Abstract
Studies treating intracerebral hemorrhage (ICH) with therapeutic hypothermia (TH) have shown inconsistent benefits. We hypothesized that TH’s anti-inflammatory effects may be responsible as inflammatory cells are essential for removing degrading erythrocytes. Here, we subjected rats to a collagenase-induced striatal ICH followed by whole-body TH (∼33℃ for 11–72 h) or normothermia. We used X-ray fluorescence imaging to spatially quantify total and peri-hematoma iron three days post-injury. At three and seven days, we measured non-heme iron levels. Finally, hematoma volume was quantified on one, three, and seven days. In the injured hemisphere, total iron levels were elevated (p < 0.001) with iron increasing in the peri-hematoma region (p = 0.007). Non-heme iron increased from three to seven days (p < 0.001). TH had no effect on any measure of iron (p ≥ 0.479). At one and three days, TH did not affect hematoma volume (p ≥ 0.264); however, at seven days there was a four-fold increase in hematoma volume in 40% of treated animals (p = 0.032). Thus, even when TH does not interfere with initial increases in total and non-heme iron or its containment, TH can cause re-bleeding post-treatment. This serious complication could partly account for the intermittent protection previously observed. This also raises serious concerns for clinical usage of TH for ICH.
Keywords: Bleeding, intracerebral hemorrhage, iron, side effects, therapeutic hypothermia
Introduction
Intracerebral hemorrhage (ICH), caused by a ruptured blood vessel in the brain, has a high mortality rate and often causes lasting impairments.1,2 There are no neuroprotective therapies for ICH with treatment relying on medical management and rehabilitation. Primary damage from an ICH is due to mechanical destruction as the blood tears through tissue creating a mass effect, which can sometimes increase intracranial pressure (ICP). This damage occurs quickly with bleeding ceasing within the first 3 h in most patients.3 Secondary damage occurs over hours and days later. Mechanisms contributing to secondary injury include inflammation, edema, raised ICP, oxidative stress, and hematoma-related factors such as thrombin production and iron release from degrading erythrocytes.4–6 Researchers target these secondary mechanisms because their protracted nature allows for later interventions compared to primary injury. Several drugs targeting iron-induced injury (e.g. iron-chelators) and/or hematoma resolution have been studied pre-clinically, but to date there has been no successful clinical translations.
Therapeutic hypothermia (TH), reducing body and/or brain temperature, typically in the range of 32–35℃, is considered a gold standard neuroprotectant. It is clinically approved to treat cardiac arrest7 and neonatal hypoxic–ischemic encephalopathy.8 The success of TH is likely due to its multifaceted effect on neurodegenerative processes such as inflammation, blood–brain barrier (BBB) damage, oxidative stress, edema, and raised ICP.9 Many of these mechanisms of injury are common to other injuries. As such, TH has been the focus of preclinical and clinical research for traumatic brain and spinal cord injury, as well as ischemic and hemorrhagic stroke, among others.10–12 In fact, there is abundant preclinical evidence supporting the use of TH for treating ischemic stroke. Studies from different labs, using multiple models (including co-morbidities), and species, have found TH to reduce cell death and improve behavioral deficits after experimental ischemic stroke.9 To date, phase II clinical trials suggest that TH is feasible and safe for treating ischemic stroke,13,14 and TH is currently being investigated in phase III clinical trials.15
Despite the overlap of neurodegenerative mechanisms between ischemic and hemorrhagic stroke, the preclinical evidence on using TH to treat ICH is mixed. In ICH, TH does generally mitigate several mechanisms of injury such as inflammation, BBB damage, edema, and raised ICP.16–20 While some TH studies have found neuroprotection and attenuated behavioral impairments after ICH,17,18,21,22 others have failed to find those effects.16,19,23 Such inconsistency is likely due to a number of factors. For instance, some studies have used the collagenase model (collagenase damages blood vessels to cause a bleed), whereas others have infused blood.16,18,19,22,24 Another factor may be treatment timing as beginning TH early can aggravate bleeding, at least in the collagenase model.22,25 Despite using an appropriate treatment delay and the same model, the beneficial effects of TH vary among studies in our lab.22,24 Clinically, small trials have suggested that TH is effective in lowering high levels of edema and mortality,26 as well as lowering inflammation27 and improving cerebral blood flow and recovery.28 The clinical studies conducted so far have been small, used historical controls, and/or participants were typically limited to those with large hemorrhages who are at risk of developing lethal levels of ICP. There may be many complications or side effects associated with using TH to treat ICH that supersede the beneficial effects. Aside from applying TH too early, no TH studies have investigated potential complications specifically associated with treating ICH.
Inflammation is a crucial mechanism for mitigating the toxic components of the hematoma. Microglia and infiltrating macrophages are essential for containing the hematoma and limiting exposure of the peri-hematoma tissue to blood and clotting factors.29,30 As well, these cells are essential in hematoma resolution and storing the iron originating from erythrocytes.31 As TH is a potent anti-inflammatory treatment, we hypothesized that TH impairs endogenous mechanisms for hematoma containment and other aspects related to hematoma resolution. In order to assess this, we completed three experiments using a collagenase model of ICH in rats. First, we evaluated the spread of iron into the surrounding parenchyma, then the rise in non-heme iron from three to seven days post-ICH, and finally the amount of blood left in the brain at one, three, and seven days. We used a collagenase model as this causes more ongoing bleeding than the whole-blood model and is reasonably consistent with greater impairments and a longer period of ongoing cell death.32 The greater bleeding is essential as it can be an important complication of using TH to treat ICH.22,25
Materials and methods
Animals and experimental conditions
All procedures were in accordance with the Canadian Council on Animal Care under the approval of the University of Alberta’s Biosciences Animal Care and Use Committee. One hundred and sixteen male Sprague-Dawley rats from the University of Alberta’s Science Animal Support Services colony (250–300 g ∼ 10 weeks old) were housed in polycarbonate cages with wood chip bedding in a temperature and humidity controlled room on a 12-h light cycle. Animals were given ad lib access to water and food (Rodent Chow, Lab Diet) and were randomized to either normothermia (NORMO) or TH (HYPO) groups by a random number generator. Group sizes were determined based on our previous experiments using the same techniques (e.g. for hemoglobin levels after ICH the average standard deviation (S.D.) was ∼15 after ICH,25 and for non-heme iron the SD was 1.2 on day 3 after ICH38). From that we roughly obtain estimates for effect sizes and variability with the goal of having 80% power to detect those effects. Most assessments were analyzed blindly except when animals were euthanized cold for non-heme iron levels and hematoma volume analysis at days 1 and 3 post-ICH. In those cases, the experimenter easily identified the cold animals by touch. These experiments are in compliance with the ARRIVE guidelines.33 Three experiments were completed for this study. First, we evaluated whether TH affected the spread of iron from the hematoma three days post-stroke (n=8/group) as well as the number of inflammatory cells. Second, we studied whether TH impacted the release of iron from heme by comparing non-heme iron levels at three and seven days post-stroke (n=10/group). In this experiment, animals euthanized on day 7 also underwent behavioral testing at baseline (i.e. prior to any surgery) and on day 7 just prior to euthanasia. Last, we evaluated the effect on TH on hematoma volume at one, three, and seven days post-ICH (n= 10/group). Animals in this experiment that were euthanized on day 7 also underwent behavioral testing at baseline and day 7 just prior to euthanasia.
Temperature probe surgery
Four to five days prior to ICH surgery rats had a core temperature telemetry probe (calibrated within 0.2℃, TAT10TA-F20 or F40 Transoma Medical; St. Paul, MN) implanted in their peritoneal cavity.19 Briefly, rats were anesthetized with isoflurane (4% induction and 1.5–2% maintenance, with 60% N2O and balance O2) and an incision was made into the abdominal cavity and a sterilized probe was inserted. The wound was sutured and Marcaine was used as a local anesthetic (∼0.1 mL infiltrating the wound area). Rats were placed in an individual clean cage and monitored after they awoke from anesthesia.
ICH surgery
The collagenase-induced ICH model is commonly used in rodents.32,34 A rectal probe measured temperature during surgery and normothermia was maintained via a water blanket. Rats were anesthetized with isoflurane and a midline incision was made along the scalp. Using a stereotaxic frame, the skull was balanced and a hole, 0.5 mm anterior and 3.5 mm lateral to Bregma, was drilled. A 26-gauge Hamilton syringe was lowered 6.5 mm from the surface of the skull and 0.14 U of type IV-S collagenase (in 0.7 µL of sterile saline) was infused over 5 min. The needle was left in place for an additional 5 min to prevent backflow and then was slowly withdrawn. A metal screw was used to plug the drilled hole. The scalp was sutured and Marcaine was applied (∼0.1 mL infiltrating the wound area). Rats were placed in a clean cage and their temperature was monitored while recovering from anesthesia. Rats were monitored frequently to ensure that they were eating and drinking after surgery and otherwise behaving as expected (at least five observations per day until euthanasia). Body weight was taken daily. Additional food was provided for up to four days after ICH in an effort to minimize weight loss (e.g. mixture of peanut butter with sunflower seeds and moistened rodent chow).
Temperature monitoring and control
All animals were confirmed to have normal baseline temperatures (average of ∼37.5℃). The rats’ cages sat on telemetry receivers (RPC-1, Transoma Medical) and body temperature was sampled every 30 s via A.R.T. 2.3 telemetry software (Transoma Medical). After ICH surgery, NORMO rats were only monitored, while HYPO rats’ temperature was regulated as follows. Twelve hours post-collagenase injection, TH (33.0℃ ± 0.5) was initiated over an hour and maintained for 72 h or until euthanasia. The delay was to ensure that TH did not worsen bleeding, which was found with earlier cooling in this model.22 HYPO rats that were euthanized on day 7 in the second and third experiments had 6 h of rewarming (0.5℃/h). A servo-regulated system consisting of a fan, water mister, and heat lamp was used to induce and maintain hypothermia in awake and freely moving animals as has been routinely used in our lab.22,35,36
Histology
Seventy-two hours after ICH, the rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and trans-cardially perfused with 0.9% saline followed by 10% formalin. The brains were removed and later cryostat sectioned (50 µm) using a Teflon-coated blade to prevent metal contamination. The series of sections with the greatest hematoma area were used for rapid-scanning X-ray fluorescence imaging (XFI), cresyl violet, and Perls’ Prussian Blue staining. The cresyl violet sections were used to define the hematoma border; however, due to edema and blood, we did not attempt to determine lesion volume assessment at day 3. The Perls’ stain was used to quantify the number of macrophages/microglia in the peri-hematoma and surrounding tissue based on iron labeling and morphology as has been previously done.19,37 Cells were counted in one section per animal at the level of maximal injury, as has been previously done and found to detect a significant effect of hypothermia.19
X-ray fluorescence imaging
This imaging technique has been previously used to quantify and localize elements, with iron of most interest, in a coronal section of tissue after ICH.38 This technique is highly sensitive and the same section of tissue can be used for other histological procedures following imaging (i.e. cresyl violet).38–40 Briefly, sections used for XFI were placed on plastic metal-free cover slips (Thermanox; Rochester, NY) and imaged at the Stanford Synchrotron Radiation Light Source on beamline 10–2 at a 50 -µm resolution and a 50-ms dwell time. The incident energy of the X-ray beam was 13 keV and the slides were mounted 45° to the incident X-ray beam and 45° to the detector. Signal strengths of calibrations standards (Micromatter Technologies Inc.; Surrey, BC) were compared to the sample signals and used to quantify iron concentrations. Images were analyzed with Sam’s Microanalysis Toolkit.41
Non-heme iron assay
The non-heme iron assay was used to determine whether TH influenced the rate of iron release from heme at survival times of three and seven days after ICH. Rats were deeply anesthetized with 4% isoflurane, decapitated, and a 6 mm thick section of both forebrains were taken (2 mm anterior and 4 mm posterior to the collagenase injection site). The cerebellum served as control. Each forebrain and the cerebellum were homogenized with dH2O and iron was released from proteins, except heme, by mixing the sample with a solution of 1 N HCl and 10% trichloroacetic acid in dH2O and heating to 95℃. The samples were centrifuged and the supernatant was collected and reacted with a ferrozine chromagen solution and compared to a standard curve to determine iron concentration.38,42
Behavioral testing
In the second experiment, the corner turn test (CTT) and neurological deficit scale (NDS) were used to evaluate deficits in rats surviving to seven days post-ICH. In the third experiment, the NDS was used on day 7. In both experiments, baseline behavioral testing occurred prior to temperature probe implantation. Both behavioral tests are sensitive to striatal injury.43,44 However, the CTT was not used in experiment three, as the test is tedious and did not add any information that the NDS did not already provide. The CTT has two walls (41 cm height by 30.5 cm width) placed at a 30° angle with a 0.5 cm gap. At baseline, the direction a rat turns coming out of the corner was averaged over two days (10 trials/day), and rats that had a turning bias (<30% in one direction) at baseline were excluded from analysis for this test. At testing, rat’s turning preference was determined by taking an average of 10 trials. The NDS is a combination of several simple motor tasks (hind-limb retraction, contralateral forelimb flexion, bilateral forepaw grasp, beam walking, and spontaneous circling) and is the most commonly used test in experimental ICH research.45 Each task ranges from 0 to 3 in score, except the contralateral forelimb flexion that ranges from 0 to 2. A score of fourteen denotes maximum impairment.
Hematoma volume
Blood volume was measured using a spectrophotometric hemoglobin assay and compared to a standard curve of known blood volumes.25,46,47 Rats were euthanized one, three, or seven days post-ICH via deep isoflurane anesthesia and decapitation. The hemispheres were separated and homogenized in double distilled water (1:4 w:v ratio), incubated on ice, and then centrifuged at 15,800 g for 35 min. Aliquots of the supernatant were reacted with Drabkin’s solution and the absorption values were obtained (measured at 540 nm; Model 4001/4; Thermo Fisher Scientific) and compared to the standard curve. Hematoma volume was calculated as injured forebrain (IPSI) – contralateral forebrain (CONTRA) blood volume. The latter was used as an estimate of the amount of blood present within the vasculature.
Statistical analysis
All data are expressed at mean ± S.D. except the NDS scores, which are presented as raw scores and medians ± interquartile range (I.Q.R.). All data were analyzed using analysis of variance (ANOVA) or independent t-tests (SPSS v.21; SPSS, Inc.) except the NDS scores, which were analyzed via the Mann–Whitney U and Wilcoxon tests. Also, we correlated the XFI analysis of distance and iron levels, and also the NDS and hematoma values, providing the Pearson r and p values. A Fischer’s exact test was used for the bleeding data of experiment three. The Levene’s test was used to test for homogeneity of variance and when there was a significant effect we used t-tests that did not assume equal variances. When a significant effect was detected, a post hoc effect size (Cohen’s d) was calculated (G × Power v3.1.3; Univerität Kiel, Germany). Significance was at p < 0.05.
Results
Experiment one: Spread of iron and number of Perls’ positive cells
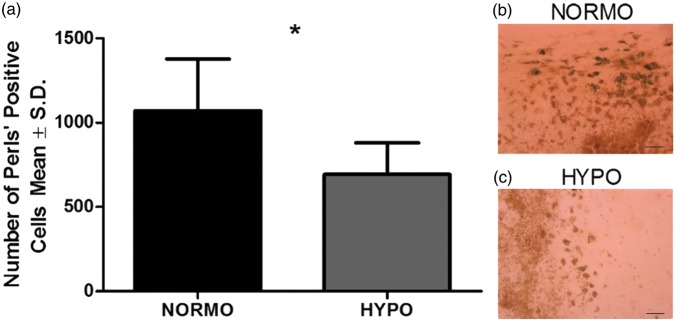
There were no exclusions or mortality in this experiment. We found TH significantly reduced the number of Perls’ positive cells in the HYPO group (p = 0.012, equal variances not assumed; Figure 1(a) to (c)). The size of the effect was large (d = 1.5).
Figure 1.
(a) TH significantly reduced the number of Perls’ positive cells by 35% (p = 0.012). (b) and (c) are representative images of tissue stained with Perls’ Prussian blue for NORMO and HYPO, respectively. Sections from the maximum hematoma were used. N = 8 animals/group; scale bar = 50 µm. *p < 0.05.
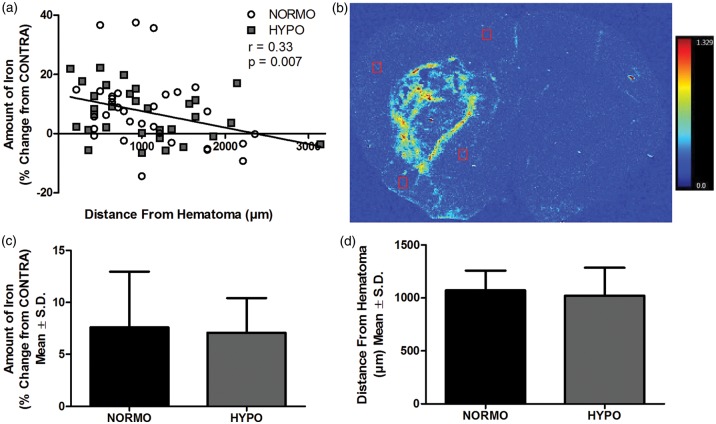
Overall, using XFI, we found a moderate but significant relationship with iron levels declining with distance from hematoma (r = 0.333, p = 0.007, Figure 2(a) and (b)). There was no significant difference between NORMO and HYPO in the average amount of iron (p = 0.825, Figure 2(c)) or distance from hematoma our samples were taken from (p = 0.674, Figure 2(d)). There was a significant increase in total iron levels in the injured hemisphere (p < 0.001 vs. CONTRA hemisphere) but no effect of group (p = 0.567) and no interaction (p = 0.419). Thus, while TH caused a significant reduction in inflammatory cells, this did not affect the spread of iron or the total amount of iron in the injured hemisphere.
Figure 2.
(a) There was a significant relationship between the amount of iron and distance from the border of the hematoma (r = 0.33 p = 0.007). (b) A representative XFI map showing the amount and location of total iron. The red boxes illustrate representative placements of the regions of interest (four regions/animal). The intensity scale bar ranges from 0.0 to 1.329 µg/cm2 of iron. (c) The average amount of iron and (d) the average distance of the regions of interest were not different between NORMO and HYPO (p ≥ 0.674). N = 8 animals/group.
Experiment two: Non-heme iron levels and behavioural impairments
In this experiment, there were two exclusions and two mortalities, with the latter cases also being excluded from our analysis. One animal was excluded from NORMO-Day 3 for a faulty probe and one from HYPO-Day 7 for being unable to self-regulate body temperature after rewarming. One animal spontaneously died during TH from HYPO-Day 7 and one animal from NORMO-Day 7 died during ICH surgery. The baseline temperatures in all groups were normal (data not shown) and Figure 3(a) and (b) shows the temperature data post-ICH in a subset of animals (n = 9 for each NORMO group and n = 6 for each HYPO group). These data are representative of the other experiments in this study.
Figure 3.
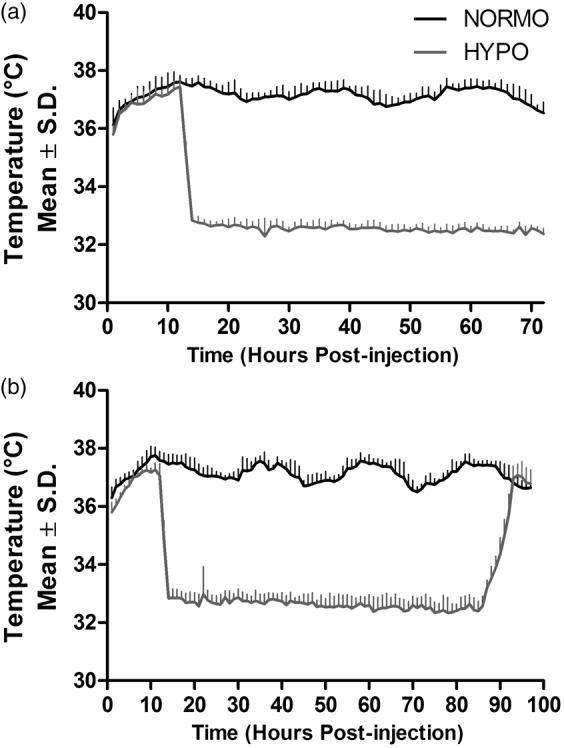
Core temperature data, measured by telemetry, of a subset of animals from experiment two, which is representative of the other experiments. The temperature control system used in all experiments significantly lowered whole-body temperature beginning 12 h after ICH and lasting 24 or 72 h in treated animals. (a) Temperature data for animals surviving to day 3. TH-treated animals were euthanized cold. (b) Four days of temperature data for animals surviving to day 7. After 72 h of TH, animals underwent 6 h of rewarming (0.5℃/h), followed by normothermia until euthanasia. N = 9 in NORMO-Day 3, N = 6 in HYPO-Day 3, N = 9 in NORMO-Day 7, and N = 6 in HYPO-Day 7.
Behavioral impairments were observed on day 7 post-stroke. For the CTT (Figure 4(a)), there was a significant time effect (p < 0.001) but no group effect (p = 0.747) or interaction (p = 0.937). As well, the NDS (Figure 4(b)) showed a significant impairment on day 7 (p < 0.001 vs. baseline) but no significant group effect at either time (p ≥ 0.316).
Figure 4.
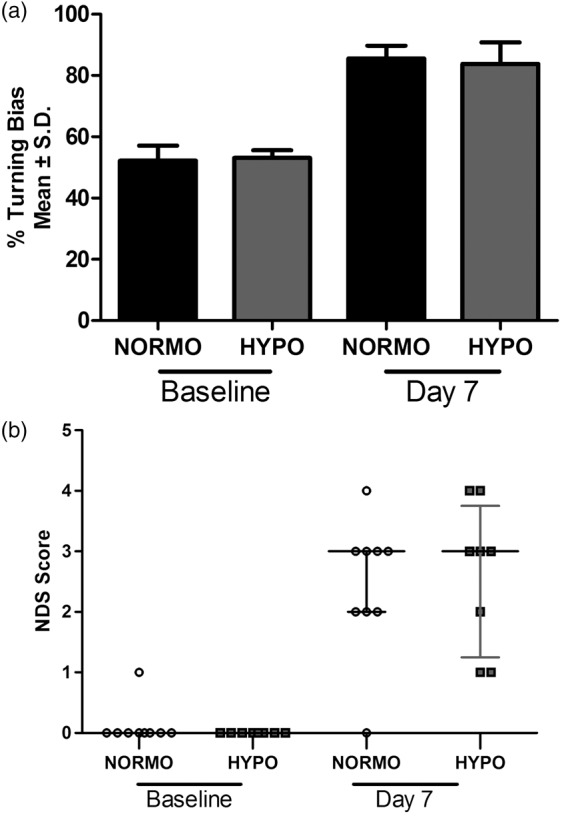
Seven days after ICH, there were significant behavioral impairments on both the (a) CTT and (b) NDS (vs. baseline, p < 0.001), but there was no effect of TH on either test (p ≥ 0.316). N = 9 in NORMO and N = 8 in HYPO. Raw NDS scores are plotted along with a horizontal bar representing the median score for each group with I.Q.R. bars extending vertically.
There was an increase in non-heme iron levels in the IPSI hemisphere between day 3 and day 7 (Figure 5) as there was a significant time effect (p < 0.001) but no group (p = 0.479) or interaction (p = 0.662) effects. The size effect of this increase was large (d = 2.3). As expected, in the CONTRA hemisphere, there were no effects of time (p = 0.071), group (p = 0.882) or interaction (p = 0.413). Likewise, there were no time (p = 0.789), group (p = 0.420), or interaction (p = 0.990) effects for cerebellum non-heme iron levels. TH had no effect on the increase of non-heme levels that occurred between three and seven days post-stroke or the behavioral impairments caused by ICH.
Figure 5.
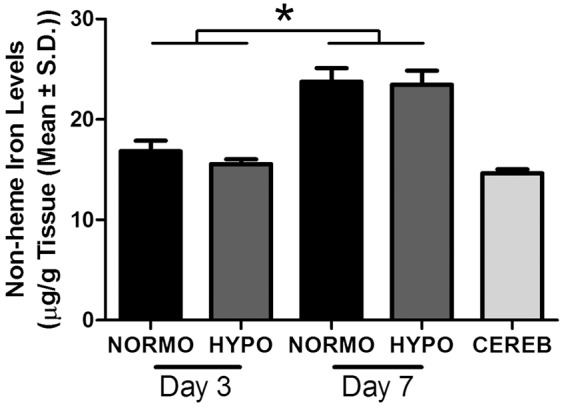
There was a significant increase in non-heme iron levels in the injured forebrain from day 3 to day 7 post-ICH (p < 0.001) but no effect of TH (p = 0.479). Non-heme iron levels at day 3 were comparable to control structure levels (i.e. CEREB: cerebellum of all animals). N = 10/group. *p < 0.05.
Experiment three: Hematoma volume and behavioural impairments
One animal was excluded from HYPO-Day 3 due to experimenter error and one animal from NORMO-Day 7 died during ICH surgery. The data from these animals were not included in our analyses. There were significant impairments detected with NDS at 7 days post-stroke (p < 0.001 vs. baseline; Figure 6) and no difference between groups at baseline (p = 0.424). However, TH caused greater impairments (p = 0.011) and the size of the effect was moderate (d = 0.7). As there was a slightly different pattern of baseline NDS scores between groups, we also analyzed the difference score (day 7 – baseline), which also showed that TH worsened impairment (p = 0.013).
Figure 6.
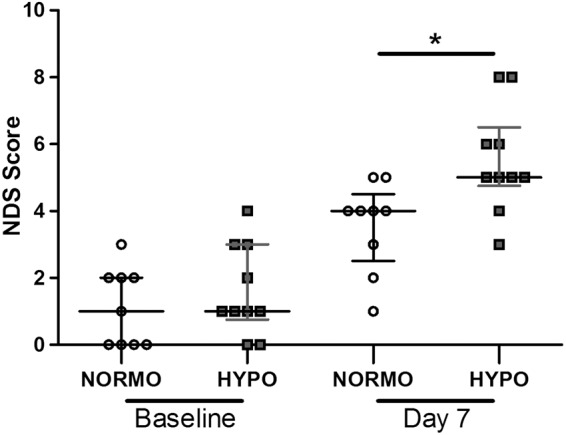
There was a significant behavioral deficit as shown by NDS on day 7 post-ICH (vs. baseline, p < 0.001) with a slight worsening of behavioral impairments in the HYPO group (p = 0.011). Raw scores are plotted along with a horizontal bar representing the median score for each group. *p < 0.05.
There was a significant difference in hematoma volume (Figure 7(a)) as there were significant time (p = 0.004), group (p = 0.014), and interaction (p = 0.046) effects. There was no difference between HYPO and NORMO at day 1 or 3 (p ≥ 0.264), but there was significantly more blood in the HYPO-Day 7 group compared to the NORMO-Day 7 group (p = 0.032, equal variances not assumed). There was a bimodal distribution of hematoma volume in the HYPO group euthanized on day 7 with 40% of the animals having a more than quadrupling in hematoma volume. This difference between NORMO and HYPO on day 7 was large (d = 1.1). The proportion of animals in this group that landed outside a cut off (i.e., three standard deviations of all other groups) was different than all other groups (p = 0.002, Fischer exact test). In fact, only one animal from all the other groups (n = 48) barely fell out of this range, while 4 of 10 from HYPO-Day 7 did. There were no time (p = 0.380), group (p = 0.589), or interaction (p = 0.677) effects for the CONTRA hemisphere blood volumes (p ≥ 0.380). Thus, while TH did not influence bleeding during treatment, there was considerable re-bleeding in a subset of animals post-treatment. Visual inspection of the injured hemispheres of these animals with the larger bleeds showed an obviously greater amount of blood that was fresher in nature (i.e. bright red vs. rust colored of an older bleed). Experimenters were blinded to treatment of all animals euthanized on day 7.
Figure 7.
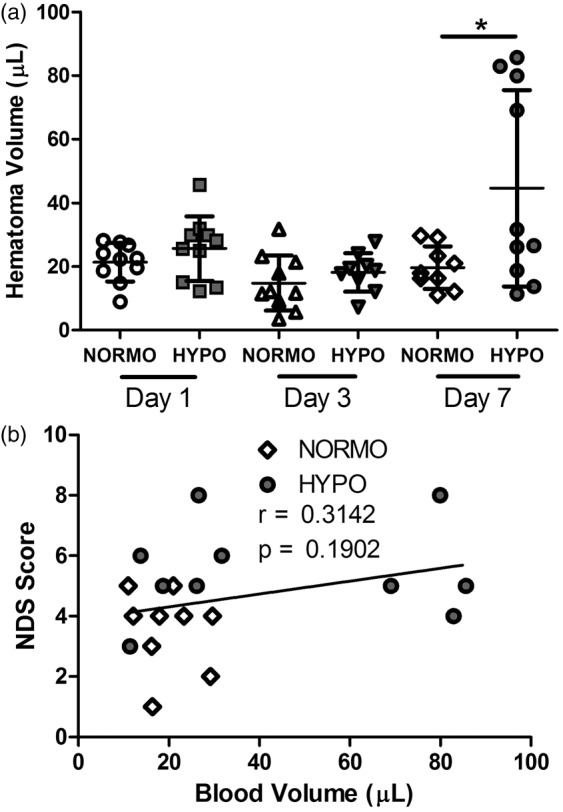
(a) There was no difference between NORMO and HYPO on days 1 and 3 post-ICH (p ≥ 0.264) but by day 7, 40% of treated animals had a significant increase in hematoma volume (p = 0.032). Data are expressed as injured (IPSI) hemisphere – uninjured (CONTRA) hemisphere blood volume and the raw score for each animal is plotted. The horizontal bar represents the average per group with S.D. bars extending vertically. *p < 0.05. (b) NDS scores did not significantly predict hematoma volume.
Although NDS scores were significantly worse in the HYPO group on day 7 post-ICH (vs. NORMO), the NDS scores did not significantly (r = 0.314, p = 0.190) predict hematoma volume (Figure 7(b)).
Discussion
Although our data do not confirm our hypothesis that the anti-inflammatory effects of TH impacts hematoma containment and components of hematoma resolution, a different complication of TH treatment was discovered. Post-treatment, 40% of HYPO animals had a large increase in hematoma volume, while during treatment there were no group differences. Whether this bleeding occurred during rewarming or over subsequent days is unclear. This treated group also had modestly worsened behavioral impairments. Our data confirm other work showing that TH is a potent anti-inflammatory,17,19,48 as we saw a 35% decrease in Perls’ positive cells (i.e., microglia/macrophages). We also found a significant relationship between distance and iron levels, with higher iron levels closer to the border of the hematoma. There was no effect of TH. On day 3, there was no difference in non-heme iron levels between the injured hemisphere and the control structure (i.e. cerebellum) suggesting that the majority of iron is still contained in heme at that time. However, from three to seven days post-ICH, we found an increase in non-heme iron levels, which TH did not influence. This increase in non-heme iron matches previous work.38 There was no effect of TH on behavioral deficits in this experiment. Overall, these results suggest that while TH did not influence endogenous mechanism of hematoma containment and clearance, there was occasionally a post-treatment worsening of bleeding. That latter effect should be considered in future animal research and is a significant concern for clinical use of TH for ICH.
Although not part of our initial hypothesis, the most interesting and concerning result of this study is the influence of TH on bleeding post-treatment, since the hematoma volume is a critical predictor of outcome and survival.49,50 Previous studies show that TH applied too early can aggravate bleeding,22,25 which we successfully avoided by delaying treatment, as we have previously done.24 As there were no group differences in hematoma volume at 1 and 3 days post-ICH, the increase seen on day 7 is likely due to re-bleeding either during rewarming or over the following days. There are a number of reasons to support that this effect was not due to chance or experimenter error. First, all the animals with a large bleed occurred in one group and all of these animals are outliers beyond the combined three standard deviations of the other groups. As well, animals were randomized to groups and the experiment was performed in eight cycles over several months with three of these cycles having animals with large bleeds. Two experimenters conducted the hemoglobin assay throughout the study and both had instances of large bleeds. Finally, control samples from each animal (i.e., contralateral hemisphere and cerebellum – data not shown) were normal in all animals. All of this suggests that the increase in hematoma volume is a genuine effect of TH. Of course, future studies will need to replicate these findings and to precisely determine the incidence rate, currently at 40%, which will require large group sizes to ensure adequate statistical power. As well, it is important to identify factors that influence the incidence and magnitude of re-bleeding complications, such as, potentially, the initial insult severity and various treatment parameters (e.g., depth and duration of cooling).
Importantly, the intermittent occurrence of re-bleeding likely contributes to the lack of a consistent neuroprotective effect in animal studies. Although not all treated animals have this increase in hematoma volume, when it occurs the effect can be substantial. In a group of 8–10 animals, the occurrence of one or two aggravated bleeding events of even half the current magnitude could easily counteract the ability to statistically detect a neuroprotective effect of cooling. Many of the studies looking at neuroprotection have histological and behavioral endpoints weeks after the initial bleed. At these times, the hematoma has resolved and it would be impossible to determine that bleeding was worsened. Even when behavioral outcome is measured at a week, as in this study, there is only a small and inconsistent worsening of impairments that can be easily missed. Although behavioral tests, such as the NDS, are commonly used in ICH studies,45 they often do not reliably detect even substantial differences in lesion size.43,44 Likewise, in this study, the NDS scores did not significantly predict hematoma size. Therefore, the impact of re-bleeding can be overlooked both histologically and functionally in rodent studies when one does not directly measure hemoglobin levels. Even the non-heme iron assay may not pick up delayed bleeding if it occurs before hemoglobin has broken down and liberated its iron, which likely explains why we did not find an increase in non-heme iron levels at day 7 in experiment two. It is also possible that re-bleeding did not occur in that study, as our last study suggests that it is an intermittent problem, and thus by chance fewer or no complications may have occurred in the non-heme experiment.
Clinical trials evaluating the use of TH for ICH have suggested benefit, which is likely due to a decrease in life threatening levels of edema where ICP is substantially increased. Only one trial evaluated hematoma volume post-treatment of TH (without hematoma evacuation) and found no increase, although this was assessed early after rewarming.26 An increase in bleeding post-treatment may be missed if quantifying hematoma volume too early, as we did not notice an effect when cooling was being maintained. There are also protocol differences between this trial and the current study, where cooling was milder (∼35℃ vs. 33℃) and rewarming was slower (0.5℃/24 h vs. 0.5℃/h) in the trial. As well, there may be physiological differences between humans and rats during and after TH (e.g., changes in blood pressure). However, the impact of TH on ICP after large ICH seen in this trial has also been seen in rats.20 While a slow increase in hematoma volume may not cause mortality, as is the case in this experiment, the increase may negate the long-term impact of TH on outcome in affected patients. As well, in patients with small to moderate sized hematomas where edema is not typically life threatening, the complications of TH may counteract any beneficial effects.
There are a number of potential causes for this unexpected increase in hematoma volume such as changes in blood pressure, cerebral blood flow, or stress response, which we did not presently measure. Such events might be especially likely to cause bleeding because of significant BBB damage that continues to worsen over days after collagenase infusion.32 In that study, we used magnetic resonance imaging to quantify gadolinium extravasation in a comparable collagenase model. It is not clear from our present data whether late re-bleeding started during rewarming or after normothermia was reached. Unfortunately, timing will have to be determined through the use of additional survival times because repeated imaging in animals would be technically difficult in rats subjected to prolonged cooling (e.g., ensuring a continuous cooling protocol) and contraindicated in some cases (e.g., if animals have implanted electronics to sense temperature). Future work will investigate the mechanism(s) of this hematoma increase, but the intermittent nature of this effect, for whatever reasons, increases the difficulty of this work and will require large sample sizes. However, as larger scale clinical study is currently underway, this complication is important to understand, even if it is an infrequent event.
The inflammatory response after an ICH is complex as this response can be both harmful and beneficial to brain tissue. We had hypothesized that the anti-inflammatory effect of TH may decrease the harmful aspects of inflammation while also impeding beneficial functions. There are several mechanisms through which the inflammatory response causes damage. Inflammatory induced cytotoxic edema and cell death occurs through exposure to cytokines and oxidative stress.51 Vasogenic edema is caused by disrupting the BBB through increased secretions of matrix metalloproteinases.51 Treatment with minocycline, a common anti-inflammatory, has shown that it lowers edema and reduces injury after ICH.52,53 Experimentally depleting neutrophils also inhibits microglia and macrophage proliferation and reduces BBB disruption, brain damage, and impairment.54,55 In contrast, others have demonstrated the importance of inflammation especially after bleeding. For example, Zhao et al.30 suggest that the transcription factor peroxisome proliferator-activated receptor gamma (PPAR-γ) is beneficial by increasing phagocytosis in microglia and macrophages, which is essential in hematoma resolution. These studies highlight the complexity of post-ICH inflammation.
The influence of TH on inflammation is known and contributes to one of the main adverse events associated with TH, pneumonia.56 Use of TH lowers pro-inflammatory cytokines after ICH in humans and animals,18,27,57 while anti-inflammatory cytokines are increased18 and inflammatory cell proliferation is decreased.17,19 The role of TH on phagocytosis is not well studied after ICH. In cell culture, after rewarming TH decreases microglia proliferation but increases microglia activity (i.e., ATP consumption) and phagocytosis behavior.58 In cerebral ischemia, Kawabori et al.59 found that TH increased the proportion of cells expressing of triggering receptor expressed on myeloid cells-2 (TREM-2), a receptor important for microglia phagocytosis. Thus, TH may not impact the inflammatory response’s abilities to contain iron or resolve the hematoma because phagocytosis is being upregulated. However, further studies on ICH are needed to test this hypothesis.
With XFI we were able to both spatially map and quantify total iron levels, which is superior to biochemical assays. Unfortunately, XFI does not distinguish the form of iron or whether it is bound to protein (e.g., heme and ferritin) or contained within a cell (e.g., microglia). The quantity of iron observed outside the hematoma was significantly above contralateral levels. Whether that peri-hematoma iron originated from hemoglobin breakdown or from microglia containing iron infiltrating or leaving the injured site is unclear. We did not find an effect of TH, but we may have missed effects in the tissue immediately bordering the hematoma. It is not easy to precisely determine that border (e.g., confounds of peri-hematoma edema). Thus, regions of interest were randomly sampled in the injured hemisphere at varying distances from the hematoma, but none were directly adjacent to the border. Thus, a border was determined as best as technically possible and regions were sampled near the border. For future studies, we recommend Raman imaging of hemoglobin in conjunction with XFI as a way of better defining that immediate border zone.4
There appears to be a discrepancy between the increase in non-heme iron from three to seven days and the lack of a decrease in hematoma volume (i.e., hemoglobin) in the normothermic groups for the same times. Both assays indirectly evaluate hematoma resolution and measure two distinct components of the hematoma, a metal (i.e., iron not contained by heme) and a protein (i.e., hemoglobin). There are a couple of explanations for this discrepancy. First, since this experiment was conducted, new data from our lab (Williamson et al. unpublished data) show that this widely used hemoglobin assay is not specific to hemoglobin. While the majority of the absorbance is for hemoglobin, this assay also detects two hemoglobin breakdown products, hemin and to a lesser extent bilirubin. The added absorbance of hemin and bilirubin detected by this assay will partially mask the reduction in hemoglobin levels over time. Also, as edema resolves from day 3 to 7, this decrease in water content make it appear that there is an increase in tissue concentration of non-heme iron.38 Given that there was a substantial non-heme iron increase, edema resolution is not likely the only factor as there is likely some release of iron from hemoglobin. As well, the increase in hematoma volume in the four treated rats who were rewarmed cannot be explained by heme and bilirubin contributing to the absorbance reading of the hemoglobin assay as these breakdown products have less absorbance than hemoglobin.
There was a significant worsening of behavioral impairments by TH in experiment three but not in experiment two. The worsening of behavioral impairments in the third experiment was significant but small, and it may not have much biological significance. More sensitive tests are needed to determine this. It is not clear why there was no worsening of deficits in HYPO rats in experiment two, but it might be due to test insensitivity or fewer animals experiencing re-bleeding. There is also a slight difference in baseline scores between experiments two and three, which is either due to chance, or the subjective nature of NDS as different experimenters conducted the behavioral testing for these two experiments. Fortunately, animals were randomized to treatment condition in all cases, experimenters were blinded, and there was still a significant impairment after ICH in both experiments. Although possible, it is not likely that the collagenase insults varied all that much among studies as this surgical procedure is highly standardized and we used the same batch of collagenase for all of the work.
In summary, the increase in hematoma volume after TH is a pressing concern for its clinical use in ICH and may help explain the lack of consistent neuroprotection in animal studies. Future studies must evaluate this re-bleeding problem and investigate possible mechanisms while varying treatment and model parameters. Contrary to our hypothesis, the decrease in inflammatory cells caused by TH does not appear to impair the brain’s ability to contain iron or influence the hematoma-related factors. This may be due to increased phagocytosis behavior caused by TH but further investigation is needed to study this.
Acknowledgements
The authors thank Sarah Jones, Andrew Chan, and Danny Abilmona for their technical help and Colby Nadeau for proof-reading this manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research support is provided by the Canadian Institutes of Health Research (CIHR) by a grant to F Colbourne who is also supported by a ‘‘Canada Research Chair in Intracerebral Hemorrhage’’ salary award. The XFI experiment was supported by a joint CIHR/Heart and Stroke Foundation of Canada team grant: Synchrotron Medical Imaging (#CIF 99472) awarded to H. Nichol, F Colbourne and others. Collection of XFI data was carried out at the Stanford Synchrotron Radiation Lightsource, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Stanford University.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
SW is lead author who contributed to the concept and experimental design and data collection, analysis, and interpretation for all three experiments. She also wrote the first draft of this manuscript along with further editing. KF contributed to experiment three by aiding in collecting hematoma volume data and collected the behavioral data. YM contributed to surgical experimental procedures for experiment one and two. HN contributed her expertise in synchrotron technology for concept and experimental design and data analysis for experiment one. She was also involved in XFI data collection and editing this manuscript. FC is the corresponding author who led concept and experimental design and data interpretation for all three experiments and contributed expertise in experimental procedures. He also contributed to the writing of this manuscript and secured funding for this project. All authors read and approved the manuscript.
References
- 1.Foulkes MA, Wolf PA, Price TR, et al. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke 1988; 19: 547–554. [DOI] [PubMed] [Google Scholar]
- 2.Sacco S, Marini C, Toni D, et al. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 2009; 40: 394–399. [DOI] [PubMed] [Google Scholar]
- 3.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 4.Hackett MJ, DeSouza M, Caine S, et al. A new method to image heme-Fe, total Fe, and aggregated protein levels after intracerebral hemorrhage. ACS Chem Neurosci 2015; 6: 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testai FD, Aiyagari V. Acute hemorrhagic stroke pathophysiology and medical interventions: blood pressure control, management of anticoagulant-associated brain hemorrhage and general management principles. Neurol Clin 2008; 26: 963–985. viii–ix. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Stezoski J, Safar P, et al. Mild hypothermia during hemorrhagic shock in rats improves survival without significant effects on inflammatory responses. Crit Care Med 2003; 31: 195–202. [DOI] [PubMed] [Google Scholar]
- 7.Howes D, Gray SH, Brooks SC, et al. Canadian Guidelines for the use of targeted temperature management (therapeutic hypothermia) after cardiac arrest: a joint statement from The Canadian Critical Care Society (CCCS), Canadian Neurocritical Care Society (CNCCS), and the Canadian Critical Care Trials Group (CCCTG). Resuscitation 2016; 98: 48–63. [DOI] [PubMed] [Google Scholar]
- 8.Peliowski-Davidovich A. Hypothermia for newborns with hypoxic ischemic encephalopathy. Paediatr Child Health 2012; 17: 41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 2012; 13: 267–278. [DOI] [PubMed] [Google Scholar]
- 10.Darwazeh R, Yan Y. Mild hypothermia as a treatment for central nervous system injuries: Positive or negative effects. Neural Regen Res 2013; 8: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marion D, Bullock MR. Current and future role of therapeutic hypothermia. J Neurotrauma 2009; 26: 455–467. [DOI] [PubMed] [Google Scholar]
- 12.Sherman AL, Wang MY. Hypothermia as a clinical neuroprotectant. Phys Med Rehabil Clin N Am 2014; 25: 519–529, vii. [DOI] [PubMed] [Google Scholar]
- 13.Lyden PD, Hemmen TM, Grotta J, et al. Endovascular therapeutic hypothermia for acute ischemic stroke: ICTuS 2/3 protocol. Int J Stroke 2014; 9: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemmen TM, Raman R, Guluma KZ, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke 2010; 41: 2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Worp HB, Macleod MR, Bath PM, et al. EuroHYP-1: European multicenter, randomized, phase III clinical trial of therapeutic hypothermia plus best medical treatment vs. best medical treatment alone for acute ischemic stroke. Int J Stroke 2014; 9: 642–645. [DOI] [PubMed] [Google Scholar]
- 16.Fingas M, Clark DL, Colbourne F. The effects of selective brain hypothermia on intracerebral hemorrhage in rats. Exp Neurol 2007; 208: 277–284. [DOI] [PubMed] [Google Scholar]
- 17.Kawanishi M, Kawai N, Nakamura T, et al. Effect of delayed mild brain hypothermia on edema formation after intracerebral hemorrhage in rats. J Stroke Cerebrovasc Dis 2008; 17: 187–195. [DOI] [PubMed] [Google Scholar]
- 18.Liu XC, Jing LY, Yang MF, et al. Enhanced neuroprotection of minimally invasive surgery joint local cooling lavage against ICH-induced inflammation injury and apoptosis in rats. Cell Mol Neurobiol 2016; 36: 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLellan CL, Davies LM, Fingas MS, et al. The influence of hypothermia on outcome after intracerebral hemorrhage in rats. Stroke 2006; 37: 1266–1270. [DOI] [PubMed] [Google Scholar]
- 20.John RF, Colbourne F. Delayed localized hypothermia reduces intracranial pressure following collagenase-induced intracerebral hemorrhage in rat. Brain Res 2016; 1633: 27–36. [DOI] [PubMed] [Google Scholar]
- 21.Fingas M, Penner M, Silasi G, et al. Treatment of intracerebral hemorrhage in rats with 12 h, 3 days and 6 days of selective brain hypothermia. Exp Neurol 2009; 219: 156–162. [DOI] [PubMed] [Google Scholar]
- 22.MacLellan CL, Girgis J, Colbourne F. Delayed onset of prolonged hypothermia improves outcome after intracerebral hemorrhage in rats. J Cereb Blood Flow and Metab 2004; 24: 432–440. [DOI] [PubMed] [Google Scholar]
- 23.MacLellan C, Shuaib A, Colbourne F. Failure of delayed and prolonged hypothermia to favorably affect hemorrhagic stroke in rats. Brain Res 2002; 958: 192–200. [DOI] [PubMed] [Google Scholar]
- 24.Klahr AC, Dietrich K, Dickson CT, et al. Prolonged localized mild hypothermia does not affect seizure activity after intracerebral hemorrhage in rats. Ther Hypothermia Temp Manag 2016; 6: 40–47. [DOI] [PubMed] [Google Scholar]
- 25.John RF, Williamson MR, Dietrich K, et al. Localized hypothermia aggravates bleeding in the collagenase model of intracerebral hemorrhage. Ther Hypothermia Temp Manag 2015; 5: 19–25. [DOI] [PubMed] [Google Scholar]
- 26.Staykov D, Wagner I, Volbers B, et al. Mild prolonged hypothermia for large intracerebral hemorrhage. Neurocrit Care 2013; 18: 178–183. [DOI] [PubMed] [Google Scholar]
- 27.Dohi K, Jimbo H, Ikeda Y, et al. Pharmacological brain cooling with indomethacin in acute hemorrhagic stroke: antiinflammatory cytokines and antioxidative effects. Acta Neurochir Suppl 2006; 96: 57–60. [DOI] [PubMed] [Google Scholar]
- 28.Su X, Zheng K, Ma Q, et al. Effect of local mild hypothermia on regional cerebral blood flow in patients with acute intracerebral hemorrhage assessed by 99mTc-ECD SPECT imaging. J X-ray Sci Technol 2015; 23: 101–109. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Sun G, Zhang J, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 2007; 38: 3280–3286. [DOI] [PubMed] [Google Scholar]
- 30.Zhao XR, Gonzales N, Aronowski J. Pleiotropic role of PPARgamma in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF-kappaB. CNS Neurosci Ther 2015; 21: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke 2011; 42: 1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLellan CL, Silasi G, Poon CC, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab 2008; 28: 516–525. [DOI] [PubMed] [Google Scholar]
- 33.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg GA, Mun-Bryce S, Wesley M, et al. Collagenase-induced intracerebral hemorrhage in rats. Stroke 1990; 21: 801–807. [DOI] [PubMed] [Google Scholar]
- 35.Colbourne F, Sutherland GR, Auer RN. An automated system for regulating brain temperature in awake and freely moving rodents. J Neurosci Meth 1996; 67: 185–190. [PubMed] [Google Scholar]
- 36.Klahr A, Nadeau CA, Colbourne F. Temperature control in rodent neuroprotection studies: methods and challenges. Ther Hypotherm Temp Manage . Epub ahead of print 21 June 2016. DOI: 10.1089/ther.2016.0018. [DOI] [PubMed] [Google Scholar]
- 37.Caliaperumal J, Colbourne F. Rehabilitation improves behavioral recovery and lessens cell death without affecting iron, ferritin, transferrin, or inflammation after intracerebral hemorrhage in rats. Neurorehabil Neural Repair 2014; 28: 395–404. [DOI] [PubMed] [Google Scholar]
- 38.Auriat AM, Silasi G, Wei Z, et al. Ferric iron chelation lowers brain iron levels after intracerebral hemorrhage in rats but does not improve outcome. Exp Neurol 2012; 234: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng W, Nichol H, Liu S, et al. Measuring iron in the brain using quantitative susceptibility mapping and X-ray fluorescence imaging. Neuroimage 2013; 78: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng W, Haacke EM, Webb SM, et al. Imaging of stroke: a comparison between X-ray fluorescence and magnetic resonance imaging methods. Magn Reson Imag 2012; 30: 1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb SM (ed.). The microanalysis toolkit: X-ray fluorescence image processing software. In: AIP conference proceedings, Chicago, 15–20 August 2011.
- 42.Rebouche CJ, Wilcox CL, Widness JA. Microanalysis of non-heme iron in animal tissues. J Biochem Biophys Methods 2004; 58: 239–251. [DOI] [PubMed] [Google Scholar]
- 43.Caliaperumal J, Ma Y, Colbourne F. Intra-parenchymal ferrous iron infusion causes neuronal atrophy, cell death and progressive tissue loss: implications for intracerebral hemorrhage. Exp Neurol 2012; 15: 363–369. [DOI] [PubMed] [Google Scholar]
- 44.MacLellan CL, Auriat AM, McGie SC, et al. Gauging recovery after hemorrhagic stroke in rats: implications for cytoprotection studies. J Cereb Blood Flow Metab 2006; 26: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 45.MacLellan CL, Paquette R, Colbourne F. A critical appraisal of experimental intracerebral hemorrhage research. J Cereb Blood Flow Metab 2012; 32: 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choudhri TF, Hoh BL, Solomon RA, et al. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke 1997; 28: 2296–2302. [DOI] [PubMed] [Google Scholar]
- 47.Wowk S, Ma Y, Colbourne F. Therapeutic hypothermia does not mitigate iron-induced injury in rat. Ther Hypothermia Temp Manag 2016; 6: 23–29. [DOI] [PubMed] [Google Scholar]
- 48.Sheng SP, Lei B, James ML, et al. Xenon neuroprotection in experimental stroke: interactions with hypothermia and intracerebral hemorrhage. Anesthesiology. 117(6): 1262–75. [DOI] [PubMed] [Google Scholar]
- 49.Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993; 24: 987–993. [DOI] [PubMed] [Google Scholar]
- 50.Rost NS, Smith EE, Chang Y, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: the FUNC score. Stroke 2008; 39: 2304–2309. [DOI] [PubMed] [Google Scholar]
- 51.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 2010; 92: 463–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu J, Yang S, Hua Y, et al. Minocycline attenuates brain edema, brain atrophy and neurological deficits after intracerebral hemorrhage. Acta Neurochir Suppl 2010; 106: 147–150. [DOI] [PubMed] [Google Scholar]
- 53.Xue M, Mikliaeva EI, Casha S, et al. Improving outcomes of neuroprotection by minocycline: guides from cell culture and intracerebral hemorrhage in mice. Am J Pathol 2010; 176: 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol 2011; 70: 218–235. [DOI] [PubMed] [Google Scholar]
- 55.Sansing LH, Harris TH, Kasner SE, et al. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochir Suppl 2011; 111: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andresen M, Gazmuri JT, Marin A, et al. Therapeutic hypothermia for acute brain injuries. Scand J Trauma Resusc Emerg Medi 2015; 23: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner KR, Beiler S, Beiler C, et al. Delayed profound local brain hypothermia markedly reduces interleukin-1beta gene expression and vasogenic edema development in a porcine model of intracerebral hemorrhage. Acta Neurochir Suppl 2006; 96: 177–182. [DOI] [PubMed] [Google Scholar]
- 58.Diestel A, Troeller S, Billecke N, et al. Mechanisms of hypothermia-induced cell protection mediated by microglial cells in vitro. Eur J Neurosci 2010; 31: 779–787. [DOI] [PubMed] [Google Scholar]
- 59.Kawabori M, Hokari M, Zheng Z, et al. Triggering receptor expressed on myeloid cells-2 correlates to hypothermic neuroprotection in ischemic stroke. Ther Hypothermia Temp Manag 2013; 3: 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]