold increase of MMP-9 in serum and CSF are both correlated to infarction size. Among the measured MMPs and TIMPs, only MMP-2, MMP-13, and TIMP-2 in CSF correlated with AR to some extent. Our data suggest there is no single MMP or TIMP fully responsible for BBB breakdown, which is regulated by a much more complicated signal network and further investigations of the mechanisms are needed.
Keywords: Blood–brain barrier, cerebrospinal fluid, brain ischemia, matrix proteins
Introduction
From 2000 to 2012, stroke was found to be the second most common cause of death worldwide.1 Tissue plasminogen activator (tPA) remains the only FDA-approved treatment for acute ischemic stroke, yet only a few patients receive such treatment due to its narrow therapeutic window and serious side effects.2 Homeostasis of blood–brain barrier (BBB) is essential to material change between brain and systemic circulation.3 Breakdown of BBB plays a pivotal role in pathophysiological process of ischemia and thrombolytic treatment.4 In general, BBB is part of the Neurovascular Unit (NVU), within which the cell–cell and cell–matrix communications hold the normal structure and function of the brain.5 BBB breakdown causes NVU integrity loss and results in irreversible damage of central nervous system (CNS).6 Despite the importance of BBB, however, our knowledge for dynamic change of BBB permeability, and its regulatory mechanism, during ischemia and reperfusion, remains very limited.7
The matrix loss and breakdown of basement membrane due to matrix metalloproteinases (MMPs) is one of the major reasons that leads to BBB permeability increase and malfunction of NVU.8 MMPs are a family of more than 20 secreted and cell surface zinc- and calcium-dependent endopeptidases that degrade extracellular matrix proteins such as collagen IV, laminin, fibronectin, and proteoglycans.9 They are involved in many pathological conditions in CNS including cerebral ischemia.10 Despite the strong redundancy of activities of different types of MMPs, there is accumulating evidence that MMP-2, MMP-3, and MMP-9 play more important roles in BBB disruption and cerebral edema during ischemic stroke.8,11–14 The activities of MMPs are tightly regulated by their inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). Recently, global responses of different types of MMPs after ischemic stroke have been reported in either brain parenchyma15–17 or blood stream.18 Nevertheless, how the secreted MMPs in extracellular space of ischemic brain changes, where they really exerted their activities, has not yet been reported.
In the current study, we used a method newly developed in our lab to sample cisterna magna cerebrospinal fluid (CSF) from monkey in a serial pattern that allowed us dynamically monitoring the permeability change of BBB in every single animal insulted by ischemia and reperfusion. Moreover, we determined the levels of MMPs and TIMPs in CSF during the acute phase (0–24 h) and subacute phase (one to seven days) following transient middle cerebral artery occlusion (tMCAO) induced by a minimally invasive catheterization method in rhesus monkeys. This longitudinal dataset from each single animal enabled us to investigate possible correlation between levels of secreted MMPs in brain side, other than the blood side, and BBB permeability change during ischemia and reperfusion.
Materials and methods
Experimental animals
Twenty-three adult, male rhesus monkeys, weighing 4–7 kg and aged three to five years, were provided by Sichuan Green-House Biotech Co., Ltd, Sichuan Province, China. They were housed in individual monkey cages under standard laboratory conditions (room temperature: 22 ± 2℃; humidity: 60%–70%; 12-h/12-h light-dark cycle; free access to standard chow and tap water) for two weeks prior to surgery. Animals were euthanized by intravenous administration of 3% pentobarbital sodium veterinary euthanasia solution (1 mL/kg) at eight days after reperfusion. All the experimental procedures were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of People’s Republic of China, as well as AAALAC-related animal ethical criteria. The protocols were approved by the Animal Care Committee of the Sichuan University West China Hospital (Chengdu, China). All the animal experiments were performed in accordance with the Animal Research: Reporting in Vivo Experiments (ARRIVE) guidelines.
tMCAO
The animals were fasted 12 h prior to surgery. Before the endovascular procedure, the animals were intubated with a cuffed endotracheal tube (inner diameter, 3.0 to 3.5 mm). Anesthesia was maintained by constant-rate propofol (AstraZeneca, 10 mg/mL) infusion (0.3–0.4 mg/kg/min) into the forearm vein. The animals were connected to monitoring equipment (BeneView T8, Mindray, China) that measured the electrocardiogram, pulse, heart rate, arterial oxygen pressure, and rectal temperature. A digital subtraction angiography (DSA) system (Allura Xper FD20, Philips, the Netherlands) was utilized to determine the conditions of cerebral perfusion. Following a routine disinfection and draping procedure, a 5F arterial sheath (Terumo Corporation, Japan) would be placed into the right femoral artery using the Seldinger technique as reported.19 Briefly, a 5F guiding catheter (Envoy, Johnson & Johnson, USA) was placed in the initial segment of the right internal carotid artery (ICA) and then the right ICA angiography was performed. Afterwards, a microcatheter (Excelsior® SL-10, Stryker, USA) would be inserted with a micro wire (Synchro® 14, Stryker, USA) into the distal end of the M1 segment of middle cerebral artery.20 The micro wire was then withdrawn and super-selective MCA angiography would be performed. After the microcatheter angiography, a Guglielmi Detachable Coil (GDC, 102 × 4, Stryker, USA) was deployed in M1 segment (but not detached) to induce embolus formation. Five minutes later, the M1 segment would be completely occluded by the micro coil which got confirmed by the ICA angiography. The MCA was occluded for 2 h and then the GDC coil would be removed from the M1 segment to restore MCA blood flow. The physiological parameters (blood pressure, heart rate, body temperature, SpO2, and glucose) in rhesus monkeys subjected to transient ischemic stroke were monitored during the whole procedure (Supplementary Table 1).
Brain infarction size determined by MRI scanning
The infarction of ischemic and reperfusion injury was determined by MRI scanning before MCAO, and 2 h, 24 h, seven days after reperfusion. The animals were anesthetized using 0.1 mg/kg Zoletil 50 (Virba, France) prior to scanning of T1, T2 fluid-attenuated inversion recovery (T2 FLAIR), and diffusion weighted image (DWI) using a 3.0-T scanner (Signa-Excite, GE, USA). The relative infarction volume was measured based on T2 FLAIR images and the data were analyzed using MRIcro software (1.40 build 1, USA). The area of abnormal hyperintensity was traced on each MRI slice. The volume was derived from the area and slice thickness. For each slice, the total brain parenchyma area was also measured, and then the total brain parenchyma volume was calculated. Each slice was measured by two technicians who were blinded to the neurologic assessments.
Neurological behavioral assessment
Neurological behavioral assessment was conducted before surgery and every day throughout the seven days of reperfusion using a neurologic deficits scoring scale for monkeys after embolization as reported.21 Of the 100 points possible, 28 are assigned to consciousness, 22 to the sensory system, and 50 to the motor system. In motor system, 18 are assigned to skeletal muscle coordination. To evaluate the neurofunctional deficit more objectively and accurately, we also used an automated non-human primate (NHP) neurobehavioral monitoring and analyzing tool (Primatescan, CleverSys, USA; Supplementary Figure 2) to capture and analyze the behavioral 24/7 throughout the experiment for each single animal as reported.22 We chose the total walking distance and resting time to indicate general motor activity, the hanging (vertical or cuddled) frequency to indicate upper limb strength, the ratio of moving left to right to indicate the coordination.
A serial cisterna magna CSF sampling method and dynamic assessment of BBB permeability
We used a minimally invasive catheterization method to sample the CSF from cisterna magna. Briefly, it includes the following steps: (1) Lumbar puncture: Monkeys were anesthetized by injecting 0.1 mL/kg Zoletil 50 (Virbac, France) intramuscularly and placed on their left side. One operator bended monkey’s neck and brought its knees toward the chest to approximate the intervertebral space as much as possible. Under aseptic conditions, another operator inserted a needle between lumbar vertebrae L3/4, L4/5, or L5/6 and pushed it until there was a “give”. This indicated the needle passed the ligamentum flavum. The needle was again pushed until a second “give” appeared, which indicated the needle was past the dura mater. The stylet from the spinal needle was then withdrawn and drops of CSF were collected. (Supplementary Figure 5(a) (2) Catheterization: The epidural catheter was inserted through the puncture needle into the subarachnoid space. The catheter tip went through the subarachnoid space and finally floated in the cisterna magna under the guidance of X-ray (Supplementary Figure 5(b)). (3) The embedding of sampling port: A 5-cm long cut was made from the puncture point to the head direction on the back, and then the skin was isolated from subcutaneous tissue to leave enough interspace for placing the port. A sampling port was inserted and connected to the other side of epidural catheter, and followed by the suturing of the incision. Monkeys can be fully recovered the day after surgery. (4) CSF sampling: To sample CSF, the monkey’s body was restrained by the bars of each cage, and its limbs were grasped downward by one experimenter to keep its back bent and fully exposed. Then a syringe would be inserted into the center of sampling port, and then the CSF from cisterna magna could be extracted through the catheter. In the current study, we collected CSF and blood simultaneously 24 h before tMCAO as control, and then at 2 h, 4 h, 6 h, 8 h, 12 h, 18 h, 24 h, two days, three days, four days, five days, six days, and seven days after reperfusion. The CSF and serum albumin were measured by enzyme-linked immunosorbent assay (ELISA) (ab108788, abcam, USA) and colorimetric method, respectively. AR = albumin in CSF (mg/L)/albumin in serum (g/L). (5) The quality assessment of CSF: (a) General observation: colorless, clear, and transparent, without clot; (b) CSF cell count: less than 5 WBC/mm3; (c) Total protein qualification of CSF: Only those CSF with total protein level the 0.21 ± 0.07 mg/L were used for this study.
Quantification of MMPs in CSF and blood
Measurement of MMPs and TIMPs from 9 out of 17 monkeys chosen at random was performed using Luminex system for three panels of human MMPs including MMP-1, -2, -3, -9, -10, -13, TIMP-1 and TIMP-2 (HMMP1MAG-55K/HMMP2MAG-55K/HTMP1MAG-54K, MILLIPLEX, Germany). Lower detection limits were as follows: MMP-1, 27 pg/mL; MMP-2, 68 pg/mL; MMP-3, 146 pg/mL; MMP-9, 14 pg/mL; MMP-10, 27 pg/mL; MMP-13, 58 pg/mL, TIMP-1, 20 pg/mL; TIMP-2, 49 pg/mL. Each sample was run in duplicate and the average value was used as the final number and was plotted against the standard curve generated with human MMP panel standard provided with each kit.
Statistical analysis
All data were expressed as mean ± standard error of mean (SEM). All graphs were generated using GraphPad Prism software (version 6.0, San Diego, CA, USA). The difference among the means of the groups is determined with the one-way ANOVA, followed by the Tukey test. Comparisons between values of each time point were performed with Student’s t-test or Mann–Whitney U test depending on the normality of distribution. The correlations between AR, injury size, and MMPs or TIMPs were analyzed statistically by using Pearson’s correlation. For correlations between injury volume and AR, the AUC or maximum value of AR for each animal was used. For correlations between MMPs and AR, their values of each time point and each animal were used. P values of less than 0.05 were considered significant in all analysis.
Results
An NHP tMCAO model induced by minimally invasive catheterization
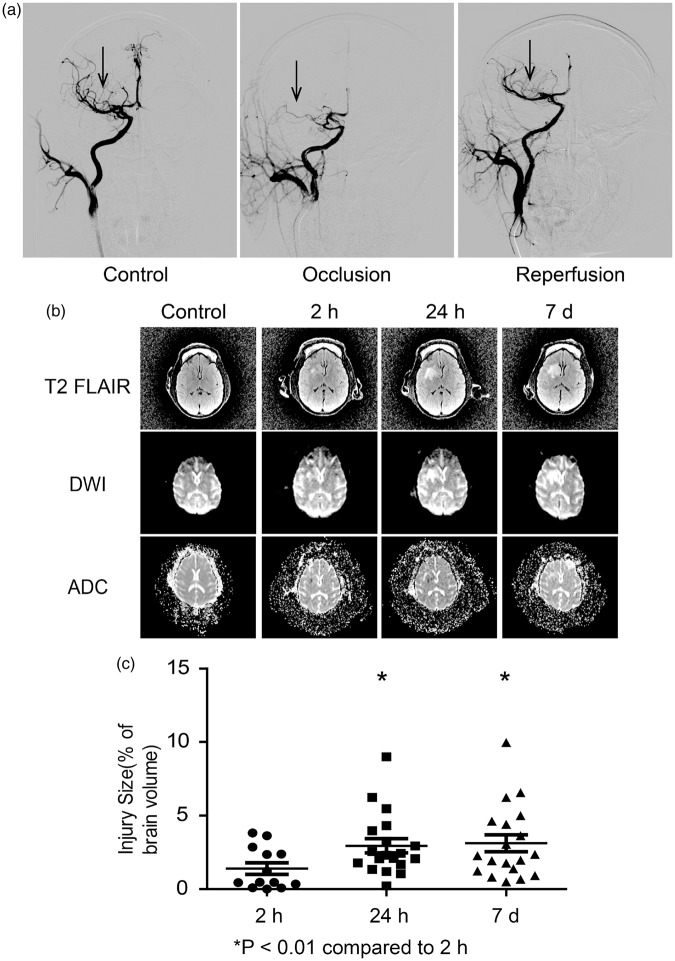
Using a minimally invasive catheterization method as described in the method, we successfully mimicked transient cerebral ischemia and reperfusion injuries in 19 rhesus monkeys (Table 1). The DSA showed the occlusion of right middle cerebral artery for the first 2 h and the reperfusion afterwards (Figure 1(a)). During the 2 h of occlusion, the monkeys were under anesthesia as described in the method. The T2 FLAIR and DWI scanning showed a gradual development of infarction size of total brain volume from 1.40% ± 0.32% at 2 h, to 2.95% ± 0.48% at 24 h, and 3.12% ± 0.57% at seven days after reperfusion (Figure 1(b) and (c)). The edema determined by apparent diffusion coefficient (ADC) map was roughly coincident with the T2 FLAIR and DWI indicated injury area at 2 h and 24 h after reperfusion, and gradually faded away (Figure 1(b)). The average neurological assessment score increased to 23.4 ± 1.5 at 24 h and gradually decreased to 20.3 ± 1.8 at seven days after reperfusion (Supplementary Figure 1(a)). As monitored and calculated by the automotive primate neurobehavioral software PrimateScan, the average walking distance dropped from the normal 486.4 ± 73.5 m/day to 48.7 ± 6.4 m/day at 24 h and increased gradually back to 192.3 ± 41.7 m/day at seven days after reperfusion (Supplementary Figure 1(b)). The resting time increased from 13.0 ± 0.6 h/day of normal to 18.1 ± 0.7 h/day at 24 h and dropped back to 16.0 ± 0.7 h/day at seven days after reperfusion (Supplementary Figure 1(c)). The hanging frequency dropped substantially from 946.9 ± 260.2 times/day to 61.2 ± 34.1 times/day at 24 h and remained relatively low at 123.7 ± 73.9 times/day at seven days after reperfusion (Supplementary Figure 1(d)). Correspondingly, the landing from the top to the ground dropped from 104.2 ± 42.7 times/day to 15.0 ± 9.1 times/day at 24 h and remained low at 17.1 ± 2.6 times/day at seven days after reperfusion (Supplementary Figure 1(e)). The body coordination was determined by calculating the ratio of moving left and moving right within each day after reperfusion. Our data indicated that it dropped from the normal of 0.99 ± 0.02 to 0.94 ± 0.03 at 24 h and 0.87 ± 0.04 at seven days after reperfusion (Supplementary Figure 1(f)).
Table 1.
The basic information of rhesus monkeys subjected to tMCAO.
| Monkey no. | Gender | Age | Weight | MRI | CSF | MMP |
|---|---|---|---|---|---|---|
| 09583 | Male | 4 | 6.4 | * | * | |
| 10399 | Male | 4 | 5.32 | * | * | * |
| 10301 | Male | 4 | 5.51 | * | * | * |
| 10497 | Male | 4 | 6.99 | * | * | * |
| 10089 | Male | 4 | 5.02 | |||
| 11387 | Male | 3 | 4.92 | * | * | |
| 11443 | Male | 3 | 6.28 | * | * | * |
| 09001 | Male | 5 | 6.55 | |||
| 09381 | Male | 5 | 6.98 | * | * | * |
| 09149 | Male | 5 | 6.92 | * | ||
| 10095 | Male | 4 | 5.11 | * | * | * |
| 10489 | Male | 4 | 4.98 | * | * | * |
| 10415 | Male | 4 | 5.28 | * | * | * |
| 10225 | Male | 4 | 5.98 | |||
| 11045 | Male | 4 | 5.49 | * | * | * |
| 11005 | Male | 4 | 4.52 | * | * | |
| 11355 | Male | 4 | 5.51 | |||
| 12163 | Male | 3 | 6.82 | * | * | |
| 11273 | Male | 4 | 6.3 | * | ||
| 11017 | Male | 4 | 5.38 | * | * | |
| 11161 | Male | 4 | 4.4 | * | * | |
| 11265 | Male | 4 | 4.34 | * | * | |
| 12317 | Male | 3 | 5.38 | * | * | |
| Total | 23 | 19 | 17 | 9 |
Notes: Monkey 10089 and 09001 died at 17 h and 30 h after reperfusion respectively, while 09149 and 11355 were dropped because of the failure of model establishment.
Figure 1.
The mild transient ischemia induced by minimally invasive catheterization. (a) Representative DSA images showing normal, occluded, and reperfused cerebral arteries of monkeys. (b) Representative MRI images of T2 FLAIR, DWI, and ADC scanning before occlusion and 2 h, 24 h, and seven days after reperfusion. (c) Quantification of infarction volume of monkey brains based on T2 FLAIR images. Mean ± S.E.M, *p < 0.01 compared to 2 h, n = 19.
The longitudinal assessment of BBB permeability
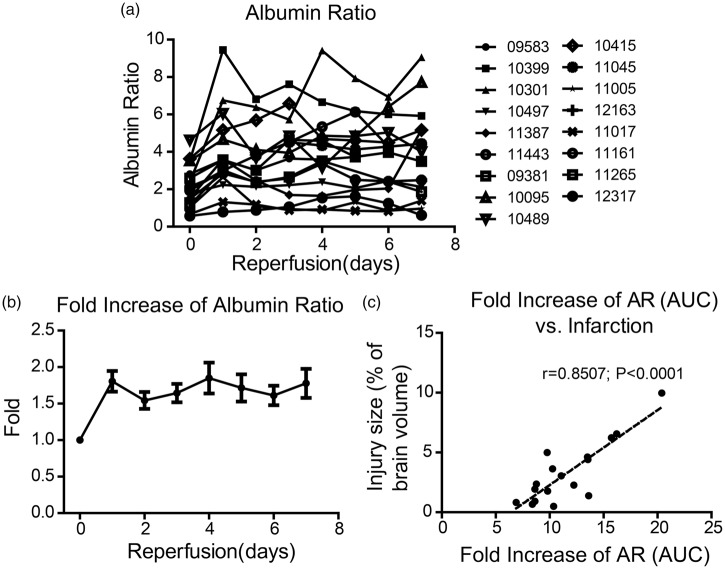
To assess the dynamic change of BBB permeability after ischemia and reperfusion, we sampled cisterna magna CSF and blood before occlusion and then at 2 h, 4 h, 6 h, 8 h, 12 h, 18 h, 24 h, 2 days, 3 days, 4 days, 5 days, 6 days, and 7 days after reperfusion. We successfully collected qualified CSF samples from 17 out of the 19 monkeys that went through the tMCAO surgery. The normal level of AR varies among the animals and the range is 2.16 ± 0.28 (Figure 2(a)). To accurately indicate the permeability change of BBB, we calculated the fold increase of AR, which increased significantly to 1.81 ± 0.14 within the first 24 h and remained relatively high in the next seven days (Figure 2(b)). The pattern of BBB permeability change varies greatly among the animals (Figure 2(a)). The AUC of the fold increase of AR showed a great correlation with the relative infarction volume seven days after reperfusion (r = 0.8507, p < 0.0001; Figure 2(c)). The maximum fold increase of AR also showed a great correlation with injury size at day 7 after reperfusion (r = 0.7560, p = 0.0004; Supplementary Figure 3).
Figure 2.
Temporal change of BBB permeability determined by AR and its correlation to brain injury volume. (a) Temporal change of AR in each monkey within the seven days after ischemia and reperfusion. (b) The fold change of AR within the seven days after ischemia and reperfusion. (c) The correlation between fold increase of AR (the value of area under curve) and brain injury volume at day 7 after reperfusion. (n = 17)
The temporal assessment of MMPs and TIMPs in CSF and blood
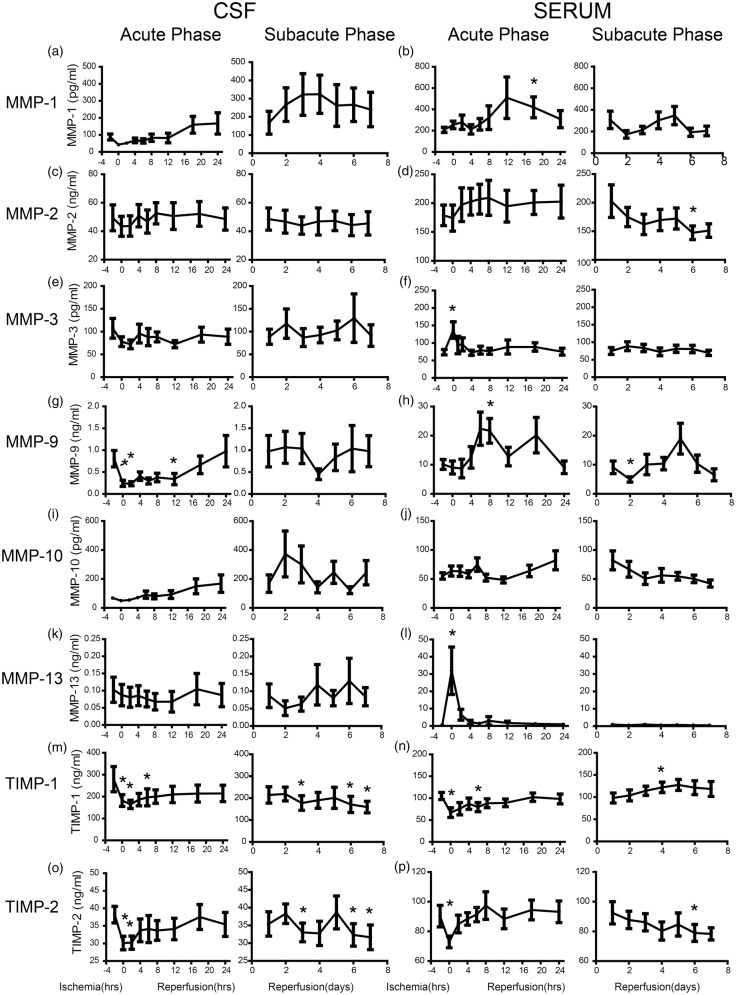
We measured concentrations of MMP-1, MMP-2, MMP-3, MMP-9, MMP-10, MMP-13, and their inhibitors TIMP-1 and TIMP-2 in both CSF and serum. All the MMPs and TIMPs showed very different changing pattern when comparing each of them between CSF and serum. In CSF, the MMP-1 dropped to half of its baseline level (88.0 ± 17.2 pg/mL) and then started increasing around 12 h after reperfusion. It reached a peak of 323.9 ± 105.1 pg/mL four days after reperfusion and remained relatively high (Figure 3(a)). In serum, MMP-1 started increasing at 4 h and reached the peak of 509.4 ± 195.1 pg/mL. It dropped back to its baseline level at two days and increased again and peaked at five days after reperfusion (Figure 3(b)). The MMP-2 in CSF only showed a mild increase and remained relatively steady (Figure 3(c)). On the contrary, the serum MMP-2 level increased rapidly within the first 24 h and dropped gradually to its baseline level (178.9 ± 17.8 ng/mL) during the subacute phase (Figure 3(d)). The MMP-3 in CSF dropped to 71.9 ± 9.6 ng/mL from its baseline level 107.1 ± 21.6 ng/mL during occlusion, and then increased mildly during the next seven days of reperfusion, although the changing pattern varied greatly (Figure 3(e)). In serum, MMP-3 increased transiently during the occlusion phase (p < 0.05 compared with control, similarly hereinafter) and returned back to its baseline level (74.2 ± 8.6 pg/mL) rapidly during the first few hours of reperfusion (Figure 3(f)). The MMP-9 in CSF first dropped significantly during ischemia from 0.8 ± 0.2 ng/mL to 0.2 ± 0.1 ng/mL (p < 0.05), and then started increasing during acute phase. The CSF level of MMP-9 during subacute phase varied greatly (Figure 3(g)). The MMP-9 in blood showed a pattern of double phase increase, once during acute phase (p < 0.05 at 8 h), the other one in subacute phase although not in every animal (Figure 3(h)). The MMP-10 in CSF increased gradually during the first 48 h and then dropped gradually afterwards (Figure 3(i)). In serum, MMP-10 increased gradually during acute phase and dropped even below its baseline level (55.2 ± 5.5 pg/mL) after three days of reperfusion (Figure 3(j)). The MMP-13 in CSF remained relatively steady while a few animals showed a mild decrease within the first three days of reperfusion (Figure 3(k)). In serum, MMP-13 increased dramatically during ischemia from 0.7 ± 0.1 ng/mL to 25.2 ± 11.3 ng/mL, (p < 0.05), and dropped rapidly afterwards (Figure 3(l)). The TIMP-1 dropped rapidly during the acute phase in both CSF (p < 0.05 at 0, 2, and 6 h) and blood (p < 0.05 at 0 and 6 h). It reached back to its baseline level gradually in serum but remained low in CSF (Figure 3(m) and (n)). The TIMP-2 also dropped rapidly during the ischemia phase in both serum and CSF. They gradually reached back to its baseline level and then dropped again during subacute phase (Figure 3(o) and (p)).
Figure 3.
Temporal analysis of MMP-1 (a and b), MMP-2 (c and d), MMP-3 (e and f), MMP-9 (g and h), MMP-10 (i and j), MMP-13 (k and l), TIMP-1 (m and n), and TIMP-2 (o and p) after ischemia and reperfusion in CSF and blood respectively. (n = 9, p < 0.05 compared to control).
Correlations between MMPs, AR, and injury size
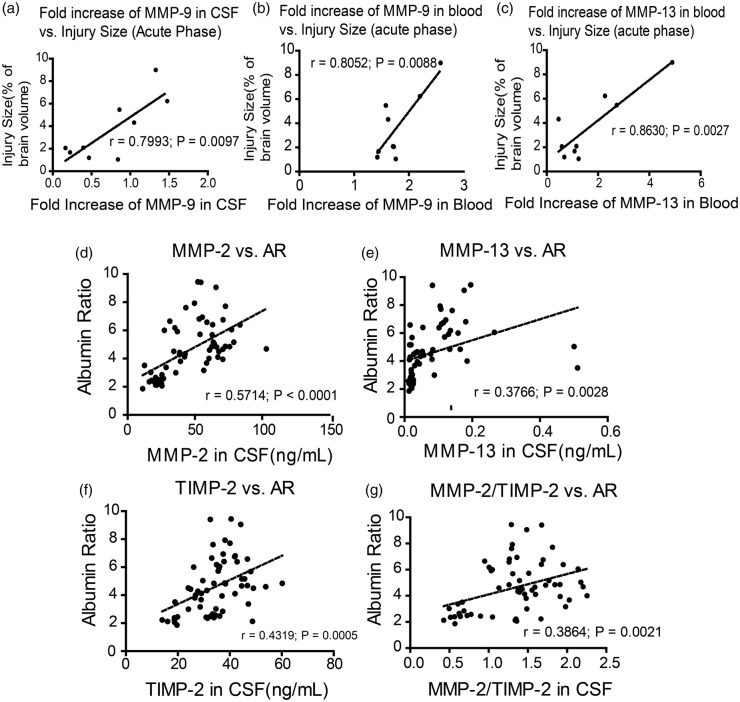
The longitudinal sampling empowered us to investigate correlations between MMP, AR, and infarction. Our data showed the fold increase of MMP-9 during acute phase is correlated with brain injury size at 24 h after reperfusion, in both CSF (r = 0.7993, p = 0.0097; Figure 4(a)) and blood (r = 0.8052, p = 0.0088; Figure 4(b)). The fold increase of MMP-13 in blood during acute phase was correlated with injury size at 24 h of reperfusion (r = 0.8630, p = 0.0027; Figure 4(c)).
Figure 4.
Correlations between MMPs, injury volume and AR. (a) The correlation of fold increase of MMP-9 in CSF and brain injury volume at 24 h after ischemia and reperfusion (b) the correlation of fold increase of MMP-9 in blood and brain injury volume at 24 h after ischemia and reperfusion (c) the correlation of MMP-13 in blood and brain injury volume at 24 h after ischemia and reperfusion. (d) The correlation of MMP-2 level in CSF and AR; (e) the correlation of TIMP-2 level in CSF and AR; (f) the correlation of MMP-13 in CSF and AR; (g) the correlation of MMP-2/TIMP-2 in CSF and AR. (n = 9)
At every sampling point of the subacute phase, we have measured value of AR and MMPs. The Pearson correlation test showed the AR change was positively correlated with MMP-2 (r = 0.5714, p < 0.0001; Figure 4(d) and Table 2(a)), MMP-13 (r = 0.3766, p = 0.0028; Figure 4(e) and Table 2(a)) and TIMP-2 (r = 0.4319, p = 0.0005; Figure 4(f) and Table 2(a)) in CSF. The AR change was also correlated with MMP-1 and MMP13 in blood. The MMP-2/TIMP-2 ratio also showed some correlation with AR (r = 0.3864, p = 0.0021; Figure 4(g)). None of the other MMPs or TIMPs that we measured, neither in CSF nor in blood, showed correlation to AR change (Table 2).
Table 2.
Correlations between MMPs and AR in CSF or blood.
| MMPs | MMP-1 | MMP-2 | MMP-3 | MMP-9 | MMP-10 | MMP-13 | TIMP-1 | TIMP-2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. Correlations between MMPs and AR in CSF (subacute phase). | AR | r | 0.240 | 0.571 | 0.119 | 0.073 | 0.199 | 0.377 | 0.083 | 0.432 | |||||||||
| p | 0.426 | <0.0001 | 0.180 | 0.288 | 0.288 | 0.003 | 0.263 | 0.001 | |||||||||||
| B. Correlations between MMPs and AR in blood (subacute phase) | |||||||||||||||||||
| AR | r | 0.370 | 0.033 | 0.067 | 0.130 | 0.282 | 0.405 | −0.077 | 0.008 | ||||||||||
| p | 0.002 | 0.401 | 0.305 | 0.159 | 0.014 | 0.001 | 0.279 | 0.475 | |||||||||||
Discussion
Developing NHP models with consistent and a reproducible ischemic lesion and syndrome has always been desirable for mechanisms and preclinical studies for ischemic stroke. Various types of NHPs such as marmosets, cynomolgus monkeys, and rhesus monkeys have been reported for modeling ischemic stroke.20,21,23 With technological advancement, early invasive surgical methods such as internal carotid ligation or MCA clipping through transorbital procedures or craniotomy have gradually faded away.24 Early endovascular method using embolus, glue, or inflatable balloon to occlude MCA usually resulted in variation of injury location and volume.25
In the current study, we delivered a microcoil which can accurately induce embolization in monkey MCA using a minimally invasive catheterization method, as previously reported.25 The relative injury size to whole brain volume of the model is very close to the mean injury size of human patients (∼4% of whole brain volume at seven days after treatment).26 Considering that rodent MCAO models usually presenting an injury size 30%–40% to its brain volume, the pathophysiological process in those models probably differ too much from real human conditions. This might explain, to some extent, the low clinical transformation of stroke drug development based on rodent models. The closer kinship of the experimental animal with human being and the likeness of the relative brain injury size might make our model closer to real human pathophysiological conditions and might have a higher clinical translational capacity.
One more advantage of using monkey as the experimental animal is that it provides much more CSF, which is one of the best biological samples to study the chemical and biological extracellular environment of cells in CNS, than rodents. Proteins concentration in CSF reflect the concentration of extracellular protein.27 However, due to technical limitations, rare studies so far have been able to use CSF samples, especially longitudinal samples from a single animal. Rodents, the most commonly used model animals in stroke study, only provide limited CSF sample (e.g. 10–15 µL for mice) after animal sacrifice. The human CSF is approachable, but the ethics and lack of control limited its applicability. In the current study, we used a novel CSF sampling method developed in our lab that empowered us to repeatedly sample CSF from cisterna magna under fully sober condition, and then calculated temporally of the AR that has been widely used as a good indicator of BBB permeability.28,29 Traditional methods for measuring BBB permeability (i.e. Evans blue or immunostaining of extracellular IgG and iron) usually require sacrificing animals that makes it impossible to do longitudinal analysis. In recent years, the molecular imaging such as dynamic contrast-enhanced MRI (DCE-MRI)29,30 enables researchers to observe the change of BBB permeability in a longitudinal way, but the cost and inconvenience using MRI prevented the widely use of this method.
The BBB is not only a barrier between CNS and systematical circulation, but also an important interface for bioactive molecules entering and exiting the brain. Thus, it is very essential to maintain the homeostasis of the CNS.5 Accumulating evidence, including our data presented in this manuscript, showed strong correlation between BBB disruption and the outcome of the ischemic stroke.8,31 Although BBB disruption has been a focus for years in the field of stroke prevention, treatment, and recovery, we still know very little of the dynamic change of BBB and its molecular regulatory mechanism during the process of ischemic stroke and reperfusion.8,32
In the current study, we were able to monitor the dynamic change of BBB permeability in a relatively convenient and inexpensive way in each single animal. However, we have to admit that the serum albumin dropped too much within the first 24 h, which may be due to the loss of blood during the surgery, and there might not be enough time for albumin to rebalance between CSF and blood. Since the cornerstone of AR as an indicator for BBB permeability is the entering of albumin from serum to brain, we chose not use the calculated ARs within the first 24 h as indicators of BBB permeability. Nevertheless, the AR calculated afterwards still provides a reliable indicator for longitudinally tracking of the BBB permeability. As to the method using AR as a surrogate for BBB permeability per se, we acknowledge that serum proteins such as albumin entering CSF move through the epithelial layer of choroidal plexus rather than the endothelial layer of BBB under physiological conditions,33 which makes AR not a perfect proxy for BBB permeability. However, it would be a bit different under pathophysiological conditions such as ischemic stroke. Firstly, strong evidence indicated that BBB does compromise under ischemic/reperfusion conditions, which theoretically would allow serum proteins entering into CSF by crossing BBB.7,34 Secondly, the occlusion of middle cerebral artery usually does not affect too much of the choroidal plexus. Therefore, we believe the fold increase of AR would serve as a good indicator of BBB breakdown here.
The longitudinally sampled CSF also enabled us to measure dynamic changes of almost any molecules in CSF concurrently with measuring AR in every single animal. This dataset enabled us to investigate possible correlation between AR and injury size, with almost any molecule that we can measure in CSF, such as MMPs and their inhibitors in this case. Here, we showed for the first time the temporal changes that all main secreted MMPs concurrently in the excellular space of the brains of stroked animals. There is accumulating evidence indicating MMPs, especially MMP-2, MMP-3, and MMP-9 play essential role in breaking down of BBB in stroke. However, no study yet has measured the change pattern of secreted MMPs in brain side, on which the MMPs truly exert their actions. To note, the substrates of MMPs are all located in the base membrane, such as the gelatins, fibronectin, laminin, and collagen.35
The multiplexing method enabled us to measure all these MMPs and their inhibitors using only 50 µL samples. The MMP-2 and MMP-9 results from the multiplexing method were validated using traditional ELISA method (Supplementary Figure 4). In the present study, we showed the change patterns of all measured MMPs and TIMPs differ greatly between CSF and blood. Although the comparably low levels of MMPs, as well as the small sample size of CSF prevented us to measure the activities of MMPs, it is certainly a drawback of the current study. Nevertheless, we found a strong correlation between the fold increase of MMP-9 (maximum MMP concentration against the baseline level) during the acute phase, in both blood and CSF, and the infarction size at seven days after reperfusion. This result is consistent with previous studies showing blood MMP-9 as a good indicator of stroke outcomes.36 However, out data showed the MMP-9 level in neither CSF nor blood has a correlation with the AR. This might suggest the MMP-9 contributes to the infarction development in a BBB-independent way. Moreover, our data showed that serum levels of MMP-1 and MMP-13 have some good correlations with AR, which is also consistent with the previous study,18 that would make them potential biomarkers for BBB damage. It would be interesting to see if they could be combined as a diagnostic panel to serve for this purpose when more data are gathered in the future. Within all the MMPs and TIMPs we measured, MMP-2, MMP-13, and TIMP-2 showed good correlations with AR to some extent. This suggests that the major players in brain side that regulated the BBB permeability are these three. Our result is contradicted to previous studies using TIMP-2 knockout mice, which showed increased Evans blue leakage.37 However, lots of studies showed double roles of TIMP-2 as a regulator of MMP-2.38–42 Also, considering the complexity of the regulating networks of MMPs, any single knockout animal could have a totally different regulating network and the butterfly effect might cause a totally different outcome.
Based on a study using only nine animals, we cannot draw any absolute conclusion on which MMP is more and which MMP is not important to the stroke outcome and BBB disruption. However, the novel NHP stroke model with a clinically related infarction size, and an easy access to cisterna magna CSF, enabled us to measure BBB permeability dynamically in a cost-effective way, and to further explore possible regulators of BBB permeability from the CNS side in the future.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Program of High Technology Research and Development of China (2012AA020702) and the National Science Foundation of China (81571177).
Acknowledgements
We thank to the Center for Information Medicine, University of Electronic Science, and Technology of China for MRI scanning.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
YZ helped to design the research, coordinated all experiment operations, conducted, and performed all AR and Luminex experiments, and helped to prepare the manuscript; FF, GZ, YZ., HJ conducted and performed all catheterization procedures. LZ conducted and performed statistical analysis. JZ and TZ helped to perform AR and Luminex experiments; DS helped on CSF analysis; CY helped Luminex experiment design; XW, KX, and HL helped on surgery design and animal welfare. ZZ designed the entire study, supervised all segments of the study, and wrote the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data.
References
- 1.The top 10 causes of death, www.who.int/mediacentre/factsheets/fs310/en/ (accessed 3 November 2016).
- 2.Chen HS, Qi SH, Shen JG. One-compound-multi-target: combination prospect of natural compounds with thrombolytic therapy in acute ischemic stroke. Curr Neuropharmacol . Epub ahead of print 19 June 2016. DOI: 10.2174/1570159X14666160620102055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Z, Nelson AR, Betsholtz C, et al. Establishment and dysfunction of the blood-brain barrier. Cell 2015; 163: 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakash R, Carmichael ST. Blood-brain barrier breakdown and neovascularization processes after stroke and traumatic brain injury. Curr Opin Neurol 2015; 28: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Leng Y, Tsai LK, et al. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 2011; 31: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013; 19: 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakhan SE, Kirchgessner A, Tepper D, et al. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol 2013; 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol 2013; 13: 649–665. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 2005; 50: 329–339. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Jin X, Liu KJ, et al. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci 2012; 32: 3044–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Thompson JF, Taheri S, et al. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cereb Blood Flow Metab 2013; 33: 1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amantea D, Certo M, Russo R, et al. Early reperfusion injury is associated to MMP2 and IL-1beta elevation in cortical neurons of rats subjected to middle cerebral artery occlusion. Neuroscience 2014; 277: 755–763. [DOI] [PubMed] [Google Scholar]
- 14.Batra A, Latour LL, Ruetzler CA, et al. Increased plasma and tissue MMP levels are associated with BCSFB and BBB disruption evident on post-contrast FLAIR after experimental stroke. J Cereb Blood Flow Metab 2010; 30: 1188–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenglet S, Montecucco F, Mach F, et al. Analysis of the expression of nine secreted matrix metalloproteinases and their endogenous inhibitors in the brain of mice subjected to ischaemic stroke. Thromb Haemost 2014; 112: 363–378. [DOI] [PubMed] [Google Scholar]
- 16.Cuadrado E, Rosell A, Borrell-Pages M, et al. Matrix metalloproteinase-13 is activated and is found in the nucleus of neural cells after cerebral ischemia. J Cereb Blood Flow Metab 2009; 29: 398–410. [DOI] [PubMed] [Google Scholar]
- 17.Orbe J, Barrenetxe J, Rodriguez JA, et al. Matrix metalloproteinase-10 effectively reduces infarct size in experimental stroke by enhancing fibrinolysis via a thrombin-activatable fibrinolysis inhibitor-mediated mechanism. Circulation 2011; 124: 2909–2919. [DOI] [PubMed] [Google Scholar]
- 18.Rosell A, Alvarez-Sabin J, Arenillas JF, et al. A matrix metalloproteinase protein array reveals a strong relation between MMP-9 and MMP-13 with diffusion-weighted image lesion increase in human stroke. Stroke 2005; 36: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 19.Tsiouris A, Elkinany S, Ziganshin BA, Elefteriades JA. Open Seldinger-Guided Femoral Artery Cannulation Technique for Thoracic Aortic Surgery. Ann Thorac Surg 2016; 101: 2231–2235. [DOI] [PubMed]
- 20.Yang JGJ, Zheng HB, Zhou MK, et al. Establishment of a rhesus monkey model of middle cerebral artery ischemia and reperfusion using a microcatheter embolization method. Neural Regen Res 2010; 5: 5. [Google Scholar]
- 21.Kito G. Experimental thromboembolic stroke in cynomolgus monkey. J Neurosci Meth 2001; 105: 45–53. [DOI] [PubMed]
- 22.Ikeda S, Harada K, Ohwatashi A, et al. A new non-human primate model of photochemically induced cerebral infarction. PloS one 2013; 8: e60037. [DOI] [PMC free article] [PubMed]
- 23.Freret T, Bouet V, Toutain J, et al. Intraluminal thread model of focal stroke in the non-human primate. J Cerebr Blood F Met 2008; 28(4): 786–796. [DOI] [PubMed]
- 24.Cook DJ, Tymianski M. Nonhuman primate models of stroke for translational neuroprotection research. Neurotherapeutics 2012; 9: 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao B, Shang G, Chen J, et al. A more consistent intraluminal rhesus monkey model of ischemic stroke. Neural Regen Res 2014; 9: 2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saunders DE, Clifton AG, Brown MM. Measurement of infarct size using MRI predicts prognosis in middle cerebral artery infarction. Stroke 1995; 26: 2272–2276. [DOI] [PubMed] [Google Scholar]
- 27.de Lange EC. Utility of CSF in translational neuroscience. J Pharmacokinet Pharmacodyn 2013; 40: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott NJ, Friedman A. Overview and introduction: the blood-brain barrier in health and disease. Epilepsia 2012; 53(Suppl 6): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Salayandia VM, Thompson JF, et al. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflamm 2015; 12: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brouns R, Wauters A, De Surgeloose D, et al. Biochemical markers for blood-brain barrier dysfunction in acute ischemic stroke correlate with evolution and outcome. Eur Neurology 2011; 65: 23–31. [DOI] [PubMed] [Google Scholar]
- 32.Moskowitz MA, Grotta JC, Koroshetz WJ, et al. The NINDS Stroke Progress Review Group final analysis and recommendations. Stroke 2013; 44: 2343–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Exp Opin Drug Deliv 2016; 13: 963–975. [DOI] [PubMed] [Google Scholar]
- 34.Pisani V, Stefani A, Pierantozzi M, et al. Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson's disease. J Neuroinflamm 2012; 9: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piccardi B, Palumbo V, Nesi M, et al. Unbalanced metalloproteinase-9 and tissue inhibitors of metalloproteinases ratios predict hemorrhagic transformation of lesion in ischemic stroke patients treated with thrombolysis: results from the MAGIC study. Front Neurol 2015; 6: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos-Fernandez M, Bellolio MF, Stead LG. Matrix metalloproteinase-9 as a marker for acute ischemic stroke: a systematic review. J Cereb Blood Flow Metab 2011; 20: 47–54. [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto M, Takagi Y, Aoki T, et al. Tissue inhibitor of metalloproteinases protect blood-brain barrier disruption in focal cerebral ischemia. J Cereb Blood Flow Metab 2008; 28: 1674–1685. [DOI] [PubMed] [Google Scholar]
- 38.Fan D, Takawale A, Basu R, et al. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc Res 2014; 103: 268–280. [DOI] [PubMed] [Google Scholar]
- 39.Ngu JM, Teng G, Meijndert HC, et al. Human cardiac fibroblast extracellular matrix remodeling: dual effects of tissue inhibitor of metalloproteinase-2. Cardiovasc Pathol 2014; 23: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kandalam V, Basu R, Moore L, et al. Lack of tissue inhibitor of metalloproteinases 2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation 2011; 124: 2094–2105. [DOI] [PubMed] [Google Scholar]
- 41.Lahat N, Bitterman H, Engelmayer-Goren M, et al. Reduced TIMP-2 in hypoxia enhances angiogenesis. Am J Physiol Cell Physiol 2011; 300: C557–C566. [DOI] [PubMed] [Google Scholar]
- 42.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 2015; 44–46: 247–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.